Antidepressants are one of the most widely prescribed drugs. Seventy million prescriptions were dispensed in 2018, amounting to nearly a doubling of prescriptions in a decade.1,Reference Iacobucci2 This striking rise in prescribing is attributed to long-term treatment rather than an increased incidence of depression,Reference Mars, Heron, Kessler, Davies, Martin and Thomas3,Reference Moore, Yuen, Dunn, Mullee, Maskell and Kendrick4 and these trends are not limited to the UK.Reference Gusmão, Quintão, McDaid, Arensman, Van Audenhove and Coffey5–Reference Moore and Mattison7 To reduce the risk of relapse, until the 2022 revision of the guidelines, maintenance treatment was recommended of at least 6 months for patients who have recovered from depression, and at least 2 years for those identified at risk of recurrent depression.8 Some patients may also stay on treatment long term because of difficulties with discontinuation and infrequent monitoring.Reference Petty, House, Knapp, Raynor and Zermansky9 One Scottish study found that over half of patients on antidepressants had been taking them for more than 2 years, with a mean treatment duration of 5.5 years.Reference Johnson, Macdonald, Atkinson, Buchanan, Downes and Dougall10 However, little is known about the health consequences of long-term antidepressant treatment. There is in vitro evidence to suggest that some antidepressants have the potential to cause adverse cardiovascular and metabolic effects.Reference Rami, Guillamat-Prats, Rinne, Salvermoser, Ring and Bianchini11–Reference Serretti and Mandelli14 Yet, most trials assessing the efficacy of antidepressants are poorly suited to examining adverse outcomes: they are often short term, are underpowered to look at most adverse outcomes, have methodological shortcomingsReference Amick, Gartlehner, Gaynes, Forneris, Asher and Morgan15,Reference Cipriani, Furukawa, Salanti, Chaimani, Atkinson and Ogawa16 and do not always report adverse effects, particularly serious ones.Reference Amick, Gartlehner, Gaynes, Forneris, Asher and Morgan15,Reference Maund, Tendal, Hróbjartsson, Jørgensen, Lundh and Schroll17–Reference Healy and Aldred21 Depression is strongly associated with adverse risk profiles such as excess adiposity, smoking, poor diet and physical inactivity.Reference Mezuk, Eaton, Albrecht and Golden22,Reference Moulton, Pickup and Ismail23 These phenotypes and behaviours are established risk factors for a number of chronic conditions, including cardiovascular disease.Reference Koene, Prizment, Blaes and Konety24 Therefore, careful assessment of the long-term cardiometabolic effects of antidepressant treatment is critical.Reference Dragioti, Solmi, Favaro, Fusar-Poli, Dazzan and Thompson25 The main challenge for observational studies examining potential adverse outcomes of long-term antidepressant use is accounting for the excess cardiovascular risk associated with depression (confounding by indication). Studies have attempted to control for this confounding by limiting analyses to patients with a diagnosis of depression. However, there are considerable challenges relating to diagnostic validity, and not all primary care physicians give or record a diagnosis of depression even when it is recognised.Reference Rait, Walters, Griffin, Buszewicz, Petersen and Nazareth26 Another approach is to identify and adjust for cardiometabolic risk factors that confound the association between depression and cardiometabolic outcomes. Meta-analyses of studies exploring the association between antidepressant use and a wide range of cardiometabolic outcomes reveal considerable heterogeneity between studies,Reference Salvi, Grua, Cerveri, Mencacci and Barone-Adesi27–Reference Trajkova, d'Errico, Soffietti, Sacerdote and Ricceri29 and the evidence base remains weak.Reference Dragioti, Solmi, Favaro, Fusar-Poli, Dazzan and Thompson25 For example, a recent meta-analysis showed a 27% increased risk of diabetes with antidepressant use, but there was considerable variation in confounder adjustment within individual studies and none fully accounted for major risk factors and predictors for diabetes, including key markers of the metabolic syndrome.Reference Salvi, Grua, Cerveri, Mencacci and Barone-Adesi27
Given the multifactorial nature of depression and cardiometabolic disease,Reference Willett, Koplan, Nugent, Dusenbury, Puska, Gaziano, Jamison, Breman, Measham, Alleyne, Claeson and Evans30,Reference Stolk, Rosmalen, Postma, de Boer, Navis and Slaets31 information on a wide range of prospectively measured confounders, including lifestyle, sociodemographic factors and baseline biomarkers for cardiometabolic disease, are needed to provide robust estimates of the risks associated with long-term antidepressant use. This requires richly phenotyped cohorts. One such cohort is UK Biobank, which is a large population-based cohort study (approximately 500 000 participants).Reference Sudlow, Gallacher, Allen, Beral, Burton and Danesh32 This open-access resource has detailed information on socioeconomic status; demographics; anthropometric, behavioural and biochemical risk factors; disability and health status with linkages to routinely available national data-sets including primary care records and deaths. We used the UK Biobank data-set to examine the association between antidepressant use and four cardiometabolic morbidity outcomes (diabetes, hypertension, cerebrovascular disease (CV), coronary heart disease (CHD)) and two mortality outcomes (cardiovascular disease (CVD) mortality and all-cause mortality).
