Binge-eating disorder (BED) is a serious psychiatric condition characterized by recurrent binge eating absent of compensatory behaviors (e.g. self-induced vomiting, excessive exercise, laxative misuse; American Psychiatric Association [APA], 2022). BED is estimated to be the most common eating disorder and is associated with significant health impairments, including metabolic syndrome, obesity, and associated medical sequalae (Kessler et al., Reference Kessler, Berglund, Chiu, Deitz, Hudson, Shahly and Xavier2013; Mitchell, Reference Mitchell2016). Binge eating is characterized by either the consumption of an objectively large amount of food (approximately >1200 kilocalories) or a subjectively large amount of food (i.e. an amount considered large by the individual) while experiencing a subjective sense of loss of control over what and/or how much one eats. Although the APA's Diagnostic Statistical Manual (DSM-5-TR; APA, 2022) necessitates weekly engagement in objective binge-eating episodes for the diagnosis of BED, individuals with clinically significant binge eating engage in both objective and subjective binge-eating episodes (e.g. Brownstone & Bardone-Cone, Reference Brownstone and Bardone-Cone2020; Kerzhnerman & Lowe, Reference Kerzhnerman and Lowe2002; Mond, Latner, Hay, Owen, & Rodgers, Reference Mond, Latner, Hay, Owen and Rodgers2010; Wolfe, Baker, Smith, & Kelly-Weeder, Reference Wolfe, Baker, Smith and Kelly-Weeder2009). In fact, the International Statistical Classification of Diseases’ (ICD-11; World Health Organization, 2019) conceptualization of binge eating encompasses any eating episodes accompanied by loss of control regardless of quantity, perhaps suggesting that loss of control itself is a critical intervention target. Moreover, when measured to approximate the underlying dimensional construct, the degree of loss of control experienced may be an indicator of binge-eating severity, as it is uniquely associated with distress, impairment, and poorer overall quality of life (Goldschmidt et al., Reference Goldschmidt, Engel, Wonderlich, Crosby, Peterson, Le Grange and Mitchell2012; Latner, Hildebrandt, Rosewall, Chisholm, & Hayashi, Reference Latner, Hildebrandt, Rosewall, Chisholm and Hayashi2007). Thus, given that varying degrees of loss of control may be experienced throughout the day among individuals with binge eating (Bottera & De Young, Reference Bottera and De Young2024), understanding diurnal shifts in loss of control even outside of discrete binge-eating episodes may provide critical insights regarding severity and risk for binge eating.
Given the importance of loss of control eating in BED, coupled with the fact that nearly half of individuals who undergo evidence-based treatments do not demonstrate sustained symptom remission (Atwood & Friedman, Reference Atwood and Friedman2019), a better understanding of the factors that contribute to loss of control eating and its severity may be crucial for improving treatment outcomes. Notably, recent research demonstrated an evening diurnal shift in loss of control eating among individuals with binge-spectrum eating disorders (i.e. eating disorders characterized by binge eating), such that the likelihood of loss of control eating and binge eating increases later in the evening (Bottera & De Young, Reference Bottera and De Young2023; Forester et al., Reference Forester, Schaefer, Dodd, Burr, Bartholomay, Berner and Wonderlich2023; Smyth et al., Reference Smyth, Wonderlich, Heron, Sliwinski, Crosby, Mitchell and Engel2007), as does overall caloric consumption (Ellison et al., Reference Ellison, Simonich, Wonderlich, Crosby, Cao, Mitchell and Peterson2016; Harvey, Rosselli, Wilson, DeBar, & Striegel-Moore, Reference Harvey, Rosselli, Wilson, DeBar and Striegel-Moore2010; Zendegui, West, & Zandberg, Reference Zendegui, West and Zandberg2014). Further, the degree or severity of loss of control eating shows the same diurnal pattern, with the likelihood of experiencing high degrees of loss of control increasing throughout the day (Bottera & De Young, Reference Bottera and De Young2024). These temporal patterns may, according to the biobehavioral circadian model of restrictive eating and binge eating, reflect disruptions to diurnal appetitive rhythms that set the stage for binge eating/loss of control eating to occur in the context of other stressors and related responses, such as elevated negative affect (De Young & Bottera, Reference De Young and Bottera2022). Treatment for BED may therefore be most effective if it can account for and influence the diurnal patterns of shifting risk throughout the day, underscoring the importance of assessing loss of control eating severity across the day regardless of the dichotomous presence or absence of binge-eating episodes. Detailed understanding of diurnal variations of loss of control severity is necessary for the enhancement of BED treatment outcomes through treatment augmentations that target maintaining mechanisms of binge eating; such augmentations may enhance longterm outcomes beyond immediate reductions in binge-eating episodes.
