Exclusive breast-feeding for 6 months with partial breast-feeding continued up to 2 years of age is recommended by the WHO due to its clear short-term benefits for decreasing morbidity and mortality from infections(Reference Victora, Bahl and Barros1–3). Infant feeding practices, mainly breast-feeding and complementary feeding, are also increasingly recognised as important in shaping brain development that may result in positive effects on cognitive development(Reference Black, Victora and Walker4,Reference Black, Walker and Fernald5) .
Mounting of observational studies examined the associations of duration of exclusive/any breast-feeding with later cognitive development(Reference Walfisch, Sermer and Cressman6–Reference Horta, de Sousa and de Mola8), but only a few studies have examined the long-term consequences of breast-feeding on cognitive development in late childhood, adolescence or beyond and reported inconsistent results(Reference Evenhouse and Reilly9–Reference Mortensen, Michaelsen and Sanders14). One study from Brazil reported that breast-feeding improved intelligence performance in adults, but the mean scores for breast-feeding duration ≥12 months were similar to those in the category of 6–11·9 months(Reference Victora, Horta and Loret15). Similar patterns, that is, the longest duration of any breast-feeding not predicting the highest cognitive test scores later in life, were evident in other studies(Reference Mortensen, Michaelsen and Sanders14–Reference Veena, Krishnaveni and Srinivasan18). Only one randomised trial of breast-feeding promotion has been conducted in Belarus that has examined child development and found a positive effect on verbal function at age 6·5 years(Reference Kramer, Aboud and Mironova19) but the effect size substantially decreased by adolescence(Reference Yang, Martin and Oken20).
The extent of the association between optimal duration of any breast-feeding and long-term cognitive development remains unclear. Exclusive breast-feeding may meet nutritional requirements of infants during the first 6 months and possibly longer, but the risk of micronutrient deficiencies among infants exclusively breast-fed for longer than 6 months has been reported in some studies(Reference Hannan, Faraji and Tanguma21–Reference Pizarro, Yip and Dallman23). One systematic evaluation found that significant associations between any breast-feeding and intelligence were observed in high-income countries but not in low- and middle-income countries(Reference Horta, Loret and Victora7), where maternal undernutrition is widely prevalent(Reference Bhutta, Das and Rizvi24) and may lead to a poor quality of human milk(Reference Lindsay25).
There are also sparse data on the relationship of complementary food composition and child development outcomes. Some studies have reported that >90 % of the Fe requirements of a breast-fed infant must be met by complementary foods(Reference Agostoni, Decsi and Fewtrell26). Recently, one review of studies mostly from developed countries concluded that complementary feeding with substantial amounts of Fe, such as meats or Fe-fortified foods, could provide an adequate amount of Fe or prevent Fe deficiency for breast-fed infants who were not receiving adequate Fe from another source(Reference Obbagy, English and Psota27). However, the corresponding effects of these Fe-rich or Fe-fortified foods on child development outcomes were inconsistent(Reference English, Obbagy and Wong28). Similarly, protein is associated with the global development of the brain(Reference Georgieff29), but the evidence on association between complementary high protein-based foods and child cognitive development is lacking. Furthermore, the association of timing of introducing complementary foods, one key aspect of complementary feeding which might be associated with micronutrient status among infants(Reference Obbagy, English and Psota27), with development outcomes also remains unclear(Reference English, Obbagy and Wong28).
According to the Chinese National Nutrition and Health Survey in 2013, the exclusive breast-feeding rate under 6 months was 18·6 % and the complementary feeding practice was also suboptimal with a prevalence of 25·1 % for minimum acceptable diet among children aged 6–23 months in China(Reference Duan, Yang and Lai30). Here, we used a birth cohort study from rural Western China. We examined the associations between the duration of exclusive or any breast-feeding, the consumption of Fe-rich or Fe-fortified foods, and the initial timing of complementary foods, and adolescent cognitive development at age 10–12 years. We also constructed an infant feeding index grounded in WHO recommendations, which comprehensively represented the feeding components above, and further examined its association with child cognitive outcomes. These results are intended to provide evidence on the long-term benefits of appropriate infant and young child feeding practices.
