INTRODUCTION
Hepatitis E (HE) is an infectious disease of the liver caused by hepatitis E virus (HEV). HEV, a sole member of the family Hepeviridae, genus Hepevirus, was first identified in 1983 by a Russian virologist [Reference Balayan1]. HEV infections occur globally, both as outbreaks and as sporadic cases [2]. An estimated 20 million infections and 3·3 million symptomatic cases of HE occur annually worldwide with an estimated 56 600 deaths [3]. Outbreak of this disease frequently occur in developing countries with limited access to safe drinking water and poor sanitation and hygiene [Reference Perez-Gracia, Suay and Mateos-Lindemann4–9]. In recent years, numerous HE outbreaks have been reported in areas of conflict and humanitarian crises, such as war zones, camps for refugees, or internally displaced populations [Reference Guthmann10–Reference Teshale12]. HE is clinically indistinguishable from other causes of acute viral hepatitis. Symptoms usually develop within 2–8 weeks of initial exposure to the virus. The primary symptoms include fever, nausea, abdominal pain, vomiting, anorexia, malaise, and hepatomegaly. HEV infection is usually self-limiting but may develop into fulminant hepatitis with high mortality [13]. HE seroprevalence increases with age. The overall case-fatality rate is estimated to be 1–3% but it can be up to 20% in HEV-infected pregnant women [Reference Emerson and Purcell14, Reference Farshadpour, Taherkhani and Makvandi15].
There are currently four known human genotypes of HEV, each displaying different epidemiological and clinical characteristics. Genotypes 1 and 2 frequently cause waterborne HE outbreaks and have been found only in humans, especially in young and middle-aged adult populations [Reference Sarguna, Rao and Sudha Ramana16–Reference Haque18]. Genotypes 3 and 4 mainly cause sporadic cases in the middle- to old-age general population and have been isolated from swine and other animal species as well as humans [Reference Kamar19]. Small-scale foodborne HE outbreaks of genotype 3 have also been reported, but outbreaks of HE caused by genotype 4 have rarely been recorded [Reference Garbuglia20].
There are four documented routes of transmission of HEV. These routes of transmission include: the faecal–oral route, ingestion of undercooked meat or meat products derived from infected animals such as boars and deer and domestic animals such as pigs, transfusion of infected blood products; and vertical transmission from a pregnant woman to her fetus. The most common source of HEV infection is the faecal–oral route due to faecal contamination of drinking water in low- and middle-income countries where outbreaks are common, while meat consumption from infected animals (pork products mainly) plays a very important role in high-income countries where sporadic cases are usual [Reference Riveiro-Barciela21]. The virus enters the body mainly through direct ingestion of contaminated water or meats. Other transmission routes that appear with less frequency are via blood and vertical transmission [Reference Perez-Gracia, Suay and Mateos-Lindemann4]. Moreover, recent studies have increasingly focused on secondary transmission. About 18% of shellfish samples collected from a coastal area of China were contaminated with 16 strains of HEV-4, which were closely related to swine and human isolates [Reference Gao22]. HEV sequences have been detected on soft fruits and vegetables, with irrigation water as the suspected vehicle of contamination [Reference Van der Poel23–Reference Kokkinos25].
From 24 January to 4 February 2015, a hospital in Jiashan County reported two laboratory-confirmed HE cases, with one death. Both these patients were residents of the same nursing home in Ganyao town, Jiashan County. Because the occurrence of HEV is rare in China, especially in a nursing home, which might have potential public health implications, an epidemiological investigation was undertaken, aiming to identify additional cases, the aetiology, source, risk factors and transmission patterns. The information drawn from this investigation will provide new insights into understanding the aetiology and epidemiology of HE infection and even a new strategy to prevent future HE outbreak.
MATERIALS AND METHODS
Study site and history of HE epidemics
The nursing home where the outbreak occurred is located in downtown Ganyao, a rural area of Jiashan County, Zhejiang Province, China (Fig. 1). There are four buildings in this nursing home. Two to five elderly individuals live in one room, sharing a separate washroom. Each floor has a public washroom. Buildings 1, 2 and 4 share the same water supply system and have only one water boiler for drinking water supplies, housed in building 1. The drinking water of the county is standardized and supplied by local drinking water companies.

Fig. 1. Location of Ganyao town in Zhejiang Province.
The population of Ganyao township is ~43 000. Sporadic HE cases were noted annually with an incidence of ~3/100 000 in Jiashan County, according to data from the National Notifiable Disease Report System. In 2014, one of the 22 HE cases reported in Jiashan County was a 45-year-old male farmer residing in a community 3·1 km from the nursing home. During the same period, a study conducted in the community-based population showed a ~40% positive rate of anti-HEV IgG in Jiashan County (N. Cao, unpublished data).