Established treatment approaches for BED include cognitive-behavioral therapy (CBT) and interpersonal therapy (Wilson, Wilfley, Agras, and Bryson, Reference Wilson, Wilfley, Agras and Bryson2010), and recent evidence points to the efficacy of Integrative Cognitive-Affective Therapy (ICAT; Peterson et al., Reference Peterson, Engel, Crosby, Strauman, Smith, Klein and Wonderlich2020). Importantly, both CBT and ICAT work to regulate diurnal appetitive rhythms (i.e. waking eating drive as reflected by eating patterns and/or daytime hormone patterns associated with appetite) through the establishment of a regular pattern of eating (i.e. 3 meals, 2–3 snacks daily) at the outset of treatment (Fairburn, Reference Fairburn2008; Peterson et al., Reference Peterson, Engel, Crosby, Strauman, Smith, Klein and Wonderlich2020; Wonderlich et al., Reference Wonderlich, Peterson, Leone Smith, Klein, Mitchell and Crow2015), which reduces overall binge-eating frequency (e.g. Ellison et al., Reference Ellison, Simonich, Wonderlich, Crosby, Cao, Mitchell and Peterson2016; Fairburn, Reference Fairburn2008; Zendegui et al., Reference Zendegui, West and Zandberg2014). Previous studies using the current dataset demonstrated the efficacy of both CBT guided self-help (CBTgsh) and ICAT in addressing binge eating as well as the role of emotion regulation and other potential maintenance factors related to its occurrence (Hazzard et al., Reference Hazzard, Peterson, Crosby, Schaefer, Smith, Engel and Wonderlich2020; Mason et al., Reference Mason, Smith, Anderson, Schaefer, Engel, Crow and Wonderlich2021a; Mason et al., Reference Mason, Smith, Williams-Kerver, Crosby, Engel, Crow and Peterson2021b; Peterson et al., Reference Peterson, Engel, Crosby, Strauman, Smith, Klein and Wonderlich2020; Smith et al., Reference Smith, Mason, Schaefer, Anderson, Critchley, Crosby and Peterson2021; Smith et al., Reference Smith, Mason, Schaefer, Anderson, Hazzard, Crosby and Peterson2022). One study also demonstrated that risk for binge eating (i.e. eating episodes including loss of control) among treatment-seeking individuals with BED is greatest in the evening (Forester et al., Reference Forester, Schaefer, Dodd, Burr, Bartholomay, Berner and Wonderlich2023). However, no studies using these data or any other dataset have examined the effect of these treatments on the timing and severity of subjective loss of control among individuals with BED.
The current study uniquely extends previous investigations by examining how the within-day patterns of loss of control eating severity change across treatment using ecological momentary assessment (EMA). Specifically, we aimed to examine whether diurnal rhythms in loss of control eating severity change from baseline (i.e. pretreatment) to end-of-treatment and at 6-month follow-up. We hypothesized that at baseline, loss of control eating severity would demonstrate an evening diurnal shift wherein loss of control eating severity would increase across the day, reaching its peak in the evening; following eating disorder treatment, we predicted that the slope of loss of control eating severity across the day would be less pronounced, with less of an evening-shifted increase in severity. In addition, we aimed to explore the differential treatment effects of CBTgsh and ICAT on within-day patterns of loss of control eating severity at baseline, end-of-treatment, and 6-month follow-up. Given limited research demonstrating differences between the two treatments, we approached this question as an exploratory aim. Importantly, the current study's aims and hypotheses, while theory-driven and stated a-priori, were part of secondary analyses and were not preregistered.
