Adipose tissue is now considered to be an endocrine organ in addition to playing its classical role in storing energy. The endocrine action of adipocytes involves secreting hormones or cytokines that are referred to as ‘adipokines’(Reference Zhang, Proenca and Maffei1). These molecules are mainly involved in energy equilibrium, lipid and glucose homeostasis, angiogenesis, adipogenesis and inflammatory response, among others. During obesity, the endocrine and metabolic functions of adipocytes are impaired(Reference Vazquez-Vela, Torres and Tovar2). Therefore, adipocyte dysfunction has been related to systemic abnormalities associated with the development of the metabolic syndrome(Reference Hajer, van Haeften and Visseren3, Reference Karastergiou and Mohamed-Ali4).
Previous efforts have focused on the identification of molecular links between adipocyte function and hypertension in obesity. Particularly, human and rodent adipocytes express all of the components of the renin–angiotensin system (RAS)(Reference Engeli, Schling and Gorzelniak5, Reference Scheen6). This means that fat cells produce the RAS precursor angiotensinogen (Agt), although the liver is generally the main source of this molecule. Agt is converted into angiotensin I due to the action of renin, and angiotensin I is converted into angiotensin II by an angiotensin-converting enzyme (ACE). Adipocytes express enzymes that synthesise angiotensin II from Agt and also express the angiotensin II receptors (ATR1 and ATR2)(Reference Crandall, Herzlinger and Saunders7). This suggests that adipocytes produce the effector hormone and are also able to sense it. Typically, angiotensin II exerts its effects in smooth vascular cells through the promotion of vasoconstriction by ATR1. In addition, angiotensin II induces aldosterone production and Ca flux in the heart(Reference Endoh8, Reference Takanashi and Endoh9). Interestingly, during obesity, the mRNA abundance of Agt in the adipose tissue and circulating concentrations are increased(Reference Massiera, Bloch-Faure and Ceiler10–Reference Van Harmelen, Ariapart and Hoffstedt12). In fact, adipose Agt can significantly contribute to the total concentration of Agt in obese subjects. Furthermore, ACE gene expression in adipose tissue has been correlated with BMI(Reference Gorzelniak, Engeli and Janke13). This evidence indicates that angiotensin produced by adipose tissue exerts systemic physiological effects but also modifies local adipocyte function.
In fact, the effects of angiotensin II on glucose metabolism have been recently studied in different cells. The infusion of angiotensin II into the abdominal subcutaneous adipose tissue reduces glucose uptake(Reference Boschmann, Ringel and Klaus14). In smooth muscle vascular cells, angiotensin II decreases insulin receptor substrate-1 phosphorylation and consequent glucose uptake(Reference Folli, Kahn and Hansen15). This evidence suggests that insulin action can be impaired by angiotensin II in the adipose tissue. Insulin regulates adipogenesis through Akt activation, which has been described as an essential process during adipocyte differentiation(Reference Baudry, Yang and Hemmings16–Reference Yun, Kim and Tucker18) and angiogenesis(Reference Gerber, McMurtrey and Kowalski19). Thus, insulin signalling in adipocytes results in the formation of mature adipocytes and blood vessels as well as glucose internalisation.
Dietary factors are known to affect the functionality of adipocytes and may influence the regulation of RAS. We have demonstrated that the type of dietary protein can change the expression of several genes in the adipose tissue of obese rats in comparison with rats fed casein (Cas). Particularly, soya protein (Soy) consumption positively modifies the adipose tissue's transcriptome and metabolism, which in turn could ameliorate the metabolic syndrome(Reference Frigolet, Torres and Uribe-Figueroa20). However, there is no evidence whether the consumption of Soy regulates the gene expression of RAS and angiogenesis in adipose tissue. For these reasons, the aim of the present study is to determine the effect of Soy consumption on rat adipose tissue gene expression of RAS and angiogenesis components, vascularisation and angiotensin II-mediated Akt phosphorylation in a model of diet-induced obesity.
