INTRODUCTION
Case-control studies of salmonellosis have traditionally used cases of laboratory-confirmed diarrhoea, ascertained through public health surveillance, with well residents of the source population as the comparison group [Reference Dwyer1]. Although laboratory confirmation provides high specificity, laboratory-based Salmonella surveillance has a low sensitivity because relatively few cases of salmonellosis are laboratory-confirmed. Of the estimated 1·4 million cases of Salmonella infection each year in the United States, <3% are laboratory-confirmed [Reference Voetsch2]. This is because few persons infected with Salmonella seek medical care and submit a stool specimen to a laboratory.
The low sensitivity of Salmonella surveillance may lead to ascertainment bias in case-control studies if factors that affect the likelihood that a case will be laboratory-confirmed and thereby ascertained are related to exposures of interest [Reference McCarthy and Giesecke3]. This may occur, for example, in persons with salmonellosis if those with medical insurance seek medical care and provide a specimen more frequently than persons without medical insurance, and, if persons with medical insurance have different diets from those without. McCarthy and Giesecke propose to minimize this potential ascertainment bias in case-control studies by using persons with similar laboratory-confirmed illnesses as the comparison group, reasoning that cases and controls would be equally likely to be ascertained [Reference McCarthy and Giesecke3].
To explore the utility of using persons infected with other Salmonella serotypes as the comparison group for cases infected with Salmonella serotype Enteritidis (SE) in a case-control study, we re-analysed data from the Centers for Disease Control and Prevention's (CDC) Foodborne Diseases Active Surveillance Network (FoodNet) Salmonella case-control study using SE cases and non-SE controls. These results were compared with the original SE case-control study analysis results, which used age-, site-, and exposure period-matched controls and comparable multivariable models [Reference Kimura4].
MATERIALS AND METHODS
To ascertain all laboratory-confirmed Salmonella cases, investigators conducted active surveillance at clinical microbiology laboratories at the FoodNet sites (Minnesota, Oregon, and selected counties in California, Connecticut, and Georgia) and large reference laboratories located outside of the FoodNet sites that tested clinical specimens from the FoodNet population [5]. Clinical laboratories forwarded Salmonella isolates to the state public health laboratory at each site for serotyping (which includes serogrouping). All SE isolates were then forwarded to CDC for phage typing [Reference Hickman-Brenner, Stubbs and Farmer6].
The case-control study was conducted for a 12-month period beginning 1 May 1996 in California, Connecticut, and Minnesota and 1 August 1996 in Georgia and Oregon. The methods of the study have been previously described [Reference Kimura4]. Briefly, investigators attempted to contact by telephone all persons aged ⩾18 years, or the parent or guardian of persons aged <18 years, with a laboratory-confirmed Salmonella serogroup B or D infection identified during the study, except in Minnesota where every third identified case was selected. SE is the predominant serotype in serogroup D and S. Typhimurium the predominant serotype in serogroup B, although both serogroups include rarer serotypes. Persons were excluded from the study if they were not residents of the FoodNet catchment area, unreachable by telephone, did not speak English or were otherwise unable to answer questions, or were not interviewed within 21 days of the specimen collection date. Persons who did not have diarrhoea, could not recall the onset date of diarrhoea, experienced diarrhoea onset ⩾10 days before their date of specimen collection, or were a secondary case of salmonellosis within a household were not included in the case-control study. Controls who did not report diarrhoea were selected from households with the same telephone exchange as cases, and matched by categorical age stratum (1–5, 6–11, 12–17, 18–39, 40–59, and ⩾60 years). Trained interviewers administered a standard questionnaire by telephone to persons aged ⩾18 years, to a parent of those aged ⩽12 years, and to either a parent or to persons aged 13–17 years depending on parental consent. Information collected during the interview included demographic details, symptoms and treatment, and pre-existing medical conditions. Persons were asked about their food history (>50 items), dining locations, drinking water sources, international travel, and animal exposures in the 5 days prior to diarrhoea onset.