Method
Participants
The study included 112 men and women, between the ages of 18 and 64, who met criteria for BED according to the Diagnostic Statistical Manual, 5th edition (DSM–5; APA, 2013). Exclusion criteria for participation were as follows: (a) inability to read English, (b) body mass index (BMI, kg/m2) less than 21.0, (c) lifetime history of psychotic symptoms or bipolar disorder, (d) substance use disorder within six months of enrollment, (e) medical instability, (f) acute suicidality, (g) purging behavior (e.g. self-induced vomiting, misuse of laxatives or diuretics) more than once per month for the previous three months, (h) current diagnosis of bulimia nervosa, (i) medical condition impacting eating or weight (e.g. thyroid condition), (j) history of gastric bypass surgery, (k) currently pregnant or lactating, (l) currently receiving weight loss or eating disorder treatment, (m) use of medication impacting eating or weight (e.g. stimulants), or (n) psychotropic medication changes in the six weeks prior to enrollment. Participants were recruited from eating disorder clinics, community advertisements, and social media postings at two locations in the midwestern United States.
Procedures
Study procedures were approved by the Institutional Review Boards at both data collection sites and all participants provided informed consent. At each assessment period (i.e. baseline, end-of-treatment, and 6-month follow-up), EMA was used to repeatedly assess participants’ current or recent eating experiences multiple times per day over the course of seven days. Specifically, eating behavior, including loss of control eating severity, and associated psychological variables were measured at five time points, semi-randomly dispersed around five anchor times occurring between 8 a.m. and 10 p.m.. Participants were also instructed to self-initiate an additional assessment each day before going to bed.
The baseline EMA protocol was completed following informed consent and diagnostic interviews, and before initiating treatment. Following the baseline EMA, participants were randomized to 17 weeks of either ICAT (n = 56) or CBTgsh (n = 56), and the same 7-day EMA protocol was then completed at end-of-treatment and at 6-month follow-up. A total of 84 participants (75%) completed the end-of-treatment assessment and 92 (82.1%) completed the 6-month follow-up. For more detail on the treatments and study procedures, see Peterson et al., Reference Peterson, Engel, Crosby, Strauman, Smith, Klein and Wonderlich2020.
Measures
DSM-5 BED diagnosis was established by trained assessors using the Eating Disorder Examination version 16.0 (Fairburn, Cooper, & O'Connor, Reference Fairburn, Cooper, O'Connor and Fairburn2008). Comorbid DSM-IV diagnoses were established with the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Version (First, Spitzer, Gibbon, & Williams, Reference First, Spitzer, Gibbon and Williams2002).
To measure loss of control severity during eating episodes using EMA, participants responded to five statements – (a) ‘Feel a sense of loss of control,’ (b) ‘Feel like you could not resist eating,’ (c) ‘Feel you could not stop eating,’ (d) ‘Feel driven or compelled to eat,’ and (e) ‘Feel disconnected – numb, zoned out, on auto pilot’ – following each eating episode recorded during an EMA protocol. Responses to each statement were given on a Likert-type scale from 1 (‘not at all’) to 5 (‘extremely’), and the sum of the five responses was used to quantify the overall degree of loss of control for each eating episode. The appropriateness of the sum score was supported by confirmatory factor analysis, which demonstrated that all five items significantly loaded (all p-values <0.001) onto a single factor (within-person loadings: 0.86a, 0.88b, 0.88c, 0.85d, 0.60e; between-person loadings: 0.89a, 0.95 b, 0.96c, 0.93d, 0.63e) and that measurement reliability was acceptable (ω within−person = 0.92; ω between−person = 0.96) (Geldhof, Preacher, & Zyphur, Reference Geldhof, Preacher and Zyphur2014) (The item about feeling disconnected (‘Feel disconnected – numb, zoned out, on auto pilot’) loaded more weakly onto the loss of control eating factor than the other four items. As a sensitivity check, we re-ran the main analyses while excluding this item. The results did not substantively change.)
Statistical analyses
Statistical analyses were conducted using R version 4.2.2. software program (R Core Team, 2022). Multilevel models (MLMs) were estimated to examine whether within-day slope trajectories of loss of control eating severity differed at baseline, end-of-treatment, and 6-month follow-up assessment periods. These models included a random intercept, fixed effects for linear (hours), quadratic (hours-squared) and cubic (hours-cubed) time components, a fixed, categorical effect of assessment period (i.e. baseline, end-of-treatment, 6-month follow-up) and interactions between time components and assessment period. For the primary analysis, the baseline assessment period was treated as the referent category for the effect of assessment period. However, to allow for comparison of the end-of-treatment and 6-month follow-up assessment periods, we also ran the model with end-of-treatment as the referent category. Additional exploratory MLMs were estimated to examine whether within-day slope trajectories of loss of control eating severity at each assessment period differed for those who completed ICAT v. CBTgsh. These models included a random intercept, fixed effects for linear (hours), quadratic (hours-squared) and cubic (hours-cubed) time components, a fixed effect of treatment condition (i.e. ICAT or CBTgsh) and interactions between time components and treatment condition.
