Introduction
It is widely recognized that biological invasions are an important component of human-induced global environmental change (Vitousek et al., Reference Vitousek, D’Antonio, Loope, Rejmánek and Westbrooks1997) and that islands are highly susceptible to ecosystem degradation and species extinction from invasion (Vitousek et al., Reference Vitousek, Walker, Whiteaker, Mueller-Dombois and Matson1987; Simberloff, Reference Simberloff1995). The severe impact of invasive species on ecosystems highlights how single species can exert profound impacts on ecosystem stability and characteristics (Vitousek et al., Reference Vitousek, Walker, Whiteaker, Mueller-Dombois and Matson1987). The effects of exotic species are diverse and can alter ecosystems by affecting overall resource availability (Vitousek et al., Reference Vitousek, Walker, Whiteaker, Mueller-Dombois and Matson1987; Vitousek, Reference Vitousek1990; Ehrenfeld et al., Reference Ehrenfeld, Kourtev and Huang2001), hydrological cycles (Dyer & Rice, Reference Dyer and Rice1999; Zavaleta, Reference Zavaleta, Mooney and Hobbs2000), trophic structure and disturbance intensity and frequency (D'Antonio, Reference D’Antonio, Mooney and Hobbs2000; Brooks et al., Reference Brooks, D’Antonio, Richardson, Grace, Keeley and Di Tomaso2004).
However, the magnitude of the impacts of most invasive species remains poorly documented and difficult to quantify (Hulme, Reference Hulme2003). Of central importance, we still do not know whether the positive correlation between native species decline and invasive species dominance in many ecosystems is cause or effect (Gurevitch & Padilla, Reference Gurevitch and Padilla2004; Didham et al., Reference Didham, Tylianakis, Hutchison, Ewers and Gemmel2005) or whether invasive species ‘actively suppress or exclude subordinates (natives) by lowering resource availability to levels that only they can tolerate … or whether invasive species are largely unaffected by recruitment barriers or environmental stressors that are highly limiting to native species…’ (MacDougall & Turkington, Reference MacDougall and Turkington2005). Because the effects of habitat loss and disturbance also heavily affect many invaded systems it is possible that exotic dominance may be less attributable to competition than has been supposed (Gurevitch & Padilla, Reference Gurevitch and Padilla2004; Didham et al., Reference Didham, Tylianakis, Hutchison, Ewers and Gemmel2005; MacDougall & Turkington, Reference MacDougall and Turkington2005). If invasive plants are passengers of environmental change, then removal or eradication of these species will have no significant impacts on native systems (Myers et al., Reference Myers, Simberloff, Kuris and Carey2000a; Zavaleta et al., Reference Zavaleta, Hobbs and Mooney2001; Mack & Lonsdale, Reference Mack, Lonsdale, Veitch and Clout2002).
It is also increasingly recognized that multiple factors interact through positive or negative feedback mechanisms and influence biodiversity loss and ecosystem processes (Callaway & Maron, Reference Callaway and Maron2006). A greater number of case studies are needed to help us understand and predict the processes that may affect native populations and communities.
Such issues are of paramount importance in Mauritius, one of the world's most invaded and degraded biodiversity hotspots (Myers et al., Reference Myers, Mittermeiter, Mittermeiter, da Fonseca and Kent2000b). Endemism is high: 45% of native plants (Strahm, Reference Strahm1993; Safford, Reference Safford1997), 50% of birds and 87% of snails (Groombridge, Reference Groombridge1992). Only 3–5% of native forest remains (Vaughan & Wiehé, Reference Vaughan and Wiehé1937; Cheke, Reference Cheke and Diamond1987; Safford, Reference Safford1997) and is consequently of high conservation value. Over the last 3 centuries Mauritius has suffered devastating impacts from large-scale deforestation and the introduction of exotic plant and animal species. More than 1,000 plant species have been introduced, nearly double the number of native species (Strahm, Reference Strahm1993).