Methods
Study design and participants
The present study was a prospective birth cohort study of the offspring born to women who participated in a trial of antenatal micronutrient supplementation (ISRCTN 08850194), which has been described in detail elsewhere(Reference Zeng, Dibley and Cheng31).
In summary, the original trial was a cluster randomised controlled trial in rural Western China from 2002 to 2006 with three treatment arms (folic acid, Fe/folic acid and multiple micronutrients). A total of 4604 singleton live births were obtained from the trial, but only 1400 births after 2004 were registered for further follow-up. Among them, twelve infants were excluded due to death (n 3), congenital diseases (n 7), maternal hearing loss (n 1) and paternal amentia (n 1). There were 1388 infants who completed the infancy follow-up stage from birth through 1, 3, 6, 9, 12, 18, 24–30 months of age. At the adolescence follow-up stage, 643 of these children were lost to follow-up as they had moved away from the study area, and thus there were 745 adolescents aged 10–12 years who were enrolled and had been followed from birth through infancy into early adolescence. Thus, to achieve 80 % power at a significance of 0·05, the minimum mean difference of test scores between groups that could be detected using our sample size was 2·60, assuming equally sized subgroups and a mean of test score at 98·1 (sd 12·5) within our sample.
Ethical approval
All procedures performed in studies were in accordance with the ethical standards of 1964 Helsinki declaration and its later amendments or comparable ethical standards. The protocol of follow-up studies including infant and adolescent development evaluation was approved by the ethics committee of Xi’an Jiaotong University Health Science Center. Written informed and oral consent were obtained from parents/caregivers and adolescents, respectively, after the purpose of the follow-up study was explained.
Measurements
Breast-feeding, timing of initiation of complementary food and consumption of iron-rich or iron-fortified foods
Detailed information about each infant’s feeding was prospectively collected by interviewer-administered questionnaires with mothers during the infant follow-up stage. At each visit from birth to 30 months of age, we asked mothers the following same questions to classify the type and duration of breast-feeding: ‘For all infants: 1. Have you ever breast-fed your baby? 2. Are you now feeding your baby any breast milk? 3. Are you now feeding your baby other food, including water, micronutrient supplements, animals’ milk or infant formula aside from breast milk?’; and ‘For weaned infants: How old was your baby (in exact days) when you stopped breast-feeding?’ Mothers who were not exclusively breast-feeding were subsequently asked detailed questions about when/which solid foods and non-breast-milk liquids were introduced using FFQ, including the following: infant formula; animals’ milk, such as cow and goat; beans, eggs or meat; vitamin/mineral supplements; just water; sugar water; rice porridge; noodles; and vegetables and fruits. The FFQ was previously validated among pregnant women in the study area(Reference Cheng, Yan and Dibley32).
According to the WHO(33), any breast-feeding (duration in exact months) was defined as receiving any breast milk and exclusive breast-feeding (duration in exact months) referred to infants receiving only breast milk from his/her mother or expressed breast milk and no other liquids or solids, with the exception of drops or syrups consisting of vitamin/mineral supplements. Regular consumption of Fe-rich food or Fe-fortified foods during 6–23 months was defined by receiving infant formula or the frequency of meat/fish consumption beyond 5–6 times/week at any visit of 6, 9, 12, 18 or 24 months of age(33). Complementary food was assessed in the following two groups: cows’/goats’ milk and high protein-based foods (beans, eggs and meats). In the present study, the high protein-based food group did not include infant formula and/or cows’/goats’ milk. The timing of introducing complementary food groups was calculated based on the birth and interview dates at every follow-up visit, and it was determined by the earliest age of introduction of items within the group (in exact days and converted into months).