In this analysis, persons with laboratory-confirmed SE infection were the cases and those with non-SE infection the comparison group. We focused the analysis on consumption of chicken due to its association with SE in the original analysis [Reference Kimura4] and with consumption of undercooked eggs (i.e. eggs with visibly runny yolk) due to its association with SE in numerous epidemiological studies [Reference Patrick7]. Exposure information was collected and analysed dichotomously (yes/no). We asked about consumption of scrambled, fried, poached, or boiled eggs prepared inside or outside the home. We coded undercooked egg consumption as a subset of egg consumption to estimate the incremental risk of undercooked eggs among those who consumed eggs.
Unconditional logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for the association of SE infection and egg or chicken consumption in the 5 days before onset of diarrhoea. Categorical age, FoodNet site, and season [winter (December, January, February); spring (March, April, May); summer (June, July, August); autumn (September, October, November)], race, household income, and factors that have been previously associated with SE infection were examined as potential confounders. These factors included diabetes and underlying immunosuppressive conditions (including medications or treatments); use of antibiotics or antacids in the 4 weeks before onset of diarrhoea; persons with diarrhoea in the same household in the 4 weeks before diarrhoea onset; contact with reptiles and travel outside the United States in the 5 days before diarrhoeal onset. Conditional logistic regression was used to re-analyse the original SE case-control data using age-, site-, and exposure period-matched controls that were not ill, using a multivariable model comparable with the present analysis. Polytomous (for non-ordered categorical variables) logistic regression was used to compare risk factors for the common SE phage types (PT) 4, 8, and 13a to the non-SE illness comparison group [Reference Hosmer and Lemeshow8, Reference Stokes, Davis and Kock9]. The upper-to-lower confidence limit ratio (CLR) was used to compare the relative precision of the odds ratios [Reference Poole10].
RESULTS
A total of 408 SE cases and 1018 non-SE controls were identified during the study period. Of these, 173 (42%) SE cases and 268 (26%) non-SE controls were interviewed (Table 1). Compared with non-SE controls, SE cases were older (median age 33 years compared with 18 years), and were more likely to reside in Connecticut, be white, and have a household income of ⩾$60 000 (Table 2). There were no important differences in the proportions of those with diabetes, underlying immunosuppressive conditions, antibiotic use, antacid use, and persons with diarrhoea in the same household in the 4 weeks prior to illness onset. SE cases were more likely than non-SE controls to have travelled internationally (predominantly to Mexico and Europe) but were not more likely to have had contact with a reptile 5 days before illness onset. The cases were less likely than controls to be hospitalized or report bloody diarrhoea, but had greater duration of diarrhoeal symptoms, and were more likely to be treated with anti-diarrhoeal medication.
Table 1. Phage-type distribution for Salmonella serotype Enteritidis cases and serotype distribution for non-Enteritidis controls by site in the Foodborne Diseases Active Surveillance Network Salmonella serogroup B and D case-control study, 1996–1997

CA, California; CT, Connecticut; GA, Georgia; MN, Minnesota, OR, Oregon; PT, Phage type; RDNC, react but do not conform.
Table 2. Covariate distribution of demographic characteristics, host factors, severity of illness, and international travel for Salmonella serotype Enteritidis cases and non-Enteritidis controls in the Foodborne Diseases Active Surveillance Network Salmonella serogroup B and D case-control study, 1996–1997
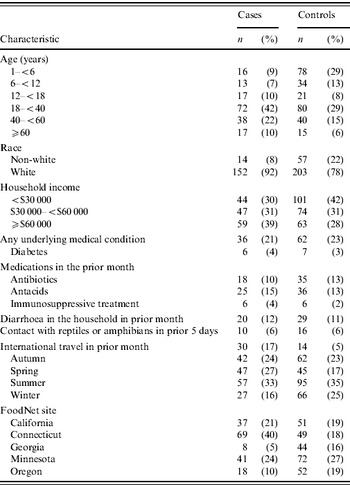
In multivariable analysis, after adjusting for age, site and international travel, SE infection was associated with eating eggs, particularly undercooked egg dishes (Table 3). SE infection was more strongly associated with eating undercooked fried eggs, and eating undercooked scrambled eggs prepared outside the home, than eating undercooked boiled or poached eggs. SE infection was also associated with eating chicken prepared outside the home. The magnitude of this association, however, was less than that with eating undercooked eggs. In a multivariable analysis including multiple risk factors and after adjusting for age, season, site and international travel, the association with eating undercooked eggs (OR 2·6, 95% CI 1·0–6·8) and chicken (OR 1·8, 95% CI 1·1–2·9) prepared outside the home remained, but the estimate for undercooked eggs was less precise.