The dominance of invasive plants in Mauritius, even in nature reserves that are relatively undisturbed, suggests that the invasives are competitively superior to native plants. For example, Psidium cattleianum (Brazilian guava, often incorrectly called Chinese guava), the dominant invader in the upland Mauritian forest, possesses traits such as prolific fruiting, shade and light tolerance, clonal regeneration, tolerance of heavy litter fall, and possible allelopathic effects that give it a competitive advantage over slow-growing native plants. Other important agents of environmental disturbances in Mauritius are cyclones, which have also been implicated anecdotally in accelerating forest degradation and invasion (Vaughan, Reference Vaughan1968; Lorence & Sussman, Reference Lorence and Sussman1986), herbivory by introduced Java deer Cervus timorensis, and seed predation by monkeys Maccaca fascicularis, rats Rattus rattus and pigs Sus scrofa (Cheke, Reference Cheke and Diamond1987). Establishing causality is crucial for achieving conservation and restoration goals (Didham et al., Reference Didham, Tylianakis, Hutchison, Ewers and Gemmel2005).
The aim of our study was to quantify the extent of forest degradation from the onset of invasion in 1939 in Macabé Reserve (Fig. 1), which has been left undisturbed by human activities such as logging, grazing and fires, to a heavily invaded system in 1999. Macabé Reserve, including most of the upland forest, is now heavily invaded by P. cattleianum and Indian privet Ligustrum robustrum. The historical survey provided data against which to compare the changes in richness, composition and abundance of native species that occurred between 1939 and 1999.
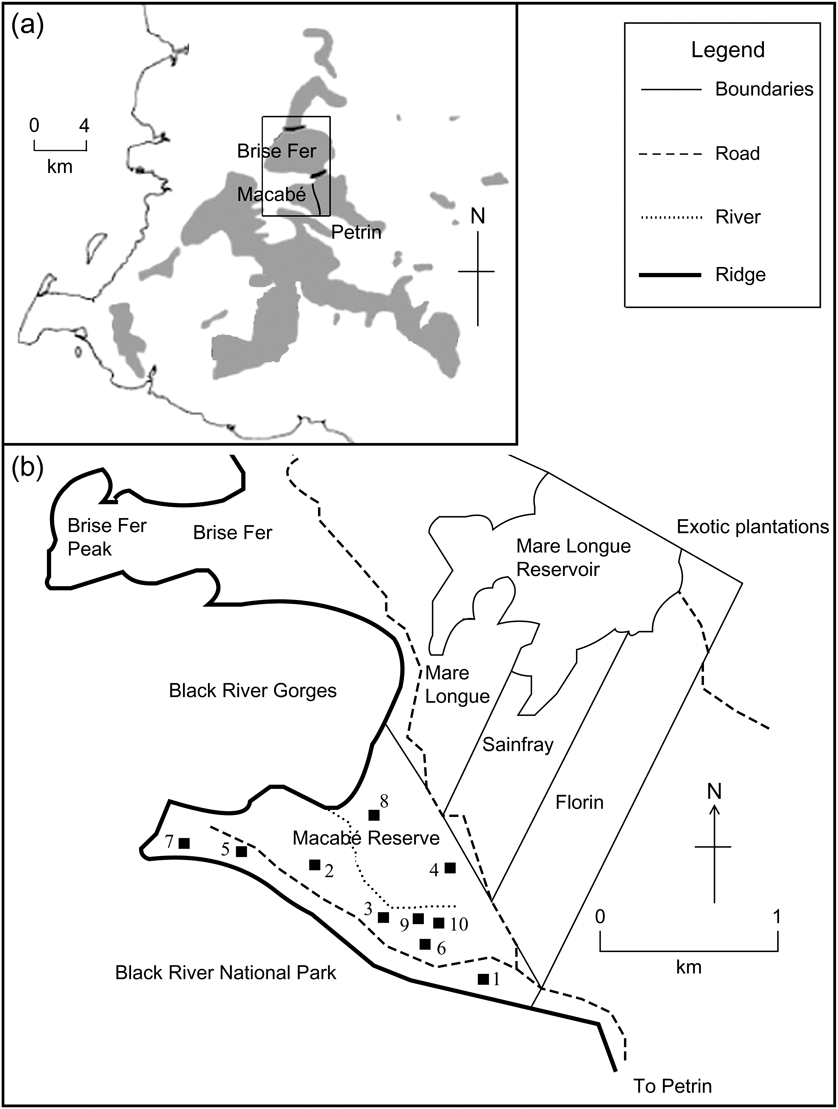
Fig. 1 (a) Location of Macabé Reserve, within the Black River National Park in south-west Mauritius, modified from Safford (Reference Safford1997), showing native vegetation remnants (shaded) and location (rectangle) of (b). (b) Macabé Reserve, based on R.E. Vaughan's map lodged at the Mauritius Herbarium, with the locations of our 10 quadrats.