Time was centered on midnight. The linear effect of time indicates whether the initial slope of the regression line (i.e. change in loss of control eating severity) increases, decreases or remains flat over the course of the day. The quadratic effect indicates whether the initial linear slope of loss of control eating severity deflects downward or upward as it moves farther away from midnight. The cubic effect indicates whether the initial deflection intensifies or decelerates in the hours most distal to midnight. Models were estimated with a first-order autoregressive covariance structure to account for serial autocorrelation. Analyses were performed using the nlme package (Pinheiro & Bates, Reference Pinheiro and Bates2022; Pinheiro & Bates, Reference Pinheiro and Bates2000).
Results
Sample characteristics and adherence with EMA procedures
Participant demographics are displayed in Table 1. As noted by Peterson et al. (Reference Peterson, Engel, Crosby, Strauman, Smith, Klein and Wonderlich2020), no significant differences were observed on demographic variables between participants who received ICAT and CBTgsh. EMA adherence rates were 76% for semi-random assessments and 52% for end-of-day assessments.
Table 1. Demographic information

Note. ICAT, Integrative cognitive-affective therapy; CBTgsh, cognitive behavioral therapy guided self-help; SCID, structured clinical interview for DSM-IV; BMI, body mass index. M, mean. SD, standard deviation.
See Peterson et al., Reference Peterson, Engel, Crosby, Strauman, Smith, Klein and Wonderlich2020 for full sample demographics.
Within-day trajectories of loss of control eating severity at baseline, end-of-treatment and follow-up periods
Results for within-day trajectories of loss of control eating severity at each time period are depicted in Table 2. Results indicated that loss of control eating severity was reduced at end-of-treatment (estimate = −3.87, s.e. = 0.18, p < 0.001) and 6-month follow-up (estimate = −3.34, s.e. = 0.18, p < 0.001), relative to baseline, whereas it was greater at 6- month follow-up relative to end-of-treatment (estimate = 0.53, s.e. = 0.19, p = 0.005). Further, main effects of the time components suggested that, overall, severity of loss of control eating increased in a curvilinear manner throughout the day (main effect linear: estimate = 64.61, s.e. = 6.08, p < 0.001; main effect quadratic: estimate = 21.23, s.e. = 6.13, p < 0.001). However, these main time of day effects were qualified by a significant interaction between the cubic time component and the end-of-treatment assessment period relative to baseline (estimate = −23.25, s.e. = 11.00, p = 0.035; Figure 1) and between the cubic time component and the 6-month follow-up assessment period relative to end-of-treatment (estimate = 26.70, s.e. = 11.25, p = 0.018; Figure 1). Specifically, the evening acceleration of loss of control eating severity was blunted at end-of-treatment compared to baseline. In addition, the evening acceleration in loss of control eating severity was greater at 6-month follow-up relative to end-of-treatment. Together, these findings suggest that treatment may have successfully reduced the typical evening increase in loss of control eating severity, but that this effect may not have been maintained at follow-up.
Table 2. Summary of multilevel models examining within-day trajectories of loss of control eating severity at baseline, end-of-treatment and follow-up periods
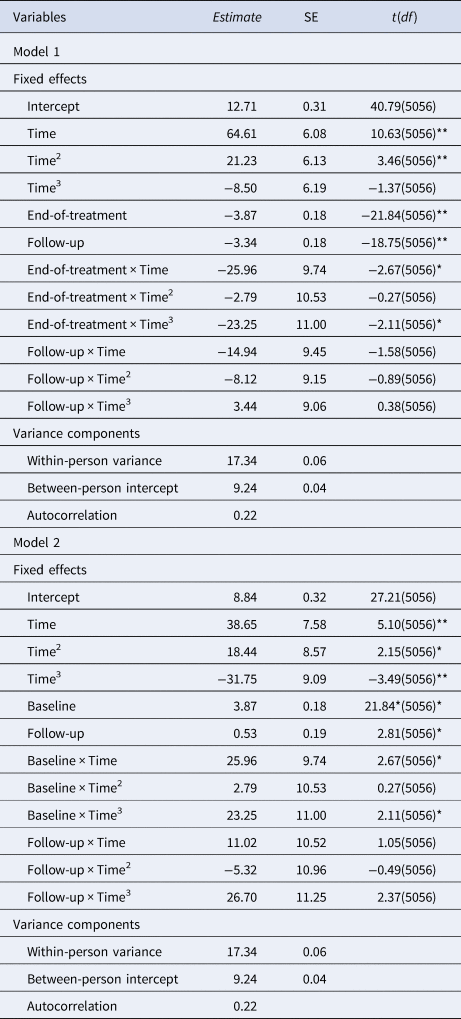
Note. Time, linear effect; Time2, quadratic effect; Time3, cubic effect. *p < 0.05, **p < 0.01.

