The detection and imaging of hydrogen peroxide (H2O2) in complex biological environments is very important because of the involvement of this destructive oxidant in signaling and cellular development. Cellular imbalance of H2O2 has been connected to aging and various severe diseases, including cancer, cardiovascular disorders, and Alzheimer’s. A nanosensor for the detection of H2O2 in intracellular environments has been developed by researchers at The University of Adelaide, Australia, by combining fluorescent nanodiamonds and organic fluorescent probes. The hybrid peroxy-nanosensor exhibits unprecedented photostability and is capable of ratiometric detection of H2O2.
Carboxy peroxyfluor-1 (PF1) is an organic fluorescent probe designed to track H2O2, and is one of the two fluorescent components of the newly synthesized hybrid material, as reported in a recent issue of Scientific Reports (doi:10.1038/s41598-017-15772-0). The probes are attached on the surface of nitrogen-vacancy (NV) nanodiamonds, which are nontoxic nanoparticles that have been engineered to contain high concentrations of NV centers. These NV centers are fluorescent point defects consisting of a nitrogen atom (which substitutes a carbon in the lattice) and an adjacent lattice vacancy.
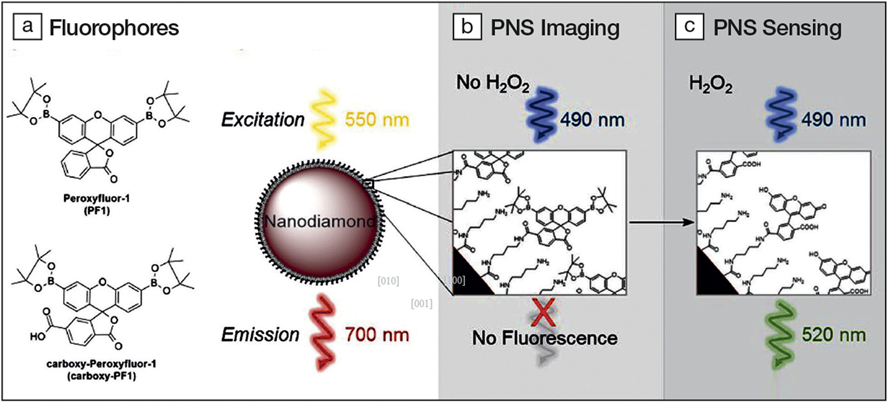
(a) Structures of organic fluorophores PF1 and carboxy-PF1. (b) Scheme of peroxynanosensor (PNS) imaging. The nanodiamond is excited at 550 nm and emits stable fluorescence around 700 nm enabling long-term imaging. (c) Scheme of H2O2 sensing by PNS. The surface bound fluorophores (carboxy-PF1) are excited at 490 nm. In the absence of H2O2 it is mostly nonfluorescent and becomes highly fluorescent (520 nm) upon exposure to H2O2. Credit: Malcolm Purdey.
Upon binding to H2O2, carboxy-PF1 increases the intensity of its green fluorescence emission. But like other organic fluorescent probes that are often used to identify biomolecules and indicate their position under the light of a fluorescence microscope, carboxy-PF1 suffers from photobleaching or fading, a phenomenon during which the fluorescence glow of even the most photostable among these molecules is reduced under continuous illumination. Scientists have tried to prevent damage of the fluorophores by reducing the intensity of the light source or the duration of light exposure. These approaches, however, usually limit the time of the experiments and the resolution of the collected images.
By combining the probes with the NV nanodiamonds, which are photostable even under intense illumination and of which far-red fluorescence remains mostly unchanged by interactions with biomolecules, the researchers created a biocompatible and highly photostable peroxynanosensor (PNS) that is trackable at any moment and does not photobleach.
To follow the PNS in a cell, the sample is monitored while being continuously excited at 550 nm. The nanodiamonds emit stable fluorescence around 700 nm. For the detection of H2O2, the surface bound carboxy-PF1 fluorophores are excited at 490 nm. In the presence of H2O2, they emit strongly at 520 nm.
“We have been able to significantly extend the time scale of bio-imaging performed with carboxy-PF1, via a reduction in laser exposure,” says Patrick Keith Capon, co-author of the study and doctoral candidate. “By using the fluorescent nanodiamond to track the location of our hybrid system, we eliminated photobleaching of the attached fluorophore, thus extending its measurement lifetime,” he adds.
The researchers evaluated the sensitivity of PNS to H2O2 by exposing it to the oxidant in a pH 7.5 phosphate buffered saline at 37°C for 45 min. They found that the fluorescence of the nanosensor was proportional to the concentration of H2O2, while that of NV nanodiamonds alone remained unchanged over the same concentration range.
To study the photostability of PNS, M1 polarized macrophages were used. These are white blood cells that produce high levels of reactive oxygen species, including H2O2, and are associated with pro-inflammatory processes. A comparative study with the free organic fluorophores PF1 and ratio-PF1 was also conducted. The experiments confirmed that PNS may be imaged by the red NV nanodiamond fluorescence in macrophage cells without photobleaching the H2O2 sensing element, while the organic fluorophores PF1 and RPF1 are photobleached by imaging only five times in half an hour.
Capon talks of their surprise, during in vitro experiments that aimed at measuring the ability of PNS to sense H2O2 over an extended period, when they inadvertently added the PNS to the cell culture media on day two of the differentiation process. “This provided us with the opportunity to assess any impact on macrophage growth, and we observed that the sensor is suitable for use during macrophage cell growth and differentiation. I think this is an exciting outcome as we can study dynamic cell processes with our new system,” he says.
According to Capon, the team believes that this technique could be extended to other fluorophores. They are also looking to optimize the nanodiamond surface attachment into a robust, broadly applicable protocol.
Mark Hutchinson of the Australian Research Council Centre of Excellence for Nanoscale BioPhotonics sees this development as a game changer in bioassay development and validation. “Usually, a probe sensor is delivered in excess with the goal that a fraction of it reaches the correct cellular or anatomical compartment for a signal to be visualized. If the signal is observed, this is a success. However, if the signal is not seen, we can’t differentiate between a false negative or a true negative result,” he says, adding that this technology will allow for validation and targeted assessment.