Table 3. Odds ratios (OR) and 95% confidence intervals (CI) for Salmonella serotype Enteritidis cases and egg and chicken consumption exposures using non-Enteritidis controls and using population-based controls in the Foodborne Diseases Active Surveillance Network Salmonella serogroup B & D case-control study, 1996–1997
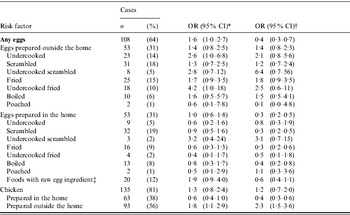
* Multivariable model, adjusted for FoodNet site, season, and international travel, using non-Enteritidis serotypes as controls.
† Multivariable model, adjusted for international travel, using population-based controls who did not report a diarrhoeal illness in the 4 weeks before interview that were matched to cases by age, 5-day exposure period, and FoodNet site.
‡ include cookie dough, batter, frosting, Caesar salad dressing, eggnog, mayonnaise, ice cream, custard, milkshakes, hollandaise or béarnaise sauce.
The estimates in the present analysis using non-SE controls varied slightly from the re-analysis of the original study data which used persons who were not ill as the comparison group. Adjusting for age, season, site, and international travel, the association between SE infection and consumption of undercooked eggs prepared outside the home was stronger in the present analysis (OR 2·6, 95% CI 1·0–6·8) than in the re-analysis (OR 1·8, 95% CI 0·60–5·4) (ratio of ORs 2·6/1·8=1·4). The estimate for consumption of undercooked eggs was more precise in the present analysis (CLR 6·8/1·0=6·8) than in the re-analysis (CLR 5·4/0·6=9·0). In contrast, using the same adjustments, the association between SE infection and consumption of chicken prepared outside the home was weaker in the present analysis (OR 1·8, 95% CI 1·1–2·9) than in the re-analysis (OR 2·8, 95% CI 1·7–4·5) (ratio of ORs 1·8/2·8=0·64). The estimate of consumption of chicken prepared outside the home in the present analysis (CLR 2·9/1·1=2·6) was equally as precise in the re-analysis (CLR 4·5/1·7=2·6).
Of 172 SE isolates from cases that were phage-typed, 33% were PT13a, 23% were PT4, 20% were PT8, 16% were other phage types, and 8% were either untypable or reacted but did not conform to any specified phage type. In a polytomous logistic regression model, adjusted for age, season, site, and international travel, SE PT4 infection was most strongly associated with undercooked egg consumption, and SE PT13a infection with chicken consumption (Table 4).
Table 4. Polytomous logistic regression analysis of risk factors by phage type for illness with Salmonella serotype Enteritidis cases using non-Enteritidis controls, adjusted for age, FoodNet site, season, and international travel in the Foodborne Diseases Active Surveillance Network Salmonella serogroup B and D case-control study, 1996–1997
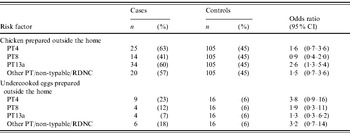
PT, Phage type; RDNC, react but do not conform.
DISCUSSION
The purpose of the present analysis of the FoodNet Salmonella Enteritidis case-control study using persons infected with other Salmonella serotypes as controls was to explore the use of an alternative control population. The results of this analysis are generally consistent with the original analysis that used healthy controls. However, in this analysis the odds ratio increased for consumption of undercooked eggs and decreased for consumption of chicken using non-SE controls.
