Reproductive biotechnologies can contribute to rapid genetic improvement of cattle. However, their efficacy depends on physiological characteristics of the female such as the antral follicle count (AFC), which can vary substantially among donors. AFC may be a good marker for selecting donors with the greatest potential to generate competent oocytes, which would increase the proportion of animals that respond well to reproductive biotechnologies (Alward and Bohlen, Reference Alward and Bohlen2020). At present, donors with high AFC are selected based on transrectal ultrasound in the framework of multiple ovulation embryo transfer (MOET) programs for assisted reproduction in cattle. However, transrectal ultrasound requires trained personnel, expensive equipment and adequate facilities for the careful evaluation of the obtained images (Perry and Cushman, Reference Perry and Cushman2016). An effective alternative to the ultrasound technique is the quantification of anti-Müllerian hormone (AMH), specifically produced by granulosa cells of growing small follicles (Monniaux et al., Reference Monniaux, Rico, Larroque, Dalbiès-Tran, Médigue, Clément and Fabre2010). Levels of this hormone are directly related to AFC in Bos taurus cows (Souza et al., Reference Souza, Carvalho, Rozner, Vieira, Hackbart, Bender and Wiltbank2015). However, the level of AMH taken at an unknown moment of the estrous cycle varies with animals, with high individual repeatability (Gobikrushanth et al., Reference Gobikrushanth, Dutra, Bruinjé, Colazo, Butler and Ambrose2017), preventing the standardized use of AMH concentration as an endocrine marker without previous evidence of the intensity of such relationship at a given moment of the estrous cycle. Moreover, whether this relationship also holds in grazing feeding systems in the high tropics is unclear.
The recommended cut-off for AMH levels to identify animals with high AFC differs across subspecies; for example, they are 0.70 ng/ml for Bos indicus (Brahaman breed) and 0.25 ng/ml for Bos taurus (Angus breed; Jaques et al., Reference Jaques, Cardoso, Chachere, Sinha, Seery, Wilkes, Forrest and Looney2020). The levels of AMH considered low or high can differ between breeds of the same B. taurus subspecies, and even in the same breed. In the Holstein breed raised under intensive production systems (in this case, Florida, USA), concentrations over 0.631 ng/ml are considered high and those under 0.085 ng/ml are considered low (Ribeiro et al., Reference Ribeiro, Bisinotto, Lima, Greco, Morrison, Kumar and Santos2014). Furthermore, different studies of the same breed have suggested different cut-off values. This variability in recommended cut-off points may be because the relationship of AMH levels with AFC in crossbred Holstein cattle depends on management and feeding conditions. In particular, we are interested in how levels of AMH and their relationship with AFC differ between animals under extensive grazing production in high tropical climatic conditions (over 2500 m above sea level) and animals under the same production system in other environments. Several considerations suggest that altitude may influence cattle fertility, although this has not been demonstrated directly. High altitude can alter physiological functions of animals (Parraguez and Gonzalez, Reference Parraguez and Gonzalez-Bulnes2020). The hypobaric hypoxia resulting from living at high altitude causes oxidative stress and affects the hypothalamus, pituitary and ovarian axis, compromising folliculogenesis of the corpus luteum and expression of the genes encoding insulin-like growth factors (IGFs) I and II. This effect of hypobaric hypoxia, in turn, reduces the fertility of animals living at high altitude compared to those living at sea level (Parraguez and Gonzalez, Reference Parraguez and Gonzalez-Bulnes2020). Criollo or also named Creole cattle in the highlands of Ecuador show differences in ovarian physiology from those reared at sea level, and these differences could be linked to altitude (Ayala et al., Reference Ayala, Pesantez, Rodas, Dután, Calle, Murillo, Vázquez, Nieto, Ortega and Samaniego2019). The agroecological conditions typical of the area and the nutritional management in high-altitude regions could alter the energy balance as well as the metabolic and endocrine activities of these Creole cattle, affecting reproductive performance.
The objective of the present study was to determine the relationship between plasma AMH concentration and the AFC, assessed during the restart of the follicular wave, in crossbred Holstein cows raised in the high tropics under an extensive grazing system. Based on our analysis, we aimed to propose cut-off values for AMH levels to select animals with high AFC for MOET protocols, currently being implemented in the frame of official breed recovery programs.