Materials and methods
Animals and protocol
Male Sprague–Dawley rats (4 weeks old) weighing an average of 100 g were obtained from Harlan Teklad (Mexico City, Mexico). All the rats were housed in individual cages in a controlled temperature environment with a 12 h light–12 h dark cycle and had free access to water and food. The rats were fed four different diets for 160 d. The diets were prepared from basic ingredients as described in Table 1. High-fat diets consisted of 25 % fat. Cas high-fat (Cas HF) and Soy high-fat (Soy HF) contained 20·9 kJ/g, and control diets (5 % fat; Cas and Soy) contained 16·7 kJ/g. The diets had 30 % of either Soy or Cas, and the dietary protein concentration was adjusted on the basis of protein purity (Cas 90·6 % and Soy 86 %). The animals fasted for 12 h and were killed with CO and a guillotine. The serum was stored at − 20°C for measuring the biochemical parameters, and the retroperitoneal adipose tissue was stored at − 70°C for the extraction of RNA. The protocol for the present study was approved by the Animal Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán.
Table 1 Composition of experimental diets
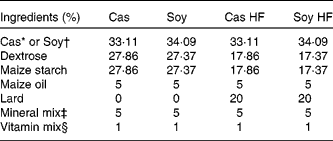
Cas, casein; Soy, soya protein; Cas HF, Cas high-fat; Soy HF, Soy high-fat.
* ‘Vitamin-free’ Cas (Harlan Teklad Research Diets, Madison, WI, USA).
† Supro 710 (Solae, México City, Mexico).
‡ Rogers-Harper (Harlan Teklad Research Diets).
§ AIN-93-VX, Harlan Teklad Research Diets (mg/kg diet): nicotinic acid, 150; calcium pantothenate, 80; pyridoxine-HCl, 35; thiamine-HCl, 30; riboflavin, 30; folic acid, 10; d-biotin, 1; vitamin B12, 0·1; dl-α tocopheryl acetate (500 IU/g; 500 mg/g) 750; vitamin A palmitate (500 000 IU/g; 150 mg/g), 40; vitamin D3 (cholecalciferol, 500 000 IU/g; 1·25 mg/g), 10; vitamin K (phylloquinone), 3·75.
Biochemical parameters
The fasting serum cholesterol and TAG concentrations were measured by colorimetric kits (DiaSys Diagnostic Systems, Holzheim, Germany). Fasting serum glucose was measured using an YSI2700 select Biochemistry Analyzer (YSI Incorporated, Yellow Spring, OH, USA). Also, fasting serum leptin and insulin were measured using a Lincoplex kit (Linco Research, St Charles, MO, USA). Culture media angiotensin I and angiotensin II concentrations were assayed by RIA kits (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA).
Real-time quantitative PCR
Total RNA was extracted from retroperitoneal fat tissues by Chomczynski's method using guanidine thiocyanate(Reference Chomczynski and Sacchi21). For integrity analysis, 15 μg of total RNA was separated by 1·0 % agarose/2·2 m-formaldehyde gel electrophoresis. Of the total RNA from each animal, 300 ng were subjected to RT before PCR amplification with the Two-Step Master Mix (Applied Biosystems, Foster City, CA, USA). Parallel non-template control reactions were run in the absence of RNA to detect nucleic acid contamination in the reaction mixtures. TaqMan fluorogenic probes and oligonucleotide primers were designed by Applied Biosystems. TaqMan PCR assays for each target gene were carried out in triplicate in ninety-six-well optical plates with an ABI Prism 7000 Sequence Detection System (Perkin-Elmer Applied Biosystems). For every PCR sample, an amplification plot was generated from the collected data, and a threshold cycle (C T) value was calculated with the software suite. Using the standard curve, C T values for each gene were used to calculate the relative initial quantity of complementary DNA. Hypoxanthine phosphoribosyltransferase (HPRT) was used as an invariant control. Taqman assays for Agt (Rn00593114), ACE (Rn01416296), ATR1 (Rn01435427), ATR2 (Rn00560677), vascular endothelial growth factor a (VEGFa; Rn01511601), angiopoietin 2 (Rn01756774), matrix metalloproteinase 2 (MMP2; Rn01538170), a tissue inhibitor of matrix metalloproteinase-3 (TIMP3; Rn00441826_m1) and HPRT (Rn01527840) were obtained from Applied Biosystems.