We examine the following questions: (1) Is there a link between invasive and native species abundance and richness? (2) Is there a link between forest degradation and landscape features (e.g. cyclones generally form in the warmer tropical latitudes of the Indian Ocean (Jury, Reference Jury1993) and reach Mauritius from the north, leaving forest on northern and western slopes more exposed)? (3) Have some native species suffered differential decline and, if so, what are their traits? We did not assess other stressors, such as impacts of exotic mammals, because their impacts are difficult to quantify unless exclusion plots are used and, as they were introduced > 300 years ago (Cheke, Reference Cheke and Diamond1987), their impact would have already been observed in the 1939 survey.
Study area
The vegetation in Macabé Reserve has been described as upland tropical evergreen climax forest (Vaughan & Wiehé, Reference Vaughan and Wiehé1937). The 152 ha Reserve is located in the Black River National Park, at an altitude of 550 m, receives 3,000–3,500 mm of rainfall annually, and is continuous with other forest reserves such as Mare Longue, Sainfray and Florin (Fig. 1). It is situated between two ridges and exposed to the heavy cyclonic winds that hit Mauritius every 6–10 years. The Reserve is situated away from human settlements and tarmac roads and has no known history of destructive human activities such as logging, fire or agriculture.
Vaughan & Wiehé (Reference Vaughan and Wiehé1941) selected Macabé Reserve as it was ‘unaffected as far as possible by local variations in topography and soils’ and ‘interference by exotic species and other secondary biotic factors had been as small as possible’. A hand-drawn map lodged by the authors at the Mauritius Herbarium shows the location and boundaries of the area surveyed. Although they noted that interference by exotic species had been minimal, Vaughan & Wiehé (Reference Vaughan and Wiehé1941) also pointed out that there was ‘no forest into which the ubiquitous Ardisia crenata and P. cattleianum have not penetrated’ (Vaughan & Wiehé, Reference Vaughan and Wiehé1941). In his field notebook Vaughan recorded only two individuals of ‘Chinese guava’ P. cattleianum, in one of the most-intensively surveyed plots (Strahm, Reference Strahm1993). We therefore concluded that introduced plant species were present in 1939 but at such low densities that the forest could be described as relatively undisturbed by these species. Therefore, 1939 appears to coincide with the onset of invasion in Macabé.
Methods
We used comparative methods to monitor forest degradation. Vaughan & Wiehé (Reference Vaughan and Wiehé1941) recorded the density and basal area (measured at breast height) of all native plant species (> 10 cm diameter at breast height, DBH) in 10 random quadrats of 50 × 20 m within the Reserve. Because they did not specify the locations of nine of their plots, we could not carry out repeat surveys of the exact locations. We did not resurvey their one intensively studied plot, which was permanently marked and has been weeded and fenced since the initial survey, and is therefore not representative of the Macabé area as a whole (which has been left unmanaged over this period; Strahm, Reference Strahm1993). From the end of 1999 to 2002 we randomly located 10 plots in the same area. We recorded density and basal area (at breast height) of all native plant species (> 1 cm DBH). Because the 1939 dataset only included individuals > 10 cm DBH we only used this subset for comparative purposes.
To compare native and invasive species abundance and richness we also recorded the abundance of introduced species (> 1 cm DBH) in 60 sub-plots of 2 × 2 m, six in each 50 × 20 m plot, to capture heterogeneities in the larger plots. We also surveyed the type of seedlings in the sub-plots to assess the relationship between invasion and regeneration. To assess the role of cyclonic disturbances we categorized plots by slope (high, medium and no slope) and aspect.
Biodiversity trends from 1939 to 1999 were analysed by comparing richness and abundance of native species (> 10 cm DBH) among the plots for all species. At the species level, stem abundance was compared between the two datasets.