Material and methods
Animals and farms
From a total of 609 farms located in the Ecuadorian high tropics, 15 farms were chosen based on altitude (over 2500 m above sea level), average environmental temperature (between 7 and 18 °C), a relative humidity of 80%, and annual rainfall between 800 and 2000 mm. Free grazing was the feeding management system, which was based on mixtures of gramineous and legumes supplemented daily with hay and mineral salts. The cows included were crossbred Holstein and the average farm size was 25.4 ± 4.1 cows, with a milk production between 8 and 12 l/day per cow. In these 15 farms there were 383 cows in production, including 152 multiparous females (3.1 ± 0.17 calvings) with a mean age of 5.9 ± 0.22 years, weight of 553.8 ± 12.82 kg, and body condition of 2.55 ± 0.05 on a scale of 1–5. The animals were an average of 75.3 ± 2.10 d postpartum and in estrus at the time of beginning the study. This requisite was particularly important to enhance the response rate to the synchronization protocol. Of these, finally 140 cows entered the experiment as they responded to the follicular wave restart protocol (described below). The cows were recruited between April 2018 and June 2020.
Animal handling and procedures were performed following the regulations of the Terrestrial Animal Health Code, chapter 7.8 ‘Use of animals in research and education’ of the World Organization for Animal Health (OIE, 2016).
Study design
Cows satisfying the inclusion criteria received the following synchronization protocol to initiate a new follicular wave. On day 1, the cows received an intravaginal progesterone-releasing device (IPD; CIDR® [1.38 g of progesterone], Zoetis, Quito, Ecuador) + 2 mg of intramuscular estradiol benzoate (EB; Gonadiol®, Zoetis). Seven days later, in the morning, an intramuscular dose of 25 mg of prostaglandin (PG; Lutalyse® [dinoprost tromethamine], Zoetis) was applied and the implant was removed. On the following day (day 8), the cows received 1 mg of intramuscular EB (Gonadiol®) and, 54 h after removal of the implant, the location of the preovulatory follicle was explored by transrectal ultrasound (Aloka ProSound 2®, Tokyo, Japan) using a 7.5-MHz linear transducer. On day 11, which was considered the day of re-initiation of the follicular wave or ‘day 0’ of the study, the coccygeal vein was punctured, and blood was sampled into evacuated tubes containing ethylenediaminetetraacetic acid (BD Vacutainer®, Franklin Lakes, NJ, USA). Samples were immediately placed in a cooler with a cooling gel at 5 °C and centrifuged in the laboratory at 1500 g for 20 min. The plasma obtained was stored in aliquots at −20 °C until subsequent analysis of AMH concentrations. On day 11, AFC was also performed (the design is shown in online Supplementary Fig. S1).
AFC
On day 9, the same technician who performed the AFC in all cows also located the preovulatory follicle by transrectal ultrasound. Two days later (day 11), the disappearance of the follicle (ovulation) was verified. In the cows that ovulated, the AFC (<4 mm in diameter) was performed in the two ovaries as described Ayala et al. (Reference Ayala, Pesantez, Rodas, Dután, Calle, Murillo, Vázquez, Nieto, Ortega and Samaniego2019) based on lateral-medial, dorso-ventral, and cranio-caudal scans. The right ovary was scanned first, followed by the left ovary.
Determination of AMH plasma concentration
The Elisa DL-AMH-b kit (DL Develop®, Tech Development, Wuxi, China) was used to measure AMH plasma levels, following the manufacturer's instructions. Optical detection was performed at a wavelength of 450 nm. The manufacturer-reported sensitivity of the assay was <0.117 ng/ml, and the inter- and intra-assay coefficients of variation were <10 and <12%, respectively.
Statistical analysis
Data were stored in Excel and subsequently analyzed using SPSS® 25 (IBM, New York, USA). General descriptive statistics were analyzed. Data were presented in tables as mean ± standard deviation (sd), while data in the figures were expressed as mean ± standard error of the mean (sem). Data that did not follow a normal distribution were log10-transformed before analysis.
The cows were categorized according to AMH concentration into three groups (low, intermediate, high). Differences in means were assessed for significance using analysis of variance (ANOVA) and compared using the Tukey test with alpha = 0.05. Differences associated with P < 0.05 were considered significant. Pearson's correlation analysis and simple linear regression were applied to explore the potential relationship between AMH levels and AFC.
Results and discussion
AMH plasma levels positively correlated to the total number of antral follicles <4 mm in diameter present at the restart of the follicular wave (r = 0.783; P < 0.001). In addition, 61% of the observed variation in average AFC could be explained by the adjusted linear regression model (r = 0.613, Fig. 1). Animals presenting plasma AMH concentrations higher than 0.09 ng/ml showed a higher follicular population at the time of restarting the follicular wave than cows with AMH concentrations lower than 0.05 ng/ml. These results confirm the strong relationship between AMH, and the AFC established in the Holstein breed by previous works under normal altitude, observed a correlation with a value of r = 0.59 (P < 0.001) in cows in stabling (Rico et al., Reference Rico, Fabre, Medigue, di Clemente, Clement, Bontoux, Touzé, Dupont, Briant, Rémy, Beckers and Monniaux2009). The correlation was even stronger among bovines raised at sea level (r = 0.79, P < 0.001; Gobikrushanth et al., Reference Gobikrushanth, Dutra, Bruinjé, Colazo, Butler and Ambrose2017).