Persons ill with other Salmonella serotypes (i.e. ill controls with laboratory-confirmed infections) have previously been used in case-control studies of laboratory-confirmed Salmonella infections. In a case-control study during a 1995 outbreak investigation of S. Newport infections in Oregon and Vancouver, two analyses were conducted; one with healthy persons from the community as controls, and one with persons infected with Salmonella serotypes other than S. Newport (and S. Stanley) as controls [Reference Van Beneden11]. Seventeen (41%) cases recalled eating alfalfa sprouts, compared with three (4%) of the community controls (OR 17, 95% CI 4·3–96·0) and 10 (12%) of the salmonellosis controls (OR 5·4, 95% CI 2·0–15). In a SE case-control study in southern Germany, two analyses were also conducted using healthy population controls and controls infected with non-SE; multivariable analyses showed similar associations with consumption of raw or undercooked eggs (OR 1·9 for population controls and 2·2 for non-SE salmonellosis controls) [Reference Kist and Freitag12]. In a FoodNet case-control study of S. Typhimurium infections, antibiotic use in the month prior to illness was a risk factor for multidrug-resistant Salmonella serotype Typhimurium definitive type 104 using both healthy controls and pan-susceptible S. Typhimurium controls [Reference Glynn13]. The results of these Salmonella case-control studies support our conclusion that the use of Salmonella cases as controls is a valid study design. Several other case-control studies have used cases diagnosed with other diarrhoeal diseases as a convenient control group to generate and test hypotheses. In New York, Ackman and colleagues identified exposure to reptiles as a risk factor for paediatric salmonellosis using shigellosis cases as controls [Reference Ackman14]. In the United Kingdom, Campylobacter jejuni cases were used as controls to generate hypotheses for C. coli infection [Reference Gillespie15]. Researchers in Minnesota and the United Kingdom used a similar method to show the increased risk of illness with quinolone-resistant Campylobacter from international travel compared to pan-susceptible Campylobacter [16, Reference Smith17].
A rationale for using ill persons as controls in a case-control study is that in a well- defined population base, such as with the FoodNet surveillance sites used in this analysis, the equal sensitivities of case ascertainment in the case and control group will cancel out and the odds ratio will be unbiased if (1) there are no false-positive cases, (2) the disease is so rare that the case under-ascertainment negligibly affects the apparent person-time at risk, and (3) the case and control Salmonella subtypes are unrelated to the exposure of interest [Reference Poole18]. For example, if the true rates of SE are R 1 in consumers of undercooked eggs and R 0 among non-consumers of undercooked eggs, then the true rate ratio (RR)=R 1/R 0. If the values for the sensitivity of the surveillance system (i.e. the proportion of all Salmonella represented by the laboratory-confirmed cases) are s 1 for the exposed and s 0 for the unexposed, then the estimated rate ratio will be s 1R 1/s 0R 0=(s 1/s 0)RR. If there is differential sensitivity between laboratory-confirmed cases and non-laboratory-confirmed cases, a study in which the controls are a random sample of the source population would produce a biased estimate of the rate ratio (s 1/s 0≠1). However, if cases infected with non-SE serotypes are used as controls and non-SE serotypes are aetiologically unrelated to consumption of undercooked eggs, a control group consisting of all non-SE cases would be equivalent to a random sample of the source population. If sensitivity of non-SE case-finding is the same as the sensitivity of SE case-finding, within categories of undercooked egg consumption, the non-SE controls who are included in the study (by becoming laboratory-confirmed) would be biased by the same factor as the SE cases: s 1/s 0. Thus, the numerator and denominator of the odds ratio would be biased by the same factor, which would be cancelled out by the division, leaving the odds ratio unbiased.
There are at least two practical advantages of using ill controls in a case-control analysis. First, there is the likely reduction in the potential for information bias from differential recall between cases and controls. The questionnaire in the FoodNet case-control study captured exposure data for controls during the matched cases' 5-day incubation period. Persons with salmonellosis were probably prompted by questions from their clinician or local public health officials to recall potential exposures. In the FoodNet Salmonella case-control study, over 25% of cases and controls had been previously contacted by officials from local health departments prior to the case-control study interview (CDC, unpublished data) which may have prompted them to recall potential exposures before symptom onset. Second, the use of ill controls obviates the need to enrol non-ill persons as controls in case-control studies. Control enrolment is generally more difficult than case enrolment and there is some evidence that participation by controls in case-control studies has declined since 1970 [Reference Morton, Cahill and Hartge19].