Adipocyte culture
Adipocytes were obtained from retroperitoneal fat pads. The fat depots were resected under aseptic conditions, and adipocytes were isolated by collagenase digestion according to the procedure of Rodbell(Reference Rodbell22) with minor modifications as described below. The fat pads were minced in the Krebs–Ringer HEPES buffer (pH 7·4) containing 5 mm-d-glucose, 2 % bovine serum albumin, 135 mm-NaCl, 2·2 mm-CaCl2.2H2O, 1·25 mm-MgSO4.7H2O, 0·45 mm-H2PO4 and 10 mm-HEPES. Adipose tissue fragments were digested in the Krebs–Ringer HEPES buffer with 1·25 mg/ml type II collagenase at 37°C with gentle shaking for 45 min at eighty cycles/min. The resultant cell suspension was diluted in 13 ml of a cold Krebs–Ringer HEPES buffer. For washing, the cells were resuspended in 50 ml of buffer and centrifuged at 400 g for 5 min. The final wash was made with 13 ml of the culture medium (Dulbecco's modified Eagle's medium (DMEM) containing 10 % fetal bovine serum). Floating cells were collected as primary adipocytes, plated on 6 cm dishes, and cultured at 37°C in 5 % CO2. Viability was calculated using trypan blue, and it always resulted in 90 % or above. Each replicate of 105 adipocytes was incubated with 2 ml of DMEM containing 10 % fetal bovine serum, and a 1 % penicillin–streptomycin mixture. The cells were stimulated with insulin (10− 7 m; Insuman, Aventis, Mexico) for 15 min, or they were stimulated with insulin followed by angiotensin II (100 nm; Sigma-Aldrich, St Louis, MO, USA) for 30 min. The unstimulated cell culture was used to collect the media and measure angiotensin concentration.
Immunoblot
After stimulation, the cells were lysed with cell lysis buffer (1 % Triton, 6·4 mm-NaCl, 1 mm-EDTA, 100 mm-NaF, 1 mm-sodium orthovanadate) and one tablet/10 ml of protease inhibitor mixture (Complete Mini; Roche Diagnostics, Werk Penzberg, Germany) and homogenised at 4°C. The homogenate was centrifuged at 4000 g for 5 min before protein determination by the Lowry's method(Reference Lowry, Rosebrough and Farr23). Equal amounts of protein (100 μg) were mixed with a loading buffer, boiled for 5 min, and separated by SDS-PAGE. After electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes (Amersham Biosciences, Chalfont St Giles, Bucks, UK) using an electrophoretic transfer system (Bio-Rad, Hercules, CA, USA). The membranes were then treated with a blocking reagent (Bio-Rad) and incubated overnight at 4°C with specific primary rabbit antibodies: anti-Akt or anti-phospho (Ser 473) Akt (sc8312, and sc7985R; Santa Cruz Biotechnology, Santa Cruz, CA, USA); primary antibody dilutions were 1:250 and 1:500, respectively. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:5000; Santa Cruz Biotechnology) for 1 h at room temperature. Finally, the blots were developed with enhanced chemiluminescence detection reagents (Millipore, Bedford, MA, USA). For quantitative analyses, a densitometric analysis of bands on immunoblots was done with the Sigma Scan software (Aspire Software International, Ashburn, VA, USA).
Histological analysis
Paraffin sections (4 μm of thickness) of formalin-fixed fat pads were stained with haematoxylin and eosin and analysed with a Leica microscope equipped with a digital camera. For each sample, ten cells or five blood vessels in three areas were evaluated. Also, vessels in each area were identified and counted. Adipocytes and blood vessel areas with blood vessels were obtained using Leica software for digital imaging processing.