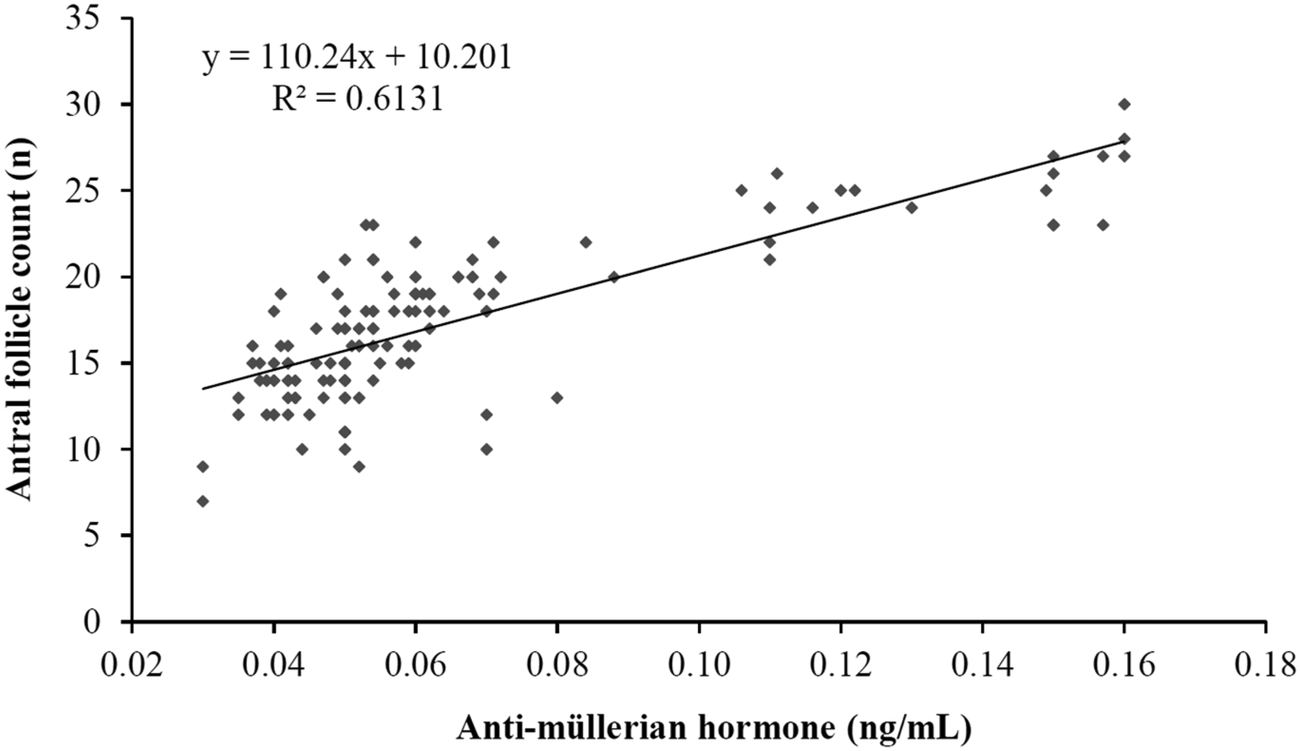
Fig. 1. Association between plasma levels of anti-Müllerian hormone (AMH) and antral follicle count (AFC) in postpartum crossbred Holstein cows at the start of a hormonally induced follicular wave.
The linear association observed between plasma AMH and AFC in our work (Fig. 1) is consistent with that described in Holstein cows (r = 0.61; P < 0.001) and heifers (r = 0.73; P < 0.001) reared in the low tropics in stabling systems and fed with formulated mixed rations (Batista et al., Reference Batista, Macedo, Sala, Ortolan, Sá Filho, Del Valle and Baruselli2014). Therefore, our data support the belief that AMH plasma concentration can be used as an endocrine indicator to indirectly identify Holstein cows with a higher follicular population at the restart of the new follicular wave under the high-tropic conditions of our study.