To examine traits of native species under greater stress from forest degradation, trends in rank abundance of abundant (> 20 individuals) native species were compared between the two time periods. Only abundant species were used because less common species could have occurred in the plots by chance. Rank abundances rather than frequency of species were used because, overall, most species had declined in absolute numbers. Trends in rank abundance were divided into two categories: unchanged and declined. Unchanged species were defined as those that had decreased by an arbitrary < 4 ranks or increased in rank. Declined species were those that had decreased by ≥ 4 ranks. Two key ecological traits for which sufficient data were available, fruit size and stratum occupied by adult trees, were analysed using binary divisions: large (> 3 cm) and small (< 3 cm) fruits, and understorey (< 7 m in height) or sub-canopy/canopy trees (> 7 m in height). Data were thus collapsed to obtain a meaningful sample size. Traits were compared for significance using Fisher's exact test in SPSS v. 11.5 (SPSS, Chicago, USA).
Non-metric multidimensional scaling (NMDS) using Sorenson's similarities were applied to the 1939 and 1999 data. NMDS was chosen because it overcomes three major weaknesses in other ordination techniques: it has greater flexibility both in definition and conversion of dissimilarity to distance, its rationale is the preservation of these relationships in low dimensional ordination space, and it does not need to meet the parametric assumptions of other ordination techniques (Carr, Reference Carr1994). Sorenson's similarity index was used because it reflects both species richness and abundance (Kent & Coker, Reference Kent and Coker1992). NMDS was conducted using Community Analysis Package v. 2 (Pisces Conservation, Lymington, UK).
Results
Comparisons between 1939 and 1999
Vegetation change (> 10 cm DBH) between 1939 and 1999 was characterized by a significant decline in the density of native trees, which was 70% lower in 1999, with a mean of 496 (range 12–102) trees per plot in comparison to 1,710 (range 163–214) in 1939 (Fig. 2). Similarly, the mean basal area in 1939 was 29.6 m2 ha-1 compared to 8.5 m2 ha-1 in 1999, i.e. a decline of 71%. Although overall native species richness only declined from 48 to 42 species, the mean richness of the 10 plots declined from 27.9 species (range 24–32) in 1939 to 15.1 (range 6–27) in 1999.
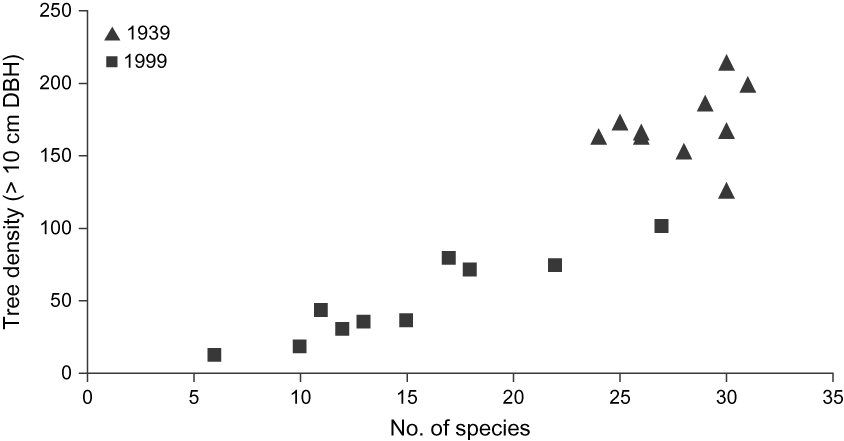
Fig. 2 Relationship between tree density (> 10 cm DBH) and number of native species in 10 random 1,000 m2 plots in 1939 and 1999 in Macabé Reserve (Fig. 1). Note the higher tree density and number of species in 1939 compared to 1999.
In 1939 the tree assemblage (> 10 cm DBH) was dominated by the native species Syzygium glomeratum (Myrtaceae), Lautembergia neraudiana (Euphorbiaceae), Mimusops maxima (Sapotaceae) and Aphloia theiformis (Flacourtiaceae), which together comprised 36.6% of all native trees. By 1999 the tree composition had changed significantly. L. neraudiana and M. maxima declined from comprising 13% of all native trees in 1939 to < 1% in 1999. The dominant trees in 1999, excluding invasive species, were Diospyros tesselaria (Ebenaceae), Labourdonnaisia spp. (Sapotaceae) and S. glomaratum, comprising together 35% of all native trees (Fig. 2).