Statistical analysis
The results obtained in the present study are presented as the means with their standard errors, and they were evaluated with one-way or two-way ANOVA followed by Fisher's protected least significant difference test to determine statistical differences. The correlations between media levels of angiotensin and mean adipocyte area were assessed by the Pearson correlation test. Differences were considered statistically significant at P < 0·05 and were indicated by the letters in the figures, where ‘a’ was the highest value (a>b>c>d).
Results
Weight gain, energy intake and biochemical parameters
At the end of the study, rats fed a Cas HF or Soy HF diet gained significantly more weight than the respective control groups; however, those fed Cas gained more weight than those fed Soy (Fig. 1(A)). These results are in agreement with a previous study from our laboratory demonstrating that Soy induces less weight gain and less total body fat(Reference Torre-Villalvazo, Tovar and Ramos-Barragan24). Differences in weight gain were not attributed to changes in energy intake because the amount of energy consumed, on average, for each group was not significantly different (Fig. 1(B)). The serum angiotensin I and II concentrations showed no statistical difference between groups (data not shown). However, adipocytes from rats fed Soy HF released lower angiotensin I (Fig. 1(C)) and angiotensin II (Fig. 1(D)) into the media culture than did rats fed Cas HF. The control groups did not show a significant difference with regard to angiotensin I or II release; however, the Cas group did have an increased media concentration when compared with the Soy group. Leptin and insulin serum concentrations were decreased by Soy consumption and by lower dietary fat content. On the other hand, serum glucose and cholesterol levels were increased in the Cas and Soy groups that were fed HF diets. The soya control group had a lower TAG serum concentration than the Cas control group (Table 2). Thus, Soy consumption resulted in a decrease in angiotensin release in cultured adipocytes and also reduced leptin, insulin and TAG serum concentration in rats.

Fig. 1 Body weight gain and angiotensin media concentration are reduced by dietary soya protein (Soy) without changes in energy consumption. (A) Body weight gain, (B) energy intake, (C) angiotensin I media concentration, (D) angiotensin II media concentration from rats fed casein (Cas, –Δ–), Cas high-fat (Cas HF, –▲–), Soy (–○–) and Soy high-fat (Soy HF, –●–) for 160 d. Values are means, with their standard errors represented by vertical bars, n 15. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
Table 2 Serum leptin, insulin, glucose, TAG and cholesterol concentration in rats fed casein (Cas) or soya protein (Soy) containing adequate or high-fat content for 160 d
(Mean values with their standard errors, n 15)

Cas HF, Cas high-fat; Soy HF, Soy high-fat.
a,b,c,d Mean values with unlike superscript letters within a row were significantly different (P < 0·05).
Angiotensin and angiogenesis-related gene expression in adipose tissue
Unexpectedly, Agt mRNA abundance was the lowest in the adipose tissue of rats fed Cas HF. No differences were observed between the remainder of the groups. However, ACE expression was three-fold higher in the adipose tissue of rats fed HF compared with the control groups; this suggests enhanced angiotensin II production during obesity. The expression of ATR1 was lower in the Soy group compared with the Cas group, but the HF groups showed the opposite pattern. This means that Soy consumption accompanied by an adequate dietary fat concentration possibly attenuates ATR1-mediated functions. On the other hand, ATR2 mRNA abundance was not modified by the experimental diets. The critical angiogenic factors, VEGFa and angiopoietin 2 mRNA expression, were increased in the adipose tissue of the Cas HF group compared with the Cas or Soy HF groups. TIMP3 and MMP2 expressions were also higher in the adipose tissue of rats fed a Cas HF diet than in rats fed a Soy HF diet (Table 3). This means that angiogenesis and extracellular matrix remodelling are induced in obese rats due to augmented adiposity and the necessity to produce more vessels or branch pre-existing vessels.
Table 3 Relative mRNA abundance of the renin–angiotensin system and angiogenesis-related genes in in rats fed casein (Cas) or soya protein (Soy) containing adequate or high-fat content for 160 d
(Mean values with their standard errors, n 5)

Cas HF, Cas high-fat; Soy HF, Soy high-fat.
a,b,c,d Mean values with unlike superscript letters within a row were significantly different (P < 0·05).