Assessment of cognitive development
The outcome of interest was adolescent cognitive development, assessed by the Wechsler Intelligence Scale for Children, fourth edition (WISC-IV)(Reference Wechsler34). Four composite indexes (Verbal Comprehension, Perceptual Reasoning, Working Memory (WMI) and Processing Speed) and the full-scale intelligence quotient (FSIQ), which represents general cognitive ability, were derived from the ten subtests and four complementary subtests. The Chinese norms of WISC-IV were established by using a nationally representative sample(Reference Chen, Keith and Weiss35) and the scores on all tests were age-standardised.
Postgraduate students administered the tests according to the Chinese WISC-IV technical manual in a school meeting room free of distractions. When the interviewers performed fully accurate administrations and agreed on the scoring of items, they were certified to collect data in the field. Scoring accuracy was reviewed by the field team leader.
All the fieldworkers administering cognitive tests were unaware of the adolescent’s infant breast-feeding and complementary feeding status.
Other data collected relevant to the analyses: covariates
All relevant covariates were grouped into seven domains: (1) socio-demographic characteristics, indicated by parental age, education, occupation and household wealth at enrolment; (2) maternal preconception nutrition, indicated by mid-upper arm circumference; (3) maternal reproductive history, indicated by parity; (4) randomised regimen (folic acid, Fe/folic acid and multiple micronutrients); (5) birth outcomes, indicated by small for gestational age and infant sex; (6) adolescent nutrition status, indicated by BMI-for-age z-score and (7) home environment, indicated by household wealth and type of school (village, town or county level) at adolescence. Data in domains 1–5, and 6 and 7 were collected in the original trial and the adolescent follow-up stage, respectively. Small for gestational age was defined as birth weight below the 10th percentile of weight-for-age and sex, as defined by INTERGROWTH standards(Reference Villar, Cheikh and Victora36). Gestational age at birth was calculated by self-reported last menstrual period. BMI was estimated as body weight divided by height squared (kg/m2). Thinness was defined as BMI-for-age z-score below 2 sd from age- and sex-specific references for WHO growth standards, and overweight was defined as BMI-for-age z-score above 1 sd (Reference Butte, Garza and de Onis37). Household wealth was established from an inventory of seventeen local household assets or the ownership of goats, cattle, horses and poultry by principal component analysis(Reference Filmer and Pritchett38), which was then classified into tertiles as an indicator of the low-, middle- and high-income households.
Statistical analysis
The baseline characteristics were described as counts/percentages for categorical variables and mean values and standard deviations or medians with interquartile ranges for continuous variables. Generalised estimating equation models were used to examine the relationships between infant and young child feeding practices and adolescent cognitive development, with an independent correlation structure and adjusting for assessors of intelligence tests and covariates from the seven domains mentioned above. We used age-standardised FSIQ as the primary outcome and aspects of WISC-IV, that is, Verbal Comprehension Index, WMI, Perceptual Reasoning Index, and Processing Speed Index, as the secondary outcomes.
We created the infant and young child feeding index scores by summing the related variables (Table 1) grounded in the principles of WHO recommendations for breast-feeding and how to assess infant and young child feeding practices (online Supplementary Table S1)(2,3,33) . Specifically, we assumed that longer durations of any/exclusive breast-feeding with starting/timely complementary feeding just after exclusively breast-feeding for 6 months would produce the largest benefits. Taking the duration of exclusive breast-feeding as an example, the infants who were exclusively breast-fed for 6 months received the highest score of 4, and other infants received relatively lower scores by the durations, that is, score 1 for less than 1 month, 2 for 2–3 months, 3 for 4–5 months and 1 for longer than 7 months. Feeding index was then divided into three levels by tertiles to indicate the appropriate level of infant and young child feeding practices.