Method
Study cohort
UK Biobank recruited approximately 500 000 participants aged 40–69 years between 2006 and 2010. Our cohort was restricted to participants (N = 222 121) whose data had been linked to primary care records during the first phase of primary care data extraction (extracted in 2018, released 2019). Biobank participants who were registered with a general practitioner (GP) practice at least 12 months before study baseline and remained registered at study entry (Biobank entry date) were eligible for inclusion. Participants were excluded from this study if they had a prior recorded prescription for antidepressants (≤12 months before baseline); any prior recorded diagnosis for the outcome of interest; any prior recorded prescription for antipsychotics, lithium or antimanic drugs; or self-reported use of cardiometabolic drugs at baseline. We also excluded participants on antidepressant polytherapy. Participants entered our cohort at the Biobank baseline assessment date. Participants who did not have the event of interest within the follow-up period were censored at the earliest of date of death, date of leaving the GP practice or end of the follow-up period (either 5 or 10 years).
Ethics and consent
UK Biobank has obtained ethics approval from the North West Multi-Centre Research Ethics Committee, which covers the UK (approval number: 11/NW/0382), and has obtained written informed consent from all participants.
Exposure assessment
We extracted information on antidepressant use (antidepressant type, strength of medication, date of prescription and quantity prescribed) from linked primary care prescribing data focusing on ten of the most commonly prescribed antidepressants in England,1 with the exclusion of amitriptyline (often prescribed for pain or sleep problems in low doses) and dosulepin (not recommended in the UK national depression guidelines by the National Institute for Health and Care Excellence).33 Information on the dosing schedule was not available for extraction. The remaining eight antidepressants were categorised by drug class as selective serotonin reuptake inhibitors (SSRIs; citalopram, sertraline, fluoxetine, paroxetine) and ‘other’ antidepressants (mirtazapine, venlafaxine, duloxetine, trazodone). Antidepressant treatment was defined as a time-varying exposure (i.e. participants were classified as unexposed before their first antidepressant prescription, and subsequently classified as exposed at the date of the first antidepressant prescription). Antidepressant use was assessed in three ways: any antidepressant treatment, SSRI antidepressant treatment or other antidepressant treatment. To explore the dose–response relationship between antidepressant use and outcome, we calculated the number of defined daily doses (DDDs) by using values on the average maintenance dose assigned by the World Health Organization Collaborating Centre for Drug Statistics Methodology (www.whocc.no/atc_ddd_index). These were categorised as ≤0.5, >0.5 to 1.0 and >1.0. For example, for citalopram the DDD is 20 mg, 0.5 DDD is 10 mg and >1.0 DDD is anything >20 mg. In our study, DDD categories are referred to as low (≤0.5), intermediate (>0.5 to 1.0) and high (>1.0). For the purposes of calculating the DDD, we used the recorded product strength and estimated the prescribed daily dose from the total amount prescribed divided by the duration of treatment. The reference category was no antidepressant use and included unexposed time periods before starting treatment and unexposed time for those who did not receive an antidepressant prescription during follow-up.