Akt phosphorylation in vitro
In order to know whether Soy consumption can ameliorate angiotensin-induced Akt inactivation, adipocytes cultured from different groups were incubated with insulin or pre-incubated with angiotensin II before stimulation with insulin. Akt phosphorylation was always increased by insulin compared with the cultured control adipocytes. Increased Akt phosphorylation by insulin was impaired by angiotensin II in all groups (Fig. 2(A), (B) and (D)) except the Cas HF group (Fig. 2(C)). The latter indicates that angiotensin II intensifies the effect of insulin on Akt activation during obesity. Thus, in the Cas HF group, angiotensin II maintains Akt activation by possibly increasing fatty acid esterification and consequent fat storage. The activation of Akt promotes fat storage via glucose uptake, preadipocyte differentiation or adipogenesis(Reference Fayard, Xue and Parcellier25). However, regarding dietary protein, the extent of Akt phosphorylation of the basal and insulin-stimulated states is higher in the adipocytes of rats fed Soy or Soy HF in contrast to the adipocytes in the Cas or Cas HF groups.

Fig. 2 Akt activation induced by insulin is altered by angiotensin II in adipocytes from rats fed soya protein (Soy). Adipocytes were prepared from rats fed the experimental diets for 160 d and were hormonally stimulated with insulin: 10− 7 m and angiotensin: 100 nm. Cell homogenates were subjected to immunoblotting with the anti-Akt antibody. Arbitrary density units of phosphorylated Akt (pAkt)/total Akt are shown for (A) casein (Cas), (B) Soy, (C) Cas high-fat (Cas HF), (D) Soy high-fat (Soy HF) adipocytes not stimulated, stimulated with insulin or with insulin and angiotensin II. Values are means, with their standard errors represented by vertical bars, n 5. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
Adipocyte size and in vitro angiotensin release
Fat cell size resulted in larger retroperitoneal fat depot of rats fed Cas compared with rats fed Soy, and also in HF groups v. controls. This indicates that the consumption of a Cas diet containing a high dietary fat concentration was associated with an increased adipocyte area (data not shown). Interestingly, adipocyte hypertrophy was directly