Table 1. Construction of infant feeding index scores*
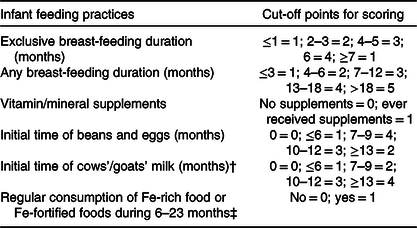
* Cut-off points for scoring were grounded in the principles of WHO recommendations for breast-feeding and how to assess infant and young child feeding practices, that is, indicators for assessing infant and young child feeding practices. Specifically, we assumed that longer durations of any/exclusive breast-feeding with starting/timely complementary feeding just after exclusively breast-feeding for 6 months would produce the largest benefits.
† Regarding the timing of introducing cows’/goats’ milk, the related recommendations differed by countries, such as 12 months in the USA and UK, 9 months in Denmark and 10 months in Sweden. As a result, we used the recommendation in China, that is, 12 months.
‡ Regular consumption of Fe-rich food or Fe-fortified foods during 6–23 months was defined by receiving the infant formula, or the frequency of meat/fish supplementation beyond 5–6 times per week at any visit of 6, 9, 12, 18 or 24 months of age.
In order to account for missing adolescent development outcome data and potential bias due to differential loss to follow-up, we conducted a sensitivity analyses using inverse probability weighting(Reference Seaman and White39), with randomised regimen, paternal age, education and job, small for gestational age and sex as predictors of missingness in the logistic model. The missingness model produced similar distributions of weights among complete and incomplete cases, no zero fitted probabilities and a reasonable model fit, that is, a P value of 0·287 for Hosmer–Lemeshow goodness-of-fit test. Statistical significance was set at α < 0·05 (two-tailed), and all analyses were performed using Stata 12.0 (StataCorp).
Results
Cohort characteristics
Fig. 1 shows the flow chart of participants. A total of 745 adolescents aged 10–12 years were followed. Of them, forty-three (5·8 %) were excluded in final analyses for never receiving breast milk (n 33) and for refusing intelligence tests (n 10). The characteristics of children and parents who were followed up were relatively similar to those lost to follow-up (online Supplementary Table S2).

Fig. 1. Participant flow chart.
Table 2. Background characteristics of participants’ parents*
(Numbers and percentages; mean values and standard deviations)

MUAC, mid-upper arm circumference.
* Data are missing for maternal education (n 2), father’s education (n 3), mother’s job (n 2), father’s job (n 2) and maternal MUAC (n 7).
Socio-demographic characteristics of participants
The socio-demographic characteristics of 745 mother–participant pairs are summarised in Tables 2 and 3. The mean age of mothers was 24·5 (sd 4·5) years. About 54·0 % of them had a secondary education. In addition, majority of families were farmers. Among the adolescents, the mean age was 11·3 (sd 0·6) years and 60·4 % of them were male.
Table 3. Background characteristics of offspring*
(Numbers and percentages; mean values and standard deviations; medians and interquartile ranges (IQR))

WISC-IV, Wechsler Intelligence Scale for Children, fourth edition; FSIQ, full-scale intelligence quotient; VCI, Verbal Comprehension Index; WMI, Working Memory Index; PRI, Perceptual Reasoning Index; PSI, Processing Speed Index; SGA, small for gestational age.
* Data are missing for birth weight (n 9), SGA (n 135), any/exclusive breast-feeding duration (n 2), feeding index scores (n 31), WISC-IV test scores (n 10), adolescent height (n 2) and weight (n 10).
† Twenty-eight did not receive complementary beans, eggs or meat in the infancy period.
‡ Feeding index scores were categorised into three-level appropriate feeding groups using their tertiles.
Infant feeding practices of adolescents
As shown in Table 3, 18·2 % of the infants were exclusively breast-fed for 4–6 months, 33·4 % had a duration of any breast-feeding of 13–18 months, while 12·8 % had a duration of any breast-feeding of >18 months. In total, 55·3 % had frequently consumed Fe-rich or Fe fortified foods between the ages of 6 and 23 months; 63·2 % had consumed cows’/goats’ milk during the first 2 years of life, and among those who did, the median age of initial consumption was 9 (interquartile range 3, 18) months. The median age of initial consumption of high protein-based food was 8 (interquartile range 6, 9) months.