Outcome assessment
We selected four morbidity (diabetes, hypertension, CHD, CV) and two mortality (CVD and all-cause mortality) outcomes. Information on study outcomes were identified with relevant Read v2 and CTV3 codes (using the Quality and Outcomes Framework version 38; https://webarchive.nationalarchives.gov.uk/ukgwa/20220117164934/https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/quality-and-outcomes-framework-qof/quality-and-outcome-framework-qof-business-rules/quality-and-outcomes-framework-qof-business-rules-v-38-2017-2018-october-code-release) extracted from linked primary care records (diabetes, hypertension, CHD, CV) and ICD-10 codes extracted from death records (CVD, all-cause mortality). Outcomes were only included if they occurred after the date of entry into the cohort. We defined first incidence as the first recorded outcome during follow-up and with no prior recorded diagnosis for the outcome in primary care records and no self-reported diagnosis at baseline assessment. We initially planned to look at risk of outcome over 5 years; however, because of the small numbers of events, we extended our follow-up period to 10 years. We have included results from the 5-year follow-up to allow for comparison.
Confounder assessment and selection
As highlighted earlier, depression – the main indication for antidepressants – is strongly associated with adverse risk profiles such as excess adiposity, smoking and physical inactivity. These are established risk factors for CVD and diabetes. To account for these shared risk factors, and given the multifactorial nature of cardiometabolic disease, we identified a wide range of personal, lifestyle, sociodemographic and biomarker covariates as potential confounders. These were age; gender; body mass index (BMI); waist/hip ratio; smoking and alcohol intake status; physical activity; parental history of outcome; biochemical and haematological biomarkers (apolipoproteins A and B, vitamin D, triglycerides, haemoglobin A1c); socioeconomic status (accommodation status, number of vehicles per household, employment status, benefits status, urban/rural status, education, household income) and self-reported long-term illness, disability or infirmity (as a generic measure of ‘ill health’). All confounders were assessed at baseline. Analyses were restricted to participants with non-missing information on confounders. Confounders for each outcome and follow-up period were selected with a stepwise approach through backward elimination, beginning with a model that included the main exposure of interest and all potential confounders. Except for age and gender (included in all models), confounders were retained where the Wald test was P≤0.05 and excluded if P > 0.05. Non-linear relationships between outcome and continuous confounders were considered by identifying, at each iterative step of the stepwise process, the best-fitting fractional polynomial terms. Details of the selected confounders for each model are shown in Supplementary Appendix 1 available at https://doi.org/10.1192/bjo.2022.563.
Sensitivity analysis
We did not have sufficient numbers to compare the effects of short-term and long-term antidepressant usage. However, we carried out a sensitivity analysis to exclude short-term usage (<90 days) and get a better sense of long-term chronic effects, i.e. related to metabolic dysfunction.
Statistical analysis
All analyses were conducted in Stata version 16 for Windows. The association between antidepressant treatment and each outcome (diabetes, hypertension, CV, CHD, CVD mortality and all-cause mortality) was quantified using Cox's proportional hazards model, with study duration as the underlying timescale. Antidepressant treatment was treated as a time-varying exposure. Hazard ratios and 95% confidence intervals were estimated for each antidepressant treatment category (any, SSRI, other) and outcome. Results are first provided from a model adjusting for baseline age and gender, and then from the fully adjusted model with the confounders selected by the multivariable selection procedure described above. We also estimated hazard ratio and 95% confidence interval by DDD category for the 10-year follow-up (insufficient number of events at 5-year follow-up). The proportional hazards assumption was assessed by means of the scaled Schoenfeld residuals, which were used to test the proportionality over time for each covariate in the final model being fitted. If there was evidence at the 5% level of a violation of the proportional hazards assumption for any covariate, the final model was refitted to include a time-varying coefficient (i.e. an interaction term between that particular covariate and time). Kaplan–Meier curves for each of the outcomes are shown in Supplementary Appendix 2.