Case definition
Individuals who lived or worked in the nursing home between 1 October 2014 and 18 April 2015 were included in a case-control study. Cases were defined as serum anti-HEV IgM positive, regardless of symptoms, and controls were defined as serum anti-HEV IgM and IgG negative. Clinically, the cases were further divided into clinical cases and sub-clinical cases. A clinical case was defined as any confirmed case with clinical symptoms or signs of acute viral hepatitis such as hypodynamia, anorexia and jaundice, accompanied by an abnormal alanine aminotransferase (ALT) level. A sub-clinical case was defined as a confirmed case with normal ALT level and lack of hepatitis-like symptoms.
Risk factors associated with HEV infections
The investigation was performed using questionnaires administered face-to-face. After consulting the literature, we designed a questionnaire composed of four parts: water situation (drinking-water source, daily potable water quantity, methods for cleaning tableware, sharing drinking glasses, gargling by tap water, etc.), dietary condition (eating raw seafood, undercooked pig livers or rotten fruits/vegetables, etc.), living habits (washing hands before meals, washing hands after visiting the toilet, activities in courtyard, etc.) and contact history (contact with hepatitis cases or suspected animals).
Case-finding
From 6 February 2015, serum samples were collected from all subjects who had lived or worked in the nursing home since 1 October 2014 and a further 111 residents in the surrounding area. Measures for case-finding included daily medical observation for the residents and work staff in the nursing home and health publicity in the areas around (within 1 km) the nursing home from 6 February to 18 April 2015.
Descriptive epidemiology
We collected information on demographics, living habits, clinical symptoms and medical records. All medical records were supplied by local hospitals. The meteorological data were provided by local meteorological departments. Basic information was supplied by the nursing home, including name, age, gender, and daily menus since October 2014. The daily menus recorded the types of food which the diners ate for each meal.
All members from the nursing home were advised to visit physicians if any symptoms developed. For this study, the overall investigation and disposal was completed on 21 May 2015. All the individuals with suspicious symptoms between 1 October 2014 and 18 April 2015 were retrospectively and prospectively recorded. We describe illnesses by onset of disease, age, sex, type of work and clinical symptoms, and calculate specific attack rates, classified by occupation, sex and residential building.
Laboratory detection and analyses
Human serum and stool sample collection
From 6 February 2015, five serological specimens for dynamic observation HEV antibodies were collected from every subject about every 2 weeks. Blood sample collections were completed on 18 April 2015. On 11 February 2015, 111 serological specimens were collected from residents in the surrounding area for serological detection. From 8 February to 15 February, stool specimens were collected from 26 cases in the nursing home.
Investigation of water supply
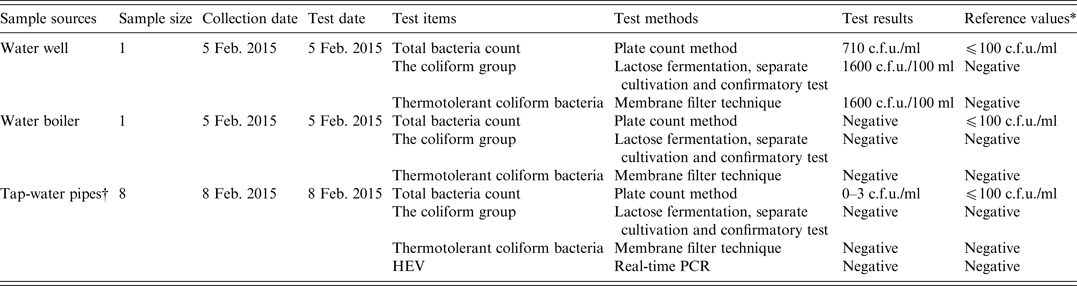
Water samples from a water well, water boilers and tap-water pipelines were collected on 5 and 8 February 2015. In accordance with national hygiene standards [standards for drinking water quality (GB 5749-2006)], water samples were tested for total bacteria count (plate count method), the coliform group (lactose fermentation, separate cultivation and confirmatory test), and thermotolerant coliform bacteria (membrane filter technique).
Investigating food supply and collection of swine serum and stool samples
Because of the relatively long incubation period of HE, it is difficult to attribute disease to consumption of a particular food and the possibly contaminated food could not be collected. Forty-four pig faecal samples for PCR detection and 30 serum samples for antibody testing were collected from surrounding pig farms, which supplied pig products to the nursing home. All the samples were stored in an ice chest at –80 °C until testing.