Duration of any or exclusive breast-feeding and cognitive development
We found that duration of exclusive/any breast-feeding was not significantly associated with any domain of adolescent cognitive development with or without adjusting for the twelve potential covariates (Table 4).
Table 4. Wechsler Intelligence Scale for Children, fourth edition (WISC-IV) test scores of adolescents with respect to duration (months) of any/exclusive breast-feeding
(Numbers; mean values and standard deviations; adjusted mean differences and 95 % confidence intervals)

FSIQ, full-scale intelligence quotient; VCI, Verbal Comprehension Index; WMI, Working Memory Index; PRI, Perceptual Reasoning Index; PSI, Processing Speed Index; MUAC, mid-upper arm circumference; SGA, small for gestational age.
* Adjusted for covariates including parental age, job and education at pregnancy enrolment, household wealth at pregnancy enrolment, maternal MUAC at pregnancy enrolment, maternal parity, randomised regimen, birth outcome (SGA), adolescent sex and school type in general estimating equation linear models.
† P for trend values are calculated in general estimating equation linear models and adjusted for covariates (parental age, job and education at pregnancy enrolment, household wealth at pregnancy enrolment, maternal MUAC at pregnancy enrolment, maternal parity, randomised regimen, birth outcome (SGA), adolescent sex and school type).
Timing and composition of complementary food introduction and cognitive development
Participants who regularly consumed Fe-rich or Fe-fortified foods at age 6–23 months had higher FSIQ scores than those who did not (adjusted mean differences 4·25; 95 % CI 1·99, 6·51; Table 5). The similar results were observed for aspects of WISC-IV, that is, Verbal Comprehension Index, WMI, Perceptual Reasoning Index and Processing Speed Index. Among those who did, we found that the timing of Fe-rich or Fe-fortified foods within the period of 6–23 months was not associated with development outcomes (online Supplementary Table S2).
Table 5. Wechsler Intelligence Scale for Children, fourth edition (WISC-IV) test scores of adolescents with respect to frequent consumption of iron-rich or iron-fortified foods during 6–23 months*
(Numbers; mean values and standard deviations; adjusted mean differences and 95 % confidence intervals)

FSIQ, full-scale intelligence quotient; VCI, Verbal Comprehension Index; WMI, Working Memory Index; PRI, Perceptual Reasoning Index; PSI, Processing Speed Index.
* Regular consumption of Fe-rich food or Fe-fortified foods during 6–23 months was defined by receiving the infant formula, or the frequency of meat/fish supplementation beyond 5–6 times per week at any visit of 6, 9, 12, 18 or 24 months of age.
† Adjusted for covariates (including parental age, job and education at pregnancy enrolment, household wealth at pregnancy enrolment, maternal mid-upper arm circumference at pregnancy enrolment, maternal parity, randomised regimen, birth outcome (small for gestational age), adolescent sex, and school type) in general estimating equation linear models.
We also examined the relationship between the timing of transition to consumption of cows’/goats’ milk with development outcomes. Among adolescents who received cows’/goats’ milk, the highest scores were found in participants who initiated consumption at 10–12 months compared with those after 13 months (Table 6) with an adjusted mean difference of 2·61 (95 % CI 0·13, 5·09) FSIQ points. Besides, adolescents who received the cows’/goats’ milk within 6 months had 2·90 (95 % CI 0·68, 5·12) points lower WMI scores as compared with those with an initial age of after 13 months.
Table 6. Wechsler Intelligence Scale for Children, fourth edition (WISC-IV) test scores of adolescents with respect to the initial age (months) of introduction of cows’/goats’ milk and high protein-based food in infancy
(Numbers; mean values and standard deviations; adjusted mean differences and 95 % confidence intervals)

FSIQ, full-scale intelligence quotient; VCI, Verbal Comprehension Index; WMI, Working Memory Index; PRI, Perceptual Reasoning Index; PSI, Processing Speed Index.