The number of native tree species and their density in the 12 most abundant families was lower in 1999 than in 1939 for all families except the Ebenaceae (Table 1). At the species level, the density and basal area of the 23 most abundant native species (i.e. with > 20 indviduals) in 1939 was lower in 1999 except for Sideroxylon puberulum (Sapotaceae), D. tesselaria and Labourdonnaisia spp.. S. puberulum maintained a similar density and D. tesselaria and Labourdonnaisia spp. were twice as dense in 1999 compared to 1939 (with an increase in numbers in most size classes) and together accounted for 25.6% of all native trees.
Table 1 The number of native species (individuals per ha > 10 cm DBH) in Macabé Reserve (Fig. 1), with their stem density and basal area, in 1939 and 1999, in the 12 families most abundant in 1939. Rank abundances are in parentheses.
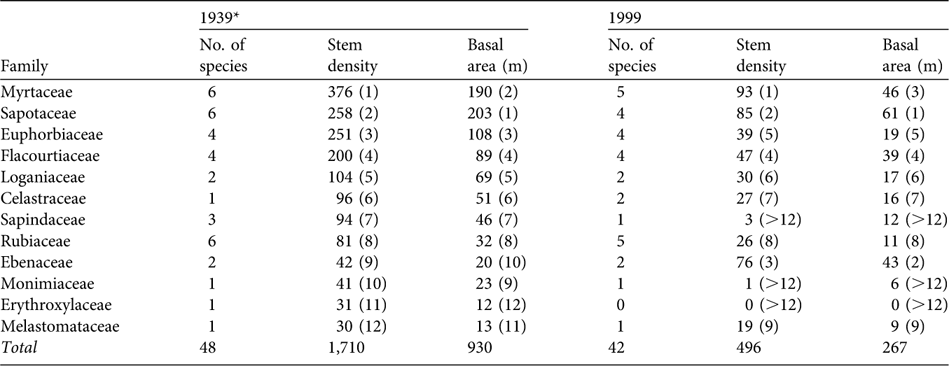
* From Vaughan & Wiehé (Reference Vaughan and Wiehé1941)
Using ranks we found that understorey trees/shrubs (shade-demanding species; n = 26, P < 0.05) and species with large fruits (n = 26, P < 0.01) have declined more significantly than sub-canopy/canopy trees (shade and light tolerant species) and small-fruited trees respectively. Thus, shade-demanding shrubs and trees such as Molinea alternifolia (Sapindaceae), Casearia coriacea (Flacourtiaceae) and Erythroxylum laurifolium (Erythroxylaceae) and large fruited species, such as Mimusops petiolaris, M. maxima, Sideroxylon grandiflorum (Sapotaceae) and Tambourissa spp. (Monimiaceae) showed the greatest decline in rank abundance. The NMDS ordination showed there was less variation in species composition among plots in 1939 than in 1999 (Fig. 3).

Fig. 3 Frequency of seven selected native taxa and all native trees (Total) in DBH classes (> 10 cm) in 1939 and 1999 (note differing y-axis scales). Whilst the density of most trees declined between 1939 and 1999 two species, Diospyros tesselaria and Labourdonnaisia spp., increased in density.
Invasive and native plants in 1999
Plots with a north or north-west facing slope had the lowest native species density (range 136–698, n = 5, DBH > 1 cm). The two plots with a south-east aspect had the highest density (range 1,096–1,188, n = 2, DBH > 1 cm) whereas plots on level ground had a moderate density of native species (range 367–518, n = 3, DBH > 1 cm).
In 1999 four introduced species (> 1 cm DBH) were recorded in all 10 plots. Extrapolating the mean density of introduced species for the sub-plots to the 10 plots, introduced species accounted for 68% of all individuals, including natives, with the dominant P. cattleianum comprising 57% of all individuals. P. cattleianum and L. robustum occurred at a density of 10,041 and 1,458 ha-1, respectively, compared to 5,611 ha-1 for all native species combined.