Results
Biobank participants who had linked primary care data were similar to participants without such data in terms of key sociodemographic and clinical characteristics (age, gender, ethnicity, socioeconomic status, BMI, long-term illness) (Supplementary Appendix 3). The number of participants in the final study cohort varied by outcome (Fig. 1). Baseline participant characteristics are presented for each outcome cohort in Table 1. On average (median) participants were aged 56–57 years, around half of the participants or just over were female, and 96% were of White ethnicity.
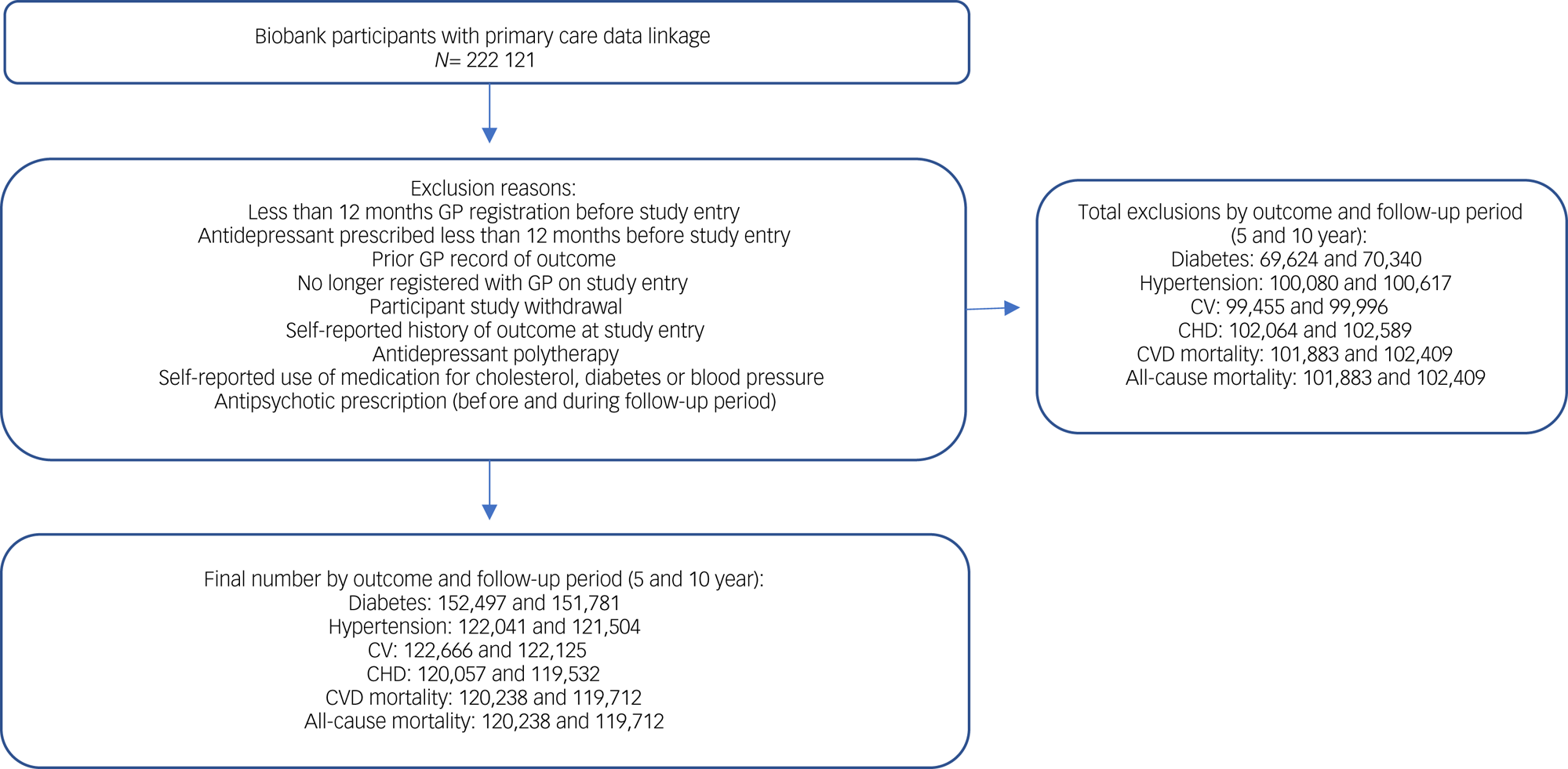
Fig. 1 Cohort selection. CHD, coronary heart disease; CV, cerebrovascular disease; CVD, cardiovascular disease; GP, general practitioner.