* Adjusted for covariates (including parental age, job and education at pregnancy enrolment, household wealth at pregnancy enrolment, maternal mid-upper arm circumference at pregnancy enrolment, maternal parity, randomised regimen, birth outcome (small for gestational age), adolescent sex and school type) in general estimating equation linear models. For high protein-based food, the potential covariates also included durations of exclusive breast-feeding.
† Number of participants who did not consume high protein-based food in infancy was 28.
In addition, adolescents who initiated high protein-based foods within 6 months had the lowest test scores as compared with those with an initial age of 7–9 months (Table 6). The adjusted mean differences were –2·42 (95 % CI –4·24, –0·61) points for the FSIQ.
Composite feeding score and cognitive development
We constructed a composite feeding score based on WHO feeding recommendations (Table 1). We identified a significant dose–response relationship of feeding index score with FSIQ, Verbal Comprehension Index, WMI and Processing Speed Index (Table 7). Adolescents in the highest tertile of feeding score (best) had higher FSIQ (adjusted mean differences 3·03; 95 % CI 1·37, 4·70) than those in the lowest tertile.
Table 7. Wechsler Intelligence Scale for Children, fourth edition (WISC-IV) test scores of adolescents with respect to tertiles of infant feeding index scores
(Numbers; mean values and standard deviations; adjusted mean differences and 95 % confidence intervals)

FSIQ, full-scale intelligence quotient; VCI, Verbal Comprehension Index; WMI, Working Memory Index; PRI, Perceptual Reasoning Index; PSI, Processing Speed Index.
* Adjusted for covariates (including parental age, job and education at pregnancy enrolment, household wealth at pregnancy enrolment, maternal mid-upper arm circumference at pregnancy enrolment, maternal parity, randomised regimen, birth outcome (small for gestational age), adolescent sex and school type) in general estimating equation linear models.
† P for trend values are calculated in general estimating equation linear models with the original feeding index scores as a continuous exposure variable, and adjusted for the same covariates as above.
Sensitivity analyses
We conducted sensitivity analyses using inverse probability weighting to account for potential bias due to dependent censoring (loss to follow-up). There were no qualitative differences in our findings using inverse probability weighting models (online Supplementary Tables S4–S7).
Discussion
Based on a prospective birth cohort study in rural China, we found that infant and young child feeding practices following WHO recommendations were associated with significantly improved cognitive development of adolescents aged 10–12 years. Specifically, regular consumption of Fe-rich or Fe-fortified foods during infancy may contribute to better early adolescence cognitive development.
Interpretations of findings and implications for public health
Our finding that the duration of any or exclusive breast-feeding was not significantly associated with adolescent cognitive test scores after rigorously controlling for covariates from multiple domains is consistent with findings from other similar studies(Reference Wigg, Tong and McMichael12,Reference Jacobson, Chiodo and Jacobson13,Reference Rochat, Houle and Stein40–Reference Huang, Peters and Vaughn42) and one systematic evaluation(Reference Walfisch, Sermer and Cressman6). Two systematic reviews that included nine studies adjusted for maternal intelligence and two studies adjusted for socio-economic variables and stimulation at home reported the significant benefits of breast-feeding on child cognitive development(Reference Horta, de Sousa and de Mola8,Reference Jenkins and Foster17) , respectively, but majority of these studies were based on populations from developed countries. Further, the few studies from low- and middle-income countries had inconsistent findings(Reference Yang, Martin and Oken20,Reference Rochat, Houle and Stein40,Reference Daniels and Adair43–Reference Tumwine, Nankabirwa and Diallo45) . Horta et al. (Reference Horta, de Sousa and de Mola8) and Huang et al. (Reference Huang, Peters and Vaughn46) summarised the possible mechanisms on the benefits of breast-feeding for child development including rich nutrients in human milk such as long-chain PUFA and DHA, nurturing and skin contact with the mother, which should also apply to populations in low- and middle-income countries. As a result, the null findings from our study and other studies in low- and middle-income countries warrant further explanations. In the present study, one possible explanation was due to the low statistical power, in which a minimum mean difference of test scores between groups that could be detected was 2·60.