A limitation of using ill controls is that Salmonella cases and controls with different serotypes will sometimes share causal exposures, resulting in bias towards the null value. It is likely that some of the ill controls in our analysis had the same causal exposures as the cases. For example, our control population included persons who were ill due to S. Heidelberg infection. The FoodNet case-control study of S. Heidelberg infections found an association with eating undercooked eggs, an exposure also found to be associated with SE infections [Reference Hennessy20]. Furthermore, outbreaks of S. Heidelberg infection have been previously associated with consumption of poultry [Reference Layton21, Reference O'Mahony22] and undercooked eggs [23, Reference Chittick24]. Similarly, our control population included persons who were ill due to S. Typhimurium infection. A case-control study of sporadic SE and S. Typhimurium infections in Minnesota in 1989–1990 showed associations between both serotypes and consumption of undercooked eggs [Reference Hedberg25]. The use of controls from a variety of Salmonella serotypes would probably reduce the impact of overlapping exposures by serotype by decreasing the proportion of controls attributable to a specific food item. Disease misclassification is also possible if a food item is contaminated with multiple Salmonella serotypes. A further limitation of using Salmonella cases as controls is that, although the estimate of effect may be valid, the precision of the estimate is limited by the size of the Salmonella patient population. In contrast, the size of the general population controls is not restricted and can be selected based on power considerations. In addition, only two risk factors, egg and chicken consumption, were considered in our analysis of SE. The results from this study design may be more interpretable in a case-control study of epidemic salmonellosis, which is usually attributable to a single source, than in a case-control study of sporadic salmonellosis, which is used to estimate the proportion of salmonellosis that is attributable to a wide variety of sources.
Regardless of the control population used, the value of using specific subtypes to define the cases in a case-control study design using only Salmonella cases is emphasized in our phage-type-specific analysis. The result that SE PT4 was more strongly associated with undercooked or undercooked eggs than other phage types suggests that a specific case definition is needed for case-only analysis of common Salmonella serotypes. In the early 1990s, PT4 increased to become the predominant SE phage type [Reference Hogue26]. Outbreak investigations in 1993 and 1995 linked SE PT4 with egg consumption in restaurants that pooled shell eggs [Reference Boyce27, Reference Sobel28]. In 1999, PT4 accounted for 49% of SE outbreaks reported to national outbreak surveillance, occurring predominantly in the western United States [Reference Patrick7]. The present analysis supports these a priori relationships between SE phage types and limited reservoirs with poultry, suggesting that the method could be used to explore other serotypes for the purpose of attributing Salmonella strains to specific animal reservoirs.
We conclude that using different Salmonella subtypes as controls in risk factor analysis provides a valid method for risk factor identification. However, the use of ill controls may produce biased results to the extent that case and control Salmonella serotypes are aetiologically related. Therefore, this study design may be of limited use to estimate the absolute risk of Salmonella infection for multiple exposures. The study design may provide an indirect method for monitoring relative changes of Salmonella in the animal reservoirs through ongoing prospective Salmonella patient interviews combined with real-time subtyping data and comparisons of risk factors between Salmonella subtypes. These data could be used to monitor changes in the association of Salmonella subtypes to food commodities (e.g. beef, poultry, pork, eggs) as part of ongoing attribution efforts [Reference Batz29] and to quickly identify risk factors during Salmonella outbreak investigations.
ACKNOWLEDGEMENTS
We thank the members of the CDC Emerging Infections Program Working Group for their contributions to this study. Financial support for this research was provided by the Centers for Disease Control and Prevention National Center for Infectious Diseases, the U.S. Department of Agriculture Food Safety Inspection Service, the Food and Drug Administration Center for Food Safety and Applied Nutrition, and National Institute of Environmental Health Sciences grant P30ES10126 (C.P.).
DECLARATION OF INTEREST
None.