Table 1 Participant baseline characteristics at 5-year follow-up

CV, cerebrovascular disease; CHD, coronary heart disease; CVD, cardiovascular disease; IQR, interquartile range; BMI, body mass index; WHR, waist/hip ratio; NVQ/HND, National Vocational Qualification/Higher National Diploma.
a. Parental history of outcome, for mortality outcomes this is parental history of cardiometabolic and vascular disease.
On average, 8% of participants in each cohort had been prescribed an antidepressant by the 5-year follow-up and 6% by the 10-year follow-up (Supplementary Appendix 4). SSRIs were the most commonly prescribed antidepressant class (80–82%), and citalopram was the most commonly prescribed SSRI (46–47%). Mirtazapine was the most frequently prescribed antidepressant in the ‘other’ category (44–46%) (Supplementary Appendix 4).
The number of events, person-years of follow-up and hazard ratios (95% confidence intervals) for the six outcomes by antidepressant class are presented in Tables 2 and 3 for the 5- and 10-year follow-ups, respectively.
Table 2 Hazard ratios by outcome and antidepressant class at 5-year follow-up

SSRI, selective serotonin reuptake inhibitor; CV, cerebrovascular disease; CHD, coronary heart disease; CVD, cardiovascular disease.
Table 3 Hazard ratios by outcome and antidepressant class at 10-year follow-up

SSRI, selective serotonin reuptake inhibitor; CV, cerebrovascular disease; CHD, coronary heart disease; CVD, cardiovascular disease.
At 5 years (Table 2), in models that adjusted for age and gender, any antidepressant use was associated with an increased risk of diabetes (hazard ratio 1.28, 95% CI 1.03–1.59), CHD (hazard ratio 1.63, 95% CI 1.22–2.17) and all-cause mortality (hazard ratio 1.79, 95% CI 1.45–2.23), with only weak evidence of an increased risk of CVD mortality (hazard ratio 1.53, 95% CI 0.89–2.61), with the confidence interval for the latter including the null. For CHD and all-cause mortality, findings were attenuated after further adjustment for confounders (CHD: hazard ratio 1.47, 95% CI 1.10–1.95; all-cause mortality: hazard ratio 1.37, 95% CI 1.10–1.70), and were no longer evident in the fully adjusted models for diabetes (hazard ratio 0.83, 95% CI 0.66–1.04) or CVD mortality (hazard ratio 1.21, 95% CI 0.70–2.10). Looking at antidepressants by class (SSRIs and ‘other’) did not change the overall pattern of results for CHD and all-cause mortality. ‘Other’ antidepressants were associated with an increased risk of diabetes in the model adjusting for age and gender (hazard ratio 2.03, 95% CI 1.47–2.81), but this was no longer evident in the fully adjusted model (hazard ratio 1.13, 95% CI 0.81–1.57). However, SSRIs were weakly associated with a reduced risk of diabetes in the model adjusted for age and gender, which became stronger following full adjustment (hazard ratio 0.64, 95% CI 0.49–0.83). There was no clear evidence of any association between antidepressant use and either hypertension or CV, although the number of CV outcomes was small (n = 31).
Antidepressant treatment was similarly associated with an increased risk of CHD and all-cause mortality at 10 years (Table 3), and these effects were only slightly attenuated in the fully adjusted model.