associated with the concentration of angiotensin I (Fig. 3(A)) and II (Fig. 3(B)) that was released into the media from cultured fat cells with significant correlations (R 0·98; P < 0·018, and 0·95; P < 0·049, respectively). Thus, increased adipocyte volume, which has been associated with adipose tissue dysfunction, can predict an augmented local angiotensin I and angiotensin II release because the systemic concentration of both hormones did not change due to dietary treatments.
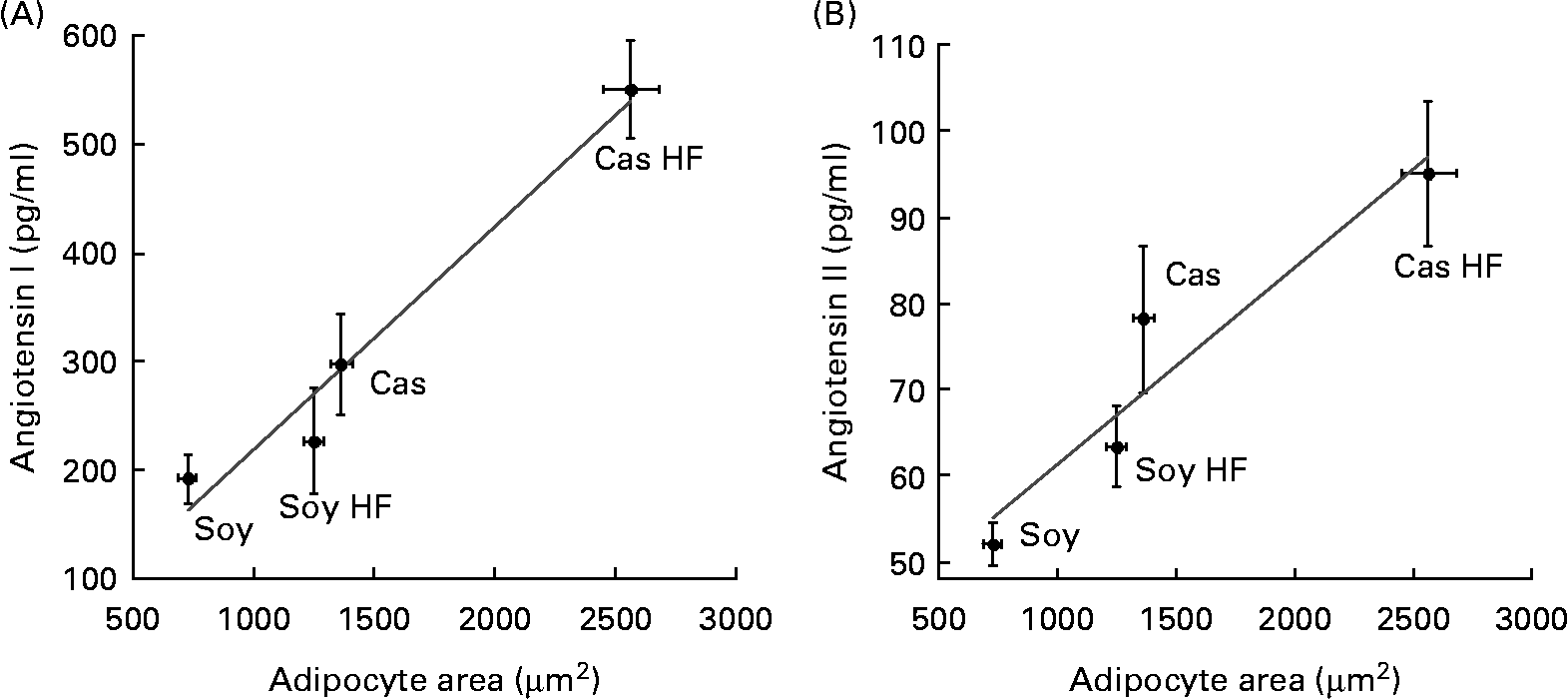
Fig. 3 Lower angiotensin release by adipocytes of rats fed soya protein (Soy) correlates with decreased adipocyte area. (A) Media concentration of angiotensin I (R 0·98; P = 0·018) and (B) angiotensin II (R 0·95; P = 0·049) are directly associated with adipocyte size in a model of diet-induced obesity in rats fed casein (Cas), Cas high-fat (Cas HF), Soy and Soy high-fat (Soy HF) for 160 d. Values are means, with their standard errors represented by vertical bars, n 5. Significant correlation is indicated (P < 0·05).
Adipose tissue vascularisation
Because angiotensin is a potent vasoconstrictor, its action may be related to the area of the blood vessels(Reference Assali and Westersten26). Furthermore, hypoxia or impaired angiogenesis is a feature of the decreased function of adipocytes(Reference Wood, de Heredia and Wang27). Therefore, we calculated the number and area of blood vessels in the adipose tissue of rats fed with dietary treatments. The area containing vessels was 37 % higher in the Soy group, which was in contrast with the Cas group (Fig. 4(A)). Also, the number of blood vessels was twofold higher in the adipose tissue of rats fed Soy HF than those fed Cas HF (Fig. 4(B)). These results indicate that Soy positively modifies adipose tissue vascularisation in different ways depending on the addition of dietary fat.

Fig. 4 Adipose tissue vascularisation is increased by dietary soya protein (Soy). (A) Blood vessel area and (B) blood vessel number in adipose tissue of rats fed casein (Cas), Cas high-fat (Cas HF), Soy and Soy high-fat (Soy HF) for 160 d. Values are means, with their standard errors represented by vertical bars, n 5. a,b Mean values with unlike letters were significantly different (P < 0·05).