Although the association between AMH and AFC in our study is similar to that previously described, the mean value of AMH in our study (0.06 ± 0.03 ng/mL), the minimum value (0.03 ng/ml), and the maximum value (0.16 ng/ml) differed substantially from those previously reported for this breed (online Supplementary Table S1). A study of cows 4–9 years old in the low tropics showed a minimum AMH concentration of 0.025 ng/ml (Rico et al., Reference Rico, Fabre, Medigue, di Clemente, Clement, Bontoux, Touzé, Dupont, Briant, Rémy, Beckers and Monniaux2009), similar to the present study. However, the maximum value in that study (0.228 ng/ml) was 1.4 times higher than in our study. The maximum value in our study differed even more from the maximum values in other studies of Holstein cows stabled in the low tropics (0.038–0.774 ng/ml; Gobikrushanth et al., Reference Gobikrushanth, Dutra, Bruinjé, Colazo, Butler and Ambrose2017).
These differences in AMH concentrations could reflect the use of different analytical methods. Although all used kits have been validated for use in cattle, they can lead to mean AMH values differing by up to 4.6 times, even if the overall results strongly correlate across the kits (r = 0.84, P < 0.001) (Fréour et al., Reference Fréour, Mirallié, Bach-Ngohou, Denis, Barrière and Masson2007). This highlights the relevance of describing the used kits at each study, as well as having previously established cut-off points by each selected diagnostic kit to work with.
Another factor that could affect AMH values is the number of antral follicles <4 mm present at the time of blood sampling for AMH assessment. In our study, we counted 17.26 ± 0.38 follicles on average (range 7–30), which was lower than the maximum values of 15–55 follicles reported previously (Rico et al., Reference Rico, Fabre, Medigue, di Clemente, Clement, Bontoux, Touzé, Dupont, Briant, Rémy, Beckers and Monniaux2009). This lower AFC might explain why our cows showed a lower maximum AMH value. Similarly, other research groups have found lower concentrations of AMH in bovine breeds with a sparse follicular population (Batista et al., Reference Batista, Macedo, Sala, Ortolan, Sá Filho, Del Valle and Baruselli2014). A reduced population of antral follicles has been found in sheep reared at high altitudes, (Parraguez and Gonzalez, Reference Parraguez and Gonzalez-Bulnes2020). Hypoxia can lead to reduced secretion of follicle-stimulating hormone (FSH) and luteinizing hormone, altering steroidogenesis, and ultimately reducing the number and growth of available follicles. While direct links between hypobaric hypoxia and fertility have not been established in bovines, our work with Creole cattle in the same region as the present study suggest changes in the ovarian physiology, including a lower number of antral follicles (Ayala et al., Reference Ayala, Pesantez, Rodas, Dután, Calle, Murillo, Vázquez, Nieto, Ortega and Samaniego2019).
The nutritional conditions typical of the area, characterized by a reduced energy intake, could also influence AFC and AMH levels. Epigenetic studies found that calves born to mothers with nutritional restriction (60% of nutritional requirements during the first 110 d of gestation) presented lower AFC (15.8 ± 1.8 follicles), lower AMH and higher FSH levels than the offspring of mothers that consumed 120% of the nutritional requirements (AFC = 23.6 ± 1.9; Mossa et al., Reference Mossa, Carter, Walsh, Kenny, Smith, Ireland, Hildebrandt, Lonergan, Ireland and Evans2013). The negative impact of poor maternal nutrition on AFC and AMH levels in the offspring has been confirmed in another study in Holstein cows under intensive production management conditions (Ribeiro et al., Reference Ribeiro, Bisinotto, Lima, Greco, Morrison, Kumar and Santos2014). When stratified by AMH levels, 13.6% (n = 19) of our cows had high AMH concentrations, 60% (n = 84) had intermediate concentrations, and 26.4% (n = 37) had low concentrations. The high AMH level (0.14 ± 0.02 ng/ml) was three times the low AHM level (0.04 ± 0.01 ng/ml; P < 0.05; Fig. 2a).