Figure 1. Within-day slope trajectories of loss of control eating severity at baseline, end-of-treatment, and follow-up assessment periods.
Note. Values on the x-axis represent hours since midnight and values on the y-axis represent the summed total loss of control scores assessed during the 7-day EMA period. Shaded regions indicate standard errors.
Trajectories of loss of control eating severity at each assessment period for ICAT v. CBTgsh
To explore whether these potential treatment effects varied by treatment type, we tested if the within-day slope trajectories of loss of control eating severity differed for those who completed ICAT v. those who completed CBTgsh, separately at each assessment period. Results for these analyses are depicted in Table 3. At baseline (when no treatment effects would be expected), we confirmed that loss of control eating severity increased in a curvilinear manner throughout the day (linear estimate = 40.51, s.e. = 6.22, p < 0.001; quadratic estimate = 15.27, s.e. = 6.10, p = 0.012) and that the time effects did not differ by upcoming treatment type (ps ⩾ 0.835; Figure 2).
Table 3. Summary of multilevel models examining trajectories of loss of control eating severity at each assessment period for ICAT v. CBTgsh

Note. Time, linear effect; Time2, quadratic effect; Time3, cubic effect. Group was coded such that ICAT represents the reference category.*p < 0.05, **p < 0.01.

Figure 2. Within-day slope trajectories of loss of control eating severity at baseline, end-of-treatment, and follow-up for ICAT and CBTgsh treatment conditions.
Note. ICAT, Integrative cognitive-affective therapy; CBTgsh, Cognitive behavioral therapy – Guided self-help. Values on the xaxis represent hours since midnight and values on the y-axis represent the summed total loss of control scores assessed during the 7-day EMA period. Shaded regions indicate standard errors.
During the end-of-treatment assessment period, loss of control eating severity increased in a linear manner throughout the day (linear estimate = 24.53, s.e. = 4.72, p < 0.001). Overall loss of control eating severity did not differ between treatment types at end-of-treatment (p = 0.722). Interactions between treatment condition and time components were non-significant (ps ⩾ 0.517), suggesting that within-day slope trajectory of loss of control eating severity at the end-of-treatment assessment period did not differ for individuals who completed ICAT v. those who completed CBTgsh (Fig. 2).
During the 6-month follow-up assessment period, loss of control eating severity increased in a curvilinear manner throughout the day overall (linear estimate = 24.11, s.e. = 4.91, p < 0.001; cubic estimate = −10.39, s.e. = 5.02, p = 0.039). Loss of control eating severity did not differ between treatment types at follow-up (p = 0.224). However, there was a significant interaction between the cubic time component and treatment condition (p = 0.038). Specifically, the inflection in the within-day trajectory of loss of control eating severity intensified more towards the end-of-the day for individuals who received CBTgsh compared to those who received ICAT (Fig. 2).
Discussion
We aimed to examine whether time of day differentially predicts severity of loss of control experienced during eating episodes at baseline, end-of-treatment, and follow-up and between individuals who received CBTgsh and ICAT. Results demonstrated that loss of control eating severity exhibited an evening diurnal shift, wherein loss of control eating severity was greater in the evening. Further, this evening diurnal shift was most pronounced prior to treatment and reduced at end-of-treatment. However, evening-shifted loss of control eating severity appeared more pronounced at 6-month follow up compared to end-of-treatment, indicating a potential return to baseline. Interestingly, while loss of control eating severity increased in a curvilinear manner both prior to treatment and at follow-up, we observed significant differences between individuals who received CBTgsh and ICAT at 6-month follow-up. Specifically, individuals who received ICAT demonstrated less of an evening-shifted increase in loss of control eating severity compared to those who received CBTgsh, suggesting that the effects of treatment on the pattern of loss of control eating severity may be more durable following ICAT relative to CBTgsh.