Overall, introduced species occurred at relatively similar densities across the 10 plots (mean 1,183 ± SD 55.6, range 917–1,542, > 1 cm DBH) compared to the more patchy distribution of native species (mean 564 ± SD 331.9, range 136–1,180, > 1 cm DBH; Fig. 4).

Fig. 4 NMDS ordination using Sorenson's similarities of native species composition (> 10 cm DBH) in 10 random 1,000 m2 plots in Macabé Reserve (Fig. 1) in 1939 and 1999. The ordination shows a distinct separation of the 1939 and 1999 samples, with less variation in species composition among plots in 1939 compared to 1999.
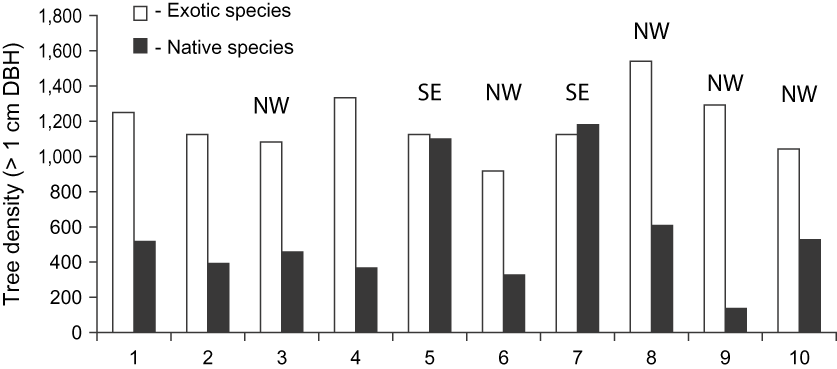
Fig. 5 The densities of exotic and native woody stems (> 1 cm DBH) in 10 random 1,000 m2 plots in Macabé Reseve (Fig. 1) in 1999, with the aspect of each (if not flat) indicated.
Richness and abundance of seedlings differed significantly between natives and exotics: a total of 479 native seedlings of 28 native species were recorded compared to 2,564 seedlings from 11 introduced species in the 60 sub-plots. There was no significant relationship between the density of native and exotic seedlings among the 60 sub-plots. The five dominant exotic seedling species, in descending order, were P. cattleianum, Ossaea marginata, Ardisia crenata, Wikstroemia indica and L. robustum. These five species constituted 83.8% of all seedlings combined. The five dominant native seedlings, in descending order, were Warneckea trinervis, Cnestis glabra, Ochna mauritiana, Acalypha integrifolia and Grangeria borbonica. These five species comprised 11.6% of all seedlings and 72% of native seedlings.
Discussion
Floristic differences between 1939 and 1999
There were significant floristic differences in Macabé between 1939 and 1999: a 70% decline in the density and biomass of trees (> 10 cm DBH) and a decline in mean species richness from 27.9 species to 15.1 (> 10 cm DBH). The composition of native species was relatively homogenous amongst the plots in 1939 (indicating that the plots were representative of Macabé Reserve at that time) compared to the large variation among the plots in 1999. In addition, although most native species have declined since 1939, certain species appear to be more affected than others. Understorey species or trees bearing large fruits experienced a greater decline.
Another significant floristic change is the rapid expansion of two invaders. Although P. cattleianum was introduced to Mauritius as early as 1822 (Lorence & Sussman, Reference Lorence and Sussman1986) it occurred only sporadically in the Reserve in 1939, with possibly 2 individuals per 0.1 ha (Strahm, Reference Strahm1993). Indian privet L. robustum, in comparison, was introduced in 1906 (Lorence & Sussman, Reference Lorence and Sussman1986) and was not present in the Reserve in 1939 (Vaughan & Wiehé, Reference Vaughan and Wiehé1941). The populations of these two invaders expanded greatly from the initial stage of establishment and in 1999 they were 300% more abundant in the Reserve than all native species together.