Antidepressants in the ‘other’ class were associated with a higher risk of these outcomes. There was a weak association between any antidepressant use and incident diabetes (hazard ratio 1.12, 95% CI 0.92–1.37), but the direction of this effect was reversed following adjustment for all confounders (hazard ratio 0.77, 95% CI 0.63–0.94). Similar findings were observed for SSRIs, but ‘other’ antidepressants were associated with a small increased risk of diabetes, although the confidence interval included the null (hazard ratio 1.17, 95% CI 0.87–1.57). There was also evidence that any antidepressant use was associated with an increased risk of both CV and CVD mortality at 10 years, which attenuated only slightly following full adjustment (CV: hazard ratio 1.26, 95% CI 0.97–1.62; CVD mortality: hazard ratio 1.89, 95% CI 1.44–2.49). Looking at antidepressant class, this effect was only observed for SSRIs and CV (fully adjusted hazard ratio 1.34, 95% CI 1.02–1.77), but was seen with both antidepressant groups for CVD mortality. In contrast, any antidepressant use was associated with a reduction in incident hypertension (fully adjusted hazard ratio 0.80, 95% CI 0.70–0.91), as was SSRI use (hazard ratio 0.77, 95% CI 0.66–0.89).
There was some evidence of a dose–response effect (Table 4) for all-cause mortality, with higher doses associated with an increased risk of this outcome. This was reflected in the analysis of the two antidepressant classes. A similar pattern was evident for CV and CHD, but the results were subject to considerable uncertainty. There was no clear evidence of a dose-response effect for the other outcomes.
Table 4 Defined daily dose hazard ratios by outcome and antidepressant class at 10-year follow-up

DDD, defined daily dose; SSRI, selective serotonin reuptake inhibitor; CV, cerebrovascular disease; CHD, coronary heart disease; CVD, cardiovascular disease.
The results of the sensitivity analysis removing individuals with short periods of antidepressant use did not have a marked effect on the associations of interest (Supplementary Appendix 5).
Discussion
Brief summary of the main findings
This population-based cohort study investigated whether commonly prescribed antidepressants were associated with a risk of developing diabetes, hypertension, CV, CHD and mortality (CVD and all-cause). Our study found that long-term antidepressant use was associated with an increased risk of CHD, CVD and all-cause mortality. These issues appear to be more problematic for antidepressants other than SSRIs (mirtazapine, venlafaxine, duloxetine, trazodone), with the use of such drugs associated with a two-fold increased risk of CHD, CVD and all-cause mortality at 10 years. There was also some evidence that antidepressants, and particularly SSRIs, were associated with a reduced risk of developing hypertension and diabetes. The findings were particularly evident after 10 years of follow-up, where we had larger numbers of events.
Comparison of hazard ratios before and after adjustment for a large number of confounders suggested that the associations between antidepressant use and increased risk of diabetes are confounded by adverse clinical phenotypes commonly associated with depression. For diabetes, this confounding appears to be driven by key metabolic risk markers and factors for this condition, mainly haemoglobin A1C and BMI.Reference Chan, Rimm, Colditz, Stampfer and Willett34,Reference Bennett, Guo and Dharmage35 This is less apparent for hypertension, where there was little difference in estimates before and after adjustment for all confounders.
Comparison with existing studies
Previous meta-analyses have highlighted the challenges of comparing work in this field because of significant heterogeneity in study design and methods, including measurement of exposures, outcomes and adjustment for confounders.Reference Dragioti, Solmi, Favaro, Fusar-Poli, Dazzan and Thompson25 A systematic review and meta-analysis of randomised trials found that SSRIs were associated with an improvement in glycemia, which was not moderated by depression status, diabetes status or change in weight across studies.Reference Tharmaraja, Stahl, Hopkins, Persaud, Jones and Ismail36 This work is consistent with our findings of a lower risk of diabetes with SSRI treatment. In contrast, a meta-analysisReference Salvi, Grua, Cerveri, Mencacci and Barone-Adesi27 and pharmaco-vigilance studyReference Siafis and Papazisis37 both found an association between treatment with antidepressants and increased rates of diabetes. The conflicting findings between these studies and our work could be explained by differences in the adjustment for confounders. For example, in the meta-analysis, none of the included studies adjusted for haemoglobin A1C and less than half adjusted for BMI.Reference Salvi, Grua, Cerveri, Mencacci and Barone-Adesi27 The lack of adjustment of key risk factors associated with depression – the main indication for treatment – and outcomes suggests a lack of sufficient control for confounding by indication in previous work. This applies to previous work related to all of our studied outcomes.