Discussion
Obesity, a disease that is characterised by increased adiposity, has become a world health problem. The presence of dysfunctional adipocytes in the adipose tissue in animal models of obesity and in obese humans has been related to the development of the metabolic syndrome(Reference Hajer, van Haeften and Visseren3). Dietary strategies to avoid the mass growth of adipose tissue and impaired metabolism include modifications in carbohydrate and lipid type as well as content(Reference Abete, Astrup and Martinez28). Nevertheless, few studies have addressed the influence of dietary protein on adipocyte function(Reference Frigolet, Torres and Uribe-Figueroa20). The results of the present study show that dietary protein also modifies the RAS and angiogenesis components in the adipose tissue, as well as Akt phosphorylation associated with angiotensin II in the adipocytes of rats.
To induce obesity in the rat, it is necessary to increase fat content in the diet above 40 % since the rat model is resistant to developing obesity with dietary treatments that include lower fat concentrations(Reference Buettner, Scholmerich and Bollheimer29, Reference Ghibaudi, Cook and Farley30). Indeed, commercial and home-made HF diets are formulated to contain 45 % energy from fat in order to induce obesity and the metabolic syndrome. It was interesting to observe that rats fed the soya diet gained significantly less weight than those fed the Cas diet. This is in agreement with previous studies in our group that demonstrate that the consumption of the Soy diet promotes lower weight gain, and this is accompanied by a significantly lesser accumulation of retroperitoneal fat pads(Reference Frigolet, Torres and Uribe-Figueroa20). However, this difference was not observed during the first 60 d of the study, when the rats had a higher requirement of protein, suggesting that differences in final body weight are not due to differences in protein quality. Consequently, there was a decrease in the circulating leptin accompanied by lower serum insulin and TAG concentrations in comparison with the Cas HF group. The prevention of hyperinsulinaemia by Soy is indicative of the maintenance of insulin sensitivity(Reference Noriega-Lopez, Tovar and Gonzalez-Granillo31) in these rats despite the consumption of a HF diet. Thus, Soy may promote an appropriate activation of the insulin pathway(Reference Paz, Hemi and LeRoith32, Reference Zick33). In contrast, the hyperinsulinaemia observed in rats fed a Cas-HF diet was associated with a high TAG serum concentration, and this is in agreement with previous observations where hyperinsulinaemia in mice has been correlated with hypertriacylglycerolaemia and reduced insulin receptor binding(Reference Shanik, Xu and Skrha34).
Soy intake was found to promote not only diminished circulating insulin concentration but also higher levels of basal and insulin-mediated Akt phosphorylation in adipocyte culture. Akt is activated in the adipose tissue to induce glucose uptake via Glut-4 membrane translocation(Reference Cong, Chen and Li35, Reference Yancopoulos, Davis and Gale36). Additionally, Akt phosphorylation is necessary and sufficient to stimulate adipogenesis as demonstrated in Akt 1 knockout mice, which are unable to induce the differentiation programme by PPAR-γ(Reference Zhang, Huang and Duvel37). Furthermore, Akt has been described as a mediator of the insulin-like growth factor-1 receptor signal cascade for inducing adipogenesis(Reference Xu and Liao38). In turn, adipogenesis regulates angiogenesis(Reference Crandall, Busler and McHendry-Rinde39, Reference Hausman40), and this process has also been related to Akt activation. VEGF and angiopoietins promote vascular homeostasis and angiogenesis through the activation of Akt signalling(Reference Shiojima and Walsh41). Akt also increases NO production and regulates vascular tone(Reference Dimmeler, Fleming and Fisslthaler42, Reference Fulton, Gratton and McCabe43). Subsequently, increased Akt activation in the isolated adipocytes of rats that were fed Soy suggests enhanced adipocyte differentiation and blood vessel formation. These results are in agreement with our findings regarding increased blood vessel area and number in the adipose tissue of the Soy groups. As we previously demonstrated, the TAG:DNA ratio was reduced in the adipose tissue of rats that consumed Soy(Reference Frigolet, Torres and Uribe-Figueroa20), thus confirming the presence of numerous smaller adipocytes in a more active adipogenic tissue.