Possible mechanisms of forest degradation
‘The search for a single invasive strategy is illusory' (Facon et al., Reference Facon, Genton, Shykoff, Jarne, Estoup and David2006)
Because the expansion of exotic species is the major change in the Macabé forest since 1939, it is possible that it is the main cause of the decline of native plant biodiversity. Introduced plant species such as P. cattleianum are known to compete with native plants for resources and may also release chemicals that prevent regeneration and growth (Strahm, Reference Strahm1993). It is also possible that understorey native species have been more affected than other species since 1939 because they occupy the same strata as P. cattleianum, which could be changing the understorey microclimatic conditions. Similar trends for shade-tolerant understorey shrubs have been observed in studies of forest fragmentation and alien species invasion in Singapore (Turner et al., 1995). In addition, invasive plants, being reproductively prolific, dominate the ground flora and could be suppressing the regeneration and recruitment of native species either by their sheer physical dominance or competition for resources. Experimental studies in Mauritius have shown that native species regenerate abundantly once plant invaders are removed (Strahm, Reference Strahm1993). However, the absence of any numerical relationship between native and exotic seedlings indicates that regeneration may also be affected by patchy distribution of adult trees.
We have also shown that the other ecological trait of trees with relatively rapid decline in abundance is the possession of large fruit. The predisposition of large fruit-bearing species to decline and small fruit-bearing species subsequently to dominate was predicted by Da Silva & Tabaralli (Reference Da Silva and Tabarelli2000) from their work in the fragmented Atlantic Forest in Brazil. Whereas in that system decline was mostly because of the extinction of large dispersers such as toucans Ramphastos spp. and curassows Mitu mitu, in Mauritius decline may be explained both by similar causation and the additional presence of pest animals. Large fruit-dispersers such as the giant tortoise Geochelone spp., broad-billed parrot Lophopsittacus mauritianus and dodo Raphus cucullatus had mostly disappeared by the end of the 18th century (Cheke, Reference Cheke and Diamond1987), leaving the Mauritian forest with no native or introduced ecological equivalents for this dispersal function. Furthermore, introduced monkeys and rats have been observed to destroy large edible fruits and seeds (Vaughan & Wiehé, Reference Vaughan and Wiehé1941; Strahm, Reference Strahm1993).
The population increases of three native species, D. tesselaria, Labourdonnaisia spp. and S. puberulum also suggest that certain traits could be conferring competitive advantage in this invaded system or that these increases could be due to natural succession. All three species are canopy trees producing small to medium sized latex fruits dispersed by the extant Mauritian fruit-bat Pteropus niger (Nyhagen et al., Reference Nyhagen, Turnbull, Olesen and Jones2005). Separation of pulp from seeds by frugivores such as bats may increase survival by reducing seed predation, in particular by rats, and microbial attack (Nyhagen et al., Reference Nyhagen, Turnbull, Olesen and Jones2005); therefore the survival of bats may have provided several advantages to the plants on which they feed.
Other mechanisms could also be influencing forest degradation and biodiversity loss. Plots on the north or north-west aspect had significantly lower diversity and density of native species in comparison to plots with other aspects. Moreover, the plot with lowest density was associated both with a north-west aspect and a steep slope. It is possible that differences in densities between north and south aspects may be due to cyclones, which reach Mauritius from the north (Jury, Reference Jury1993), leaving forest on northern and western slopes more exposed. Although cyclones are a natural phenomenon in forest disturbance dynamics, they could be enhancing forest degradation by forming gaps, which are quickly exploited by invasive species and then in turn prevent recruitment of native species (M. Virah-Sawmy, pers. obs., 1999). Invasive species could also be altering the disturbance regime. For example, MacDonald et al. (Reference MacDonald, Thebaud, Strahm and Strasberg1991) observed that native trees show heavier damage after a cyclone when certain invasive species dominated the forest type on the nearby island of Réunion. Overall, these results indicate that although invasive species could be dominant because of their superior traits, the patchy decline of native forest may be due to positive feedback mechanisms between cyclonic disturbances and invasion. The role of cyclones in invasion and degradation processes merits further research.