There is some evidence that depression is associated with lower blood pressure,Reference Licht, de Geus, van Dyck and Penninx38,Reference Hildrum, Romild and Holmen39 although this is contradicted by a meta-analysis of prospective cohort studies that concluded depression ‘is probably a risk factor for hypertension’.Reference Meng, Chen, Yang, Zheng and Hui40 Licht et al also found that antidepressant use increased the risk of developing hypertension, whereas the other studies mentioned did not adjust for antidepressant use.Reference Licht, de Geus, van Dyck and Penninx38,Reference Hildrum, Romild and Holmen39 Our study is not in line with the findings of Licht et al regarding the effect of antidepressant treatment. We found a reduction in the risk of developing hypertension for the ‘any’ antidepressants and ‘SSRI’ categories, although it was less convincing for the category of ‘other’ antidepressants.
Coupland et alReference Coupland, Dhiman, Morriss, Arthur, Barton and Hippisley-Cox41,Reference Coupland, Hill, Morriss, Moore, Arthur and Hippisley-Cox42 described an increased risk of CV with antidepressants in those with incident prescriptions who were aged >65 years. However, these studies were based on primary care data and adjusted for a limited range of confounders. A meta-analysis also found that SSRIs were associated with an increased risk of CV (relative risk 1.24, 95% CI 1.15–1.34).Reference Biffi, Scotti and Corrao28 The results of this meta-analysis should be treated with caution because the estimates are characterised by a high between-study heterogeneity. Moreover, it was not possible to distinguish between the effects of antidepressants and depression itself. Our fully adjusted model at 10-year follow-up, although showing a trend toward increased risk, also includes the possibility of no association (hazard ratio 1.26, 95% CI 0.97–1.62) for all antidepressants. However, there was some evidence of an increase in risk for those on SSRIs (hazard ratio 1.34, 95% CI 1.02–1.77). Although this may reflect a genuine risk from this class of drug, it may also be because SSRIs are widely perceived as safer than other antidepressants, and are therefore more likely to be prescribed to those who are already at risk.
Our finding of an increase in the risk of developing CHD and of CVD mortality is broadly in line with published work.Reference Maslej, Bolker, Russell, Eaton, Durisko and Hollon43 However, there are some differences. Oh et alReference Oh, Kim, Myung, Hwang and Yoon44 highlighted the risk of tricyclic antidepressants in CHD and found that SSRIs did not increase risk, although most of their evidence came from case–control designs and studies that were scored as low quality. Our findings are more nuanced. We found an increase in the risk of CHD and CVD mortality in the 10-year fully adjusted model, except for CHD in those taking SSRIs, where confidence intervals included the null. We did not replicate Coupland et al's finding of a reduced risk of myocardial infarction in a younger cohort.Reference Coupland, Dhiman, Morriss, Arthur, Barton and Hippisley-Cox41
Our finding of an increase in all-cause mortality at 10-year follow-up is supported by Almeida et al.Reference Almeida, Alfonso, Hankey and Flicker45 They described a risk that increases with the severity of depression. In their study, those who were currently well and taking antidepressants were at lower risk than those who were depressed, irrespective of whether they were taking antidepressants. This suggests that other factors related to depression (for example, suicidality) may be more important contributors to all-cause mortality than antidepressants. Our study design does not allow us to determine this.