Interestingly, the expression of VEGF, angiopoietin 2, MMP2 and TIMP3 mRNA was greater in the adipose tissue of rats fed a HF Cas diet in comparison with a HF Soy diet. Indeed, VEGF is the most critical factor initiating adipose angiogenesis in response to hypoxia. Blood vessel sprouting is accompanied by extracellular matrix degradation by MMPs and TIMP(Reference Pepper44). Subsequently, these angiogenic genes can be up-regulated in response to adipose tissue growth and adipocyte enlargement when alternative vascularisation is mandatory. Together, these results show that obesity in Cas HF rats induces angiogenesis-associated gene expression.
Angiogenesis in obesity conditions can be influenced by adipokine-mediated Akt activation. For instance, adiponectin stimulation of human macrophages promotes TIMP1 release through IL-10 induction(Reference Kumada, Kihara and Ouchi45), new vessel growth through AMP-activated protein kinase and Akt in endothelial cells(Reference Ouchi, Kobayashi and Kihara46). Also, leptin influences angiogenesis in the cornea and endothelial cell migration, proliferation and MMP and VEGF expression(Reference Bouloumie, Drexler and Lafontan47–Reference Sierra-Honigmann, Nath and Murakami49). Thus, the hyperleptinaemia observed in the Cas HF group coincides with the augmented angiogenic gene expression. Angiotensin II is also an adipokine (not exclusively secreted by adipocytes) that has been implicated in the regulation of angiogenesis(Reference Fernandez, Tarlatzis and Rzasa50, Reference Le Noble, Hekking and Van Straaten51) via ATR1(Reference Sasaki, Murohara and Ikeda52, Reference Shimizu, Okamoto and Chiba53) and the consequent augmented expression of VEGF. When we incubated adipocytes with angiotensin II, the hormone decreased insulin-stimulated Akt phosphorylation in all groups except in the obese group that was fed the Cas HF diet. Thus, it is possible that angiogenic genes can be up-regulated in the adipose tissue of obese animals due to angiotensin-mediated Akt activation. In the non-obese groups, angiotensin II reversed the effects of insulin phosphorylating Akt, probably because of adequate adipose vascularisation, regulated recruitment of new adipocytes, and decreased flux towards TAG accumulation. In contrast, adipocytes from obese rats locally released increased concentrations of angiotensin II, possibly to maintain vascularity and blood flow(Reference Goossens, Blaak and Saris54, Reference Goossens, McQuaid and Dennis55) while the adipose tissue was being expanded.
Angiotensin has been proposed as a molecular link between hypertension, insulin resistance and obesity because RAS components are extensively expressed during obesity. The expression of RAS components was also studied in this model. Increased ATR1 and ACE mRNA in the adipose tissue of rats that were fed HF diets is in agreement with the fructose-fed rat model(Reference Giacchetti, Sechi and Griffin56) and human obesity(Reference Van Harmelen, Ariapart and Hoffstedt12). In fact, ATR1a deficiency protects rodents from high-fat-induced obesity(Reference Kouyama, Suganami and Nishida57). These results may imply that increased local angiotensin II release and high expression of ATR1 greatly stimulate ATR1 signalling in an autocrine fashion in adipocytes. Angiotensin II via ATR1 inhibits adipocyte differentiation and promotes adipocyte hypertrophy(Reference Sharma, Janke and Gorzelniak58). These metabolic adaptations agree with our results in the adipose tissue of obese rats where increased ATR1 mRNA, angiotensin release and adipocyte hypertrophy concur. Interestingly, Soy ameliorates the abnormalities promoted by angiotensin during obesity.
In summary, Soy consumption promotes increased basal and insulin-stimulated Akt activation, beneficially modifies the expression of RAS components and maintains the vascularity and cell size of adipocytes in a diet-induced obesity model. Therefore, the type of dietary protein can maintain adipocyte functionality.
Acknowledgements
The present study was supported by the National Council of Science and Technology, grant 46135-M. M. E. F. conducted the experimental research, analysed the data, and contributed to the manuscript. N. T. contributed to the analysis of the data and to the discussion of the manuscript. A. R. T. designed the study, analysed the data, and wrote the manuscript. The authors had no conflicts of interest.