Results discussed herein should be interpreted in the context of several limitations. First, the study sample was demographically homogenous and cannot fully represent the efficacy of eating disorder treatment on reducing loss of control eating severity in more diverse populations. Given the prevalence of BED across genders, racial/ethnic identities, and sexual identities (Keski-Rahkonen, Reference Keski-Rahkonen2021; Striegel-Moore & Franko, Reference Striegel-Moore and Franko2003), future research is needed to better understand the effects of ICAT and CBTgsh on daily patterns of loss of control eating severity in more diverse samples. Second, our treatment group sample sizes were small (n = 56, respectively), limiting our power to examine the three-way interaction between time, treatment modalities (i.e. ICAT and CBTgsh), and measurement timepoint (i.e. baseline, end-of-treatment, and 6-month follow-up). While results from our exploratory analyses indicate potential differences between ICAT and CBTgsh, studies including larger sample sizes are needed to assess whether trajectories of loss of control eating severity differentially change across these two treatments. Third, although examining loss of control eating severity represents a significant strength of our study that provides nuanced information apart from discrete occurrence of binge eating, a floor effect following post-treatment reductions in loss of control eating severity remains possible. Finally, a 6-month follow-up period is relatively short and cannot fully demonstrate the long-term effects of treatment on daily trajectories of loss of control eating severity. Although it appears that the effect of treatment on evening-shifted loss of control eating severity was more durable following ICAT relative to CBTgsh, it is possible that these effects would diminish over longer follow-up periods, requiring booster treatments to maintain effects. Such possibilities necessitate treatment studies with longer follow-up periods as well as novel treatment delivery models.
Our results aligned with previous findings demonstrating the presence of an evening diurnal shift in loss of control eating severity (Bottera & De Young, Reference Bottera and De Young2024), such that individuals with BED reported within-day increases and greater severity of loss of control eating later in the evening. Our findings partially supported our hypothesis that evening-shifted loss of control eating severity would be dampened following treatment for binge eating. Compared to pretreatment baseline, the evening increase in loss of control eating severity was blunted post-treatment; however, the effects of treatment on loss of control eating severity appeared to partially wane at 6-month follow up. Thus, for some, treatment's efficacy in reducing the severity of loss of control eating may be less durable. For example, it is possible that individuals with more frequent or severe binge eating at treatment initiation require more intensive treatment or intermittent treatment boosters following a full dose of treatment to maintain the effects. Interestingly, previous results demonstrated that binge-eating frequency was reduced following both ICAT and CBTgsh and that these reductions were maintained at 6-month follow up (Peterson et al., Reference Peterson, Engel, Crosby, Strauman, Smith, Klein and Wonderlich2020), suggesting that measuring loss of control eating severity in addition to discrete binge-eating episodes provides additional insights into treatment's effects. That is, changes in diurnal patterns of loss of control eating severity may serve as a unique risk factor for lapse of binge-eating behavior that could serve as a novel relapse prevention target for individuals following treatment for BED. Further, screenings that assess loss of control eating severity in addition to binge-eating frequency may better capture prodromal risk of binge eating and identify individuals who may benefit from prevention efforts. Importantly, greater degrees of loss of control may signify greater severity of binge eating (Bottera & De Young, Reference Bottera and De Young2024; Goldschmidt, Reference Goldschmidt2017; Latner, Mond, Kelly, Haynes, & Hay, Reference Latner, Mond, Kelly, Haynes and Hay2014), potentially pointing to the importance of reducing severity of loss of control eating within treatment. Given the clinically significant distress associated with loss of control (Goldschmidt et al., Reference Goldschmidt, Engel, Wonderlich, Crosby, Peterson, Le Grange and Mitchell2012), understanding within-day changes in loss of control eating severity, apart from binge-eating episodes, across treatment may have critical implications for proactive problem-solving around at-risk time periods during treatment and sustaining behavioral changes following treatment. While loss of control severity may index momentary distress, explicit examination of the relation between momentary ratings of loss of control severity and concurrent momentary distress is warranted. Further, the impact of various within-person factors (e.g. comorbid presentations, binge-eating frequency) on treatment response as it relates to evening-shifted loss of control eating severity should be investigated using longitudinal and EMA study designs, as observation of within-person differences may aid in the development of personalized, momentary interventions (e.g. Just-in-Time adaptive interventions; Juarascio, Parker, Lagacey, and Godfrey, Reference Juarascio, Parker, Lagacey and Godfrey2018) that facilitate sustained symptom remission.