Generally, timely Fe supplementation or complementary feeding with Fe-rich/Fe-fortified foods was necessary for the depletion of infant Fe stores after exclusive breast-feeding for 6 months(Reference Agostoni, Decsi and Fewtrell26). However, the results of Fe supplementation trials during infancy on long-term cognitive development are inconsistent and appear to vary by different population(Reference Jáuregui-Lobera47–Reference Agrawal, Berggren and Marks49). There is also a concern of adverse effects of Fe supplementation, including hampering brain development, due to the excess intake of Fe(Reference Lönnerdal50). Thus, some studies suggest that timely/starting complementary Fe-rich/Fe-fortified foods just after exclusive breast-feeding for 6 months is more practical than the use of Fe supplements(Reference Jáuregui-Lobera47). In the present study, we found positive associations of the consumption of Fe-rich/Fe-fortified foods with adolescent cognitive development. Although this finding may be context-specific such as the different vitamin C-rich diets influencing the uptake of nonheme Fe as the reviewer commented, it implies that promotion or provision of Fe-rich/Fe-fortified complementary foods during the first 2 years of life can meet Fe requirements of infants and contribute to their long-term cognitive development.
Unmodified cows’/goats’ milk does not contain sufficient Fe and folate to meet infant dietary requirements, and early introduction of these foods is negatively associated with Fe status(Reference Razafindrakoto, Ravelomanana and Rasolofo51,Reference Turck52) . Some studies also reported that Fe deficiency was negatively associated with the development of white matter, hippocampal–frontal and striatal–frontal areas of the brain(Reference Georgieff29). Consequently, early introduction of animals’ milk may hamper the child development; however, to our knowledge, no studies have examined the associations of initial age of cows’/goats’ milk with child cognitive development. We observed that among adolescents who ever received cows’/goats’ milk at infancy, those who initiated before 6 months had insignificantly lower FSIQ and significantly lower WMI scores. In addition, introducing high protein-based foods before 6 months was also significantly associated with lower FSIQ scores, which may be due to that introducing high-protein foods before 6 months was the interference of exclusive breast-feeding and digestion, resulting in protein deficiency. Protein deficiency was found to be negatively associated with the global development of the brain(Reference Georgieff29). Taken together, these findings suggest that introducing complementary feeding before 6 months may not only comprise the exclusive breast-feeding but also hamper the long-term development of children. Regarding the optimal initial age, the highest FSIQ scores for cows’/goats’ milk were found in participants who initiated between 10 and 12 months compared with an initial age of beyond 13 months, which was conflicted in the ongoing Chinese guideline of waiting until 12 months. The possible explanation may be due to the prevalent undernutrition and poor resource in study area where animals’ milk was used as the primary food of providing protein requirements for infant and young child. In our study area, the proportions of initiating cows’/goats’ milk before 10 and 12 months were 34·4 and 44·7 %, respectively. In terms of high protein-based food, participants who initiated between 7 and 9 months had higher test scores, which was in line with the Chinese guideline on complementary feeding.