With respect to whether invasive plants are taking opportunistic advantage of other forms of ecosystem change or whether they are the drivers of change (MacDougall & Turkington, Reference MacDougall and Turkington2005), our study sugests that invaders in Macabé Reserve could be both triggering and driving forest degradation. They could be driving degradation by their numerical dominance, through competition affecting regeneration and access to resources, and by responding to disturbances from cyclones or other environmental stressors that negatively affect slow-growing native trees. Invaders could drive forest degradation by promoting disturbances to native species during cyclones, and later by preventing natural succession by being able to colonize ‘cyclonic’ gaps more successfully.
Conservation implications
Although the data analysed in this study came from a relatively small number of plots, this 1999–2002 survey provides a benchmark for a future re-survey. Our survey indicates that although forest degradation has been severe, there are nevertheless some pockets of good quality forest in Macabé Reserve that are comparable to 1939 pre-invasion baselines. The control of invasive plant species in such areas is key to the conservation and management of natural ecosystems in Mauritius. Based on the results of our survey the following actions may be among those that need to be considered if ecosystem integrity is to be maximized in Mauritian native forest: selecting areas to be restored that are buffered from cyclones, designing restoration strategies that can enhance resistance of native forest to disturbances and re-invasions, and planting of vulnerable native groups e.g. shade-loving understorey specialists and large-fruited species in restored forest reserves.
Since 2002 the National Park and Conservation Service (NPCS) and Forestry Service have been expanding restoration activities in existing and new conservation areas, selected because they contain many rare species. Criteria suggested here, such as selection of conservation areas sheltered from cyclones, have not been taken into account, as more experimentation is needed to compare degradation patterns over time in sheltered and unsheltered areas. However, NPCS is expanding the size of conservation areas to enhance the resistance of these restored areas to reinvasion and disturbances. With regard to vulnerability of large-fruited species, the Mauritian Wildlife Foundation is working with analogue species, such as introduced giant tortoises from the Seychelles, on Iles aux Aigrettes to enhance the dispersal of large-fruited species. It may be possible in the future to release these analogue tortoises in conservation areas in the upland forest.
This research has also indicated that relatively few invasive species are dominant in undisturbed areas of Macabé Reserve. It may thus be useful to look at the possibility of using biocontrol for several of the principal plant invaders in Mauritian upland forests. The US Forest Service has proposed the release of a highly host-specific scale insect from Brazil for the control of P. cattleianum in Hawaii (Hawaiin Ecosystems at Risk, 2009), and research on possible biological control agents for L. robustum and Rubus alceifolius have been undertaken on Réunion (Kueffer & Lavergne, Reference Kueffer and Lavergne2004). Release of biocontrol agents in Mauritius must only be done after appropriate host-specificity testing and would need to be accompanied by management measures to ensure that a reduction in vigour of one or more alien species did not result in its replacement by other invaders. Finally, the severe forest degradation that has occurred since 1939 calls for more research, funding and political will to restore the last vestiges of Mauritian forest.
Acknowledgements
The authors would like to thank the National Park and Conservation Service for granting us permission to work in the National Park. Data collection would not have been possible without the logistic help of the Mauritian Wildlife Foundation and the Mauritius Herbarium, based at the Mauritius Sugar Research Institute, and the botanical expertise of G. D'Argent. We would like to thank J. Millett, L. Gillson and B. Kunin for reviewing earlier drafts of this article.
Biographical sketches
Malika Virah-Sawmy has carried out conservation management and ecological restoration for local NGOs and government bodies on a range of islands including Mauritius, the Seychelles, New Zealand and Madagascar. She is now the Terrestrial and Freshwater Programmes coordinator for WWF Madagascar and Western Indian Ocean Islands. John Mauremootoo worked as Plant Conservation Manager for the Mauritian Wildlife Foundation for 7 years, where he led projects such as the restoration of Round Island, Iles aux Aigrettes and Rodrigues, and he is now a freelance consultant. Doreen Marie studied agriculture at the University of Mauritius and now works for the Ministry of Environment. Saoud Motala specializes in insect taxonomy for conservation and works for the Mauritian Wildlife Foundation and the Natural History Museum, London. Jean-Claude Sevathian is the Rare Plant Coordinator for the Mauritian Wildlife Foundation and a consultant in forest restoration for the private sector. He is also a member of the IUCN/Species Survival Commission Indian Ocean Plants Specialist Group.