Finally, results from our exploratory aim highlight the potentially unique efficacy of ICAT in maintaining decreases in evening diurnal shifts in loss of control eating severity long-term. That is, for individuals who received ICAT, relative to CBTgsh, their within-day loss of control eating severity appeared to remain dampened 6-months posttreatment, suggesting that ICAT may not only acutely blunt evening-shifted loss of control eating severity, but that these effects may be uniquely durable. Further, since average daily loss of control eating severity did not differ between individuals who received ICAT or CBTgsh at any timepoint, including the 6-month follow-up, ICAT's potentially unique effect may be specific to the within-day diurnal shift in loss of control eating severity. While our findings are preliminary in nature and necessitate replication with longer follow-up periods, the durability of ICAT's effects on evening-shifted loss of control eating severity may point to an important treatment target for individuals with binge eating. In addition to establishing a regular pattern of eating, ICAT uniquely includes structured meal planning and an affective intervention component (Peterson et al., Reference Peterson, Engel, Crosby, Strauman, Smith, Klein and Wonderlich2020). In accordance with the biobehavioral circadian model of restrictive eating and binge eating (De Young & Bottera, Reference De Young and Bottera2022), structured meal planning likely serves to regulate diurnal appetitive disruptions that may set the stage for binge eating in the context of negative emotions. While CBTgsh emphasizes the importance of regular eating (i.e. 3 meals and 2–3 snacks daily), the structure and extra accountability provided by ICAT compared to CBTgsh may have contributed to consistency and maintanence of regular eating. Further, and perhaps more significantly, ICAT uniquely emphasizes the role emotions play in the onset of binge-eating episodes, targeting negative emotions through adaptive coping strategies and addressing elicitors of negative affect such as excessive self-control and self-neglect, interpersonal difficulties, and self-discrepancy and evaluative standards (Peterson et al., Reference Peterson, Engel, Crosby, Strauman, Smith, Klein and Wonderlich2020; Wonderlich et al., Reference Wonderlich, Peterson, Leone Smith, Klein, Mitchell and Crow2015). On the other hand, CBTgsh does not explictely address emotional anticedents of binge eating. Thus, the large emphasis placed on the role of affect in eliciting binge eating in ICAT may contribute to the longevity of decreased within-day loss of control eating severity. That is, even when present, loss of control eating severity may not demonstrate the same evening-shifted increase if individuals engage in adaptive coping and emotion regulation techniques emphasized in treatment when negative emotions arise. Such integration of treatment components that specifically target negative emotional states that often precipitate binge eating (Haedt-Matt & Keel, Reference Haedt-Matt and Keel2011) may explain the observed differences between ICAT and CBTgsh at 6-month follow-up and could underscore the importance of addressing affective maintanence mechnisms in treatment for binge eating. Further study of the role of momentary emotions on loss of control eating severity, specifically, and the impact affect-focused interventions have on relapse prevention is needed.
Our study is the first to examine how within-day patterns of loss of control eating severity changed across treatment for BED, indicating that both ICAT and CBTgsh reduce evening-shifted loss of control eating severity. Alongside recent literature characterizing the evening diurnal shift of loss of control (Bottera & De Young, Reference Bottera and De Young2023, Reference Bottera and De Young2024), our findings provide a foundation for future investigation of whether explicitly targeting diurnal shifts in loss of control severity impacts illness course for individuals with binge eating. Further, while exploratory and necessitating replication, ours is the first to indicate potential differences in the durability of treatment effects on within-day increases in loss of control eating severity between ICAT and CBTgsh, suggesting that ICAT's effects may be more long-lasting. Overall, current treatments appear to blunt evening-shifted loss of control eating severity immediately following treatment; however, such effects may lose their potency over time, necessitating further understanding of long-term treatment effects on loss of control eating severity and perhaps emphasizing the utility of targeting diurnal patterns of loss of control eating severity and related emotional states in treatment.
Data availability statement
The data that support the findings of this study are available from author CBP ([email protected]) upon reasonable request.
Funding statement
This work was supported by the National Institute of Mental Health (grant numbers: R34MH099040, R34MH098995, T32MH082761), the National Institute of General Medical Sciences (grant number: P20GM134969), and the National Institute of Diabetes and Digestive Kidney Diseases (grant number: P30DK60456).
Competing interests
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