We examined the relationship between a composite feeding practice score and development outcomes. This analysis may be the most informative as crudely dividing feeding practices by breast-feeding duration and complementary food introduction may not adequately capture the nuances of infant and young child feeding experience. We found that adolescents in the highest tertile of feeding scores had 3·03 higher points on general cognitive ability as compared with those in the lowest tertile. In our sample, from grade 4 to grade 7, FSIQ scores increased on average by 4·51 points per school year (Table 3). Consequently, the effect sizes of improving appropriate feeding during the early years of life are relatively large and are approximately equivalent to an additional 8-month schooling. As discussed above, some studies explained the mechanisms for the individual component of the composite score such as breast-feeding, Fe and protein(Reference Horta, de Sousa and de Mola8,Reference Georgieff29,Reference Huang, Peters and Vaughn46,Reference Jáuregui-Lobera47) , but our study could not provide evidence for the mechanisms behind the observed association of the feeding index with development outcome. Generally, the brain still develops rapidly during the first 2 years of life including the visual/auditory cortex, angular gyrus/Broca’s area and the prefrontal cortex, which may thus be vulnerable to early undernutrition, Fe deficiency and other environmental factors that lay the foundation for brain architecture and functional capacity, and that extend the effects to adolescence or beyond(Reference Thompson and Nelson53).
Although great efforts to increase breast-feeding duration have been made, our findings indicate that programmes should also equally focus on high-quality complementary foods to harness the potential long-term development benefits of optimal infant and young child feeding practices.
Strengths and limitations
The strengths of our study include the prospective birth cohort design with regular visits at infancy, the length of follow-up to early adolescence and the standardised, culturally appropriate intelligence evaluation scales (WISC-IV) for Chinese children. To our knowledge, this is the first study in which associations between infant and young child feeding practices, comprehensively emphasising on the durations of exclusive/any breast-feeding and initial ages and types of complementary foods, and adolescent cognitive development have been examined.
Our study was limited by a few facts. Firstly, participants in our study were born to women who had participated in a micronutrient supplementation trial. Although we adjusted for randomised regimen in the analysis, the generalisability to mothers who would not participate in a clinical trial should be considered. Secondly, 643 (46·3 %) out of 1388 moved away from the study areas and were not able to be followed up. Nevertheless, the majority of the background characteristics between followed and lost to follow-up were balanced (online Supplementary Table S2), and the sensitivity analyses of using inverse probability weighting to account for missing observed similar result patterns. Thirdly, although the infant feeding index was constructed by principles of appropriate feeding practices, it was not validated and needs replication in future studies. Besides, we did not consider the weights or the relative importance of different feeding practices and specify the type of supplements (Table 1) the participants received in the data collection form. In addition, the effect sizes of categorising the index into tertiles should cautiously be generalised to other populations as they may have different distributions of infant feeding factors that contributed to our score. Finally, we did not have information on maternal intelligence which may be associated with feeding practices and cognitive development and can allow for residual confounding(Reference Walfisch, Sermer and Cressman6,Reference Jenkins and Foster17) . Randomised trials of breast-feeding and complementary feeding promotion are needed to determine causal effects.
Conclusion
Appropriate infant feeding practices (breast-feeding plus appropriate timing of the introduction of high-quality complementary foods), based on the current WHO recommendations, were associated with significantly improved early adolescence cognitive development in rural China. Specifically, consumption of Fe-rich or Fe-fortified foods during infancy may contribute to better cognitive development.
Acknowledgements
The authors thank all the field workers for helping to collect data. The authors are also grateful for all the women, adolescents and their families who participated in the study.
This work was supported by the National Natural Science Foundation of China (L. Z., grant no. 81872633); the National Key Research and Development Program of China (H. Y., grant no. 2017YFC0907200, 2017YFC0907201) and the China Scholarship Council (Z. Z., grant no. 201806280188). All the founders had no role in the design, analysis or writing of this article.
Z. Z., Y. C., H. Y., M. J. D. and L. Z. planned and designed the study; Z. Z., Y. C., Y. L., S. M., M. E. and S. T. conducted the study; Z. Z., Q. Q., S. L., H. L., W. W. F. and C. R. S. analysed data and interpreted results; Z. Z. wrote the paper; L. Z. had primary responsibility for final content and all authors reviewed, revised and approved the final paper.
There are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114519003271