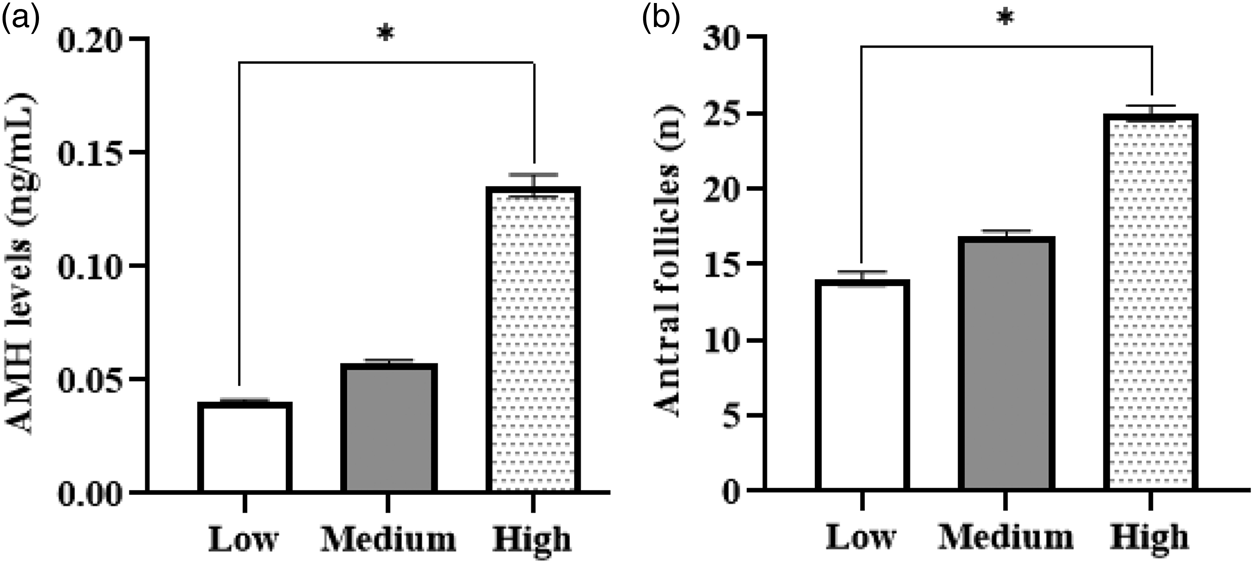
Fig. 2. Plasma levels of anti-Müllerian hormone (AMH) (a) and antral follicle count (b) on the day of onset of the follicular wave in crossbred Holstein cows. Cows were classified into three groups according to the level of AMH, using the cut-off values of 0.09 and 0.05 ng/ml.
Considering that our cattle were exposed to a mixture of different risk factors that have not previously been studied simultaneously, we could not directly infer results on AMH and AFC relationship coming from other studies. The lowest AMH concentration in the present study was similar to that determined previously in Holstein animals with similar parity (Ireland et al., Reference Ireland, Smith, Scheetz and Folger2011). However, the high concentration group in that study showed 42.8% higher AMH (>0.20 ng/ml) and 58.4% more antral follicles (39.61 ± 2.3 follicles) than our high concentration group. Substantial differences in AFC between animals with ‘high’ or ‘low’ AMH were also observed in a study of younger Holsteins (Batista et al., Reference Batista, Macedo, Sala, Ortolan, Sá Filho, Del Valle and Baruselli2014), where the high AMH group had an AMH level of 0.57 ± 0.26 ng/ml and AFC of 34.3 ± 3.12, while the low AMH group had values of 0.06 ± 0.02 ng/ml and 13.4 ± 1.40. The lower AFC and AMH in our high AMH animals may reflect the grazing feeding system traditionally provided to herds in the Ecuadorian highlands. The hypobaric hypoxia in the region may then reinforce the effects of this diet on fertility.
Our results suggest that 0.09 ng/ml may be a useful AMH cut-off value for the selection of animals with a sizeable follicular population in the high tropics. With this cut-off, only 13.6% of the cows in our study would be selected, but they would have 1.8 times more antral follicles than the rest. The optimal cut-off point depends on the reproductive technology to be applied. Dairy cows and heifers managed at around sea level, with a follicular population >25 may have the highest number of viable embryos in vivo. Animals with a mean AFC of 20.40 ± 1.63 present the best results after ovum pick-up and in vitro embryo production (Ghanem et al., Reference Ghanem, Jin, Kim, Choi, Lee, Ha and Kong2016). We propose that applying reproductive technologies such as MOET, ovum pick-up and in vitro embryo production to crossbred Holstein cows in the high tropics is more likely to be successful if the animals have plasma AMH >0.09 ng/ml and AFC of at least 25.0 ± 2.21 follicles.
In conclusion, crossbreed Holstein cows fed on grass living in the Ecuadorian high tropics show a direct relationship between the concentration of AMH and the follicular population. AMH >0.09 ng/ml may be useful for selecting such animals for reproductive biotechnologies. This relationship differed in intensity and shape when copmpared to those previously observed in other cattle breeds and under different environmental circumstances.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029922000140
Acknowledgements
We thank the farm owners for their support during the experiment. We are also grateful to the veterinarian Ernesto Cobos for his help in sample collection. This work was funded by the Research Directorate of the University of Cuenca (Project No. DIUC_XVII_2018_37_Ayala_Luis).