Strengths and limitations
The major strength of this study is the linkage of a richly phenotyped national prospective cohort study to primary care records. This has enabled us to examine multiple cardiometabolic outcomes, and thus obtain a more complete picture on the potential long-term effects of taking antidepressant medication. Importantly, the use of the linked data from Biobank has enabled adjustment for a wide range of prospectively measured confounders – both clinical and socioeconomic – in our analytical models. In addition, this data linkage ensured the availability of high-quality data in terms of measures of exposure and outcomes. We have therefore been able to overcome the limitations of previous work that have relied solely on prospective cohorts (with poorly measured exposures/outcomes) and those that utilised data from primary care records (with limited information on confounders).
Limitations include the time lag between measurement of confounders and outcomes (up to 5 and 10 years). We did not have enough events to carry out a sensitivity analysis at 1-year follow-up to allow us to assess the impact of this. Furthermore, although we adjusted for a wide range of potential confounders, we cannot rule out the possibility of residual confounding. The low number of events meant that we could not compare outcomes by individual antidepressants. However, we were able to explore findings by antidepressant class. Like previous work, we did not have information on the severity of depression at the time of prescription and outcome. This information is not included routinely in primary care records. No information on dosing schedule was available. However, we calculated DDDs by using WHO data to explore dose–response effects. Patients may not have taken their antidepressant medication as prescribed, and therefore it is possible that there may be some misclassification of the antidepressant exposure. Type 1 errors might be inflated from testing of multiple outcomes, and significant findings should be interpreted with caution.
Finally, only 44% of the Biobank had linked primary care data available at the time of our analyses and this could have introduced bias. However, comparison of baseline characteristics between those with and without linked primary care data suggested few differences. Our study cohort is mostly of White British ethnic origin and our findings require replication in more ethnically diverse cohorts, particularly given ethnic differences in cardiometabolic risk and disease.
Clinical implications
Antidepressants, and especially SSRIs, may have a good safety profile in the short term, but are associated with adverse outcomes in the long term. This is important because most of the substantial increase in prescribing in the past 20 or more years is in long-term repeat prescribing. Although we cannot establish causality, we have described concerning associations with increases in CHD, CVD and all-cause mortality that are broadly in line with earlier findings, but have been undertaken in a cohort that has had detailed prospectively recorded information, enabling us to adjust for important confounders. The increase in all-cause mortality is also worrying, although, as we note above, other factors related to depression (for example suicidality) may be more important contributors to all-cause mortality than antidepressants. Some of our findings are less concerning. We found some evidence that, once other clinical and socioeconomic factors are adjusted for, antidepressants – and particularly SSRIs – may reduce the risk of developing hypertension and diabetes. This is intriguing and, if it is supported, suggests directions for research into the mechanisms involved in the association between antidepressants and CHD and CVD mortality. Since this is an observational study, our findings do not imply causality, and highlight the importance of further work to investigate and elucidate potential mechanisms. In the meantime, the message for clinicians is that prescribing of antidepressants in the long term may not be harm-free, and it is particularly important to review the cardiovascular health of patients on antidepressants more proactively and have discussions around stopping treatment for those on long-term treatment, particularly those with CVD.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2022.563
Data availability
The data was created under UK Biobank application number 46704 and is available directly from UK Biobank.
Author contributions
N.B. was the study Principal Investigator; was responsible for study conception, funding acquisition, data curation, formal analysis, methodology and project administration; wrote the original manuscript and reviewed and edited the manuscript. M.H. was responsible for study methodology, formal analysis and reviewed and edited the manuscript. R.A.P., D.J.S., D.K. and N.W. were responsible for study conception, funding acquisition and reviewed and edited the manuscript.
Funding
This research is funded by the National Institute of Health Research (NIHR) School for Primary Care Research (grant number 422 awarded to Bansal (Principal Investigator), Payne, Kessler and Wiles). The views expressed are those of the author(s) and not necessarily those of the NIHR, NHS or Department of Health. This study was also supported by the NIHR Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol (Kessler and Wiles). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
This research has been conducted with the UK Biobank Resource under application number 46704.
Declaration of interest
None.









eLetters
No eLetters have been published for this article.