Serological and molecular assay of HEV
Enzyme-linked immunosorbent assay (ELISA)
All the human and animal serum samples were tested for HEV antibodies (IgG and IgM) by using commercially available enzyme immunoassay kits (Wantai Biologic Pharmacy Enterprise, China). Assays were run in accordance with the manufacturer's instructions.
Reverse transcription-polymerase chain reaction (RT–PCR)
Nucleic acids were extracted from stool suspensions using the RNeasy mini kit (Qiagen, Germany) in accordance with the manufacturer's instructions. The first-round PCR was conducted using One-Step PrimeScript RT–PCR templates (Takara, Japan). A total of 20 µl reaction mixture was set up, which contained the RNA (8·4 µl), 2 × PCR buffer (10 µl), Takara Ex Taq HS (5 U/µl, 0·4 µl), PrimeScript RT enzyme mix (0·4 µl) and external primers [HEo44-os (20 µ m, 0·4 µl) and HEo44-oas (20 µ m, 0·4 µl)] (Table 1) [Reference Shrestha26]. The reaction conditions: RT at 42 °C for 20 min, then initial denaturation at 94 °C for 1 min, followed by 40 cycles in the first round (94 °C for 30 s; 55 °C for 30 s; 72 °C for 1 min, with an additional 5 min in the last cycle). A total of 50 µl reaction mixture was included in the second-round PCR, which contained the PCR products (5 µl), 10 × Ex Taq buffer (5 µl), dNTP (4 µl), Ex Taq HS (0·25 µl), sterile water (33·75 µl) and internal primers [HEo44-is (20 µ m, 1 µl) and HEo44-ias (20 µ m, 1 µl)] (Table 1). The PCR amplification was carried out for 25 cycles in the second round (94 °C for 30 s, with an additional 2 min in the first cycle; 55 °C for 30 s, 72 °C for 45 s, with an additional 5 min the last cycle). Agarose gel electrophoresis showed that the PCR fragment was ~450 bp. The sequence of the PCR-positive product was the partial sequence of ORF2 of HEV. HEV negative stool detected previously was used as a negative control. The obtained PCR samples were subjected to DNA sequencing (Sangon Biotech Co. Ltd, China).
Table 1. Primers used for detection of HEV-RNA
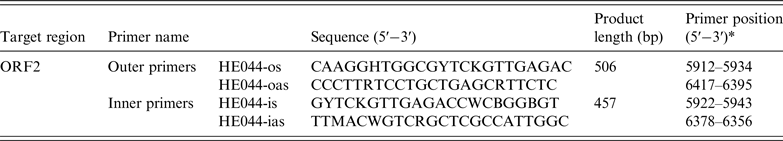
* Primer positions are based on GenBank sequence accession no. M73218.
Sequence and phylogenetic analysis
Subsequently, the RT–PCR products amplified from HEV-positive samples were sequenced by Sangon Biotech (Shanghai) Co. Ltd using the Sanger sequencing method. The sequences of different HEV sub-genotype strains were retrieved from Genbank for comparison. Genetic analyses of HEV sequences were conducted using the neighbour-joining method with the MEGA v. 5.1 software package (www.megasofeware.net). The sequences of the three Jiaxing isolates were compared with 39 reference strains from the Genbank database using MegAlign software (http://www.dnastar.com/t-megalign.aspx).
Statistical analysis
EpiData v. 3.0 (epidata.software.informer.com) was used for data entry and SPSS v. 18.0 (SPSS Inc., USA) was usedfor data analysis. Testing of hypotheses was two-sided with an α-value of 0·05. The χ 2 test or Fisher's exact test were used to analyse categorical data. A measure of multiplicative interaction for risk ratios was used to analyse interaction effects.
Ethical statement
This investigation was undertaken in response to a public health emergency and approved by the ethics committee of Zhejiang Provincial Centre for Disease Control and Prevention. Written informed consent for participation was obtained from all subjects or their legal guardians.
RESULTS
Descriptive epidemiology
During the outbreak period, there were 185 residents (88 men, 97 women, aged from 40 to 102 years) in the nursing home. In addition, 24 staff members worked in the nursing home. Of these, eight lived in the nursing home. Of note, four infected workers lived outside the nursing home except for duty days.
Overall, 37 HEV cases [12 symptomatic (8 men, 4 women), 25 asymptomatic (9 men, 16 women)] were found between 13 January and 18 April 2015. These cases were all distributed in the nursing home. The overall attack rate was 17·70% (37/209). The case-fatality rate was 8·1% (3/37) in this outbreak (Fig. 2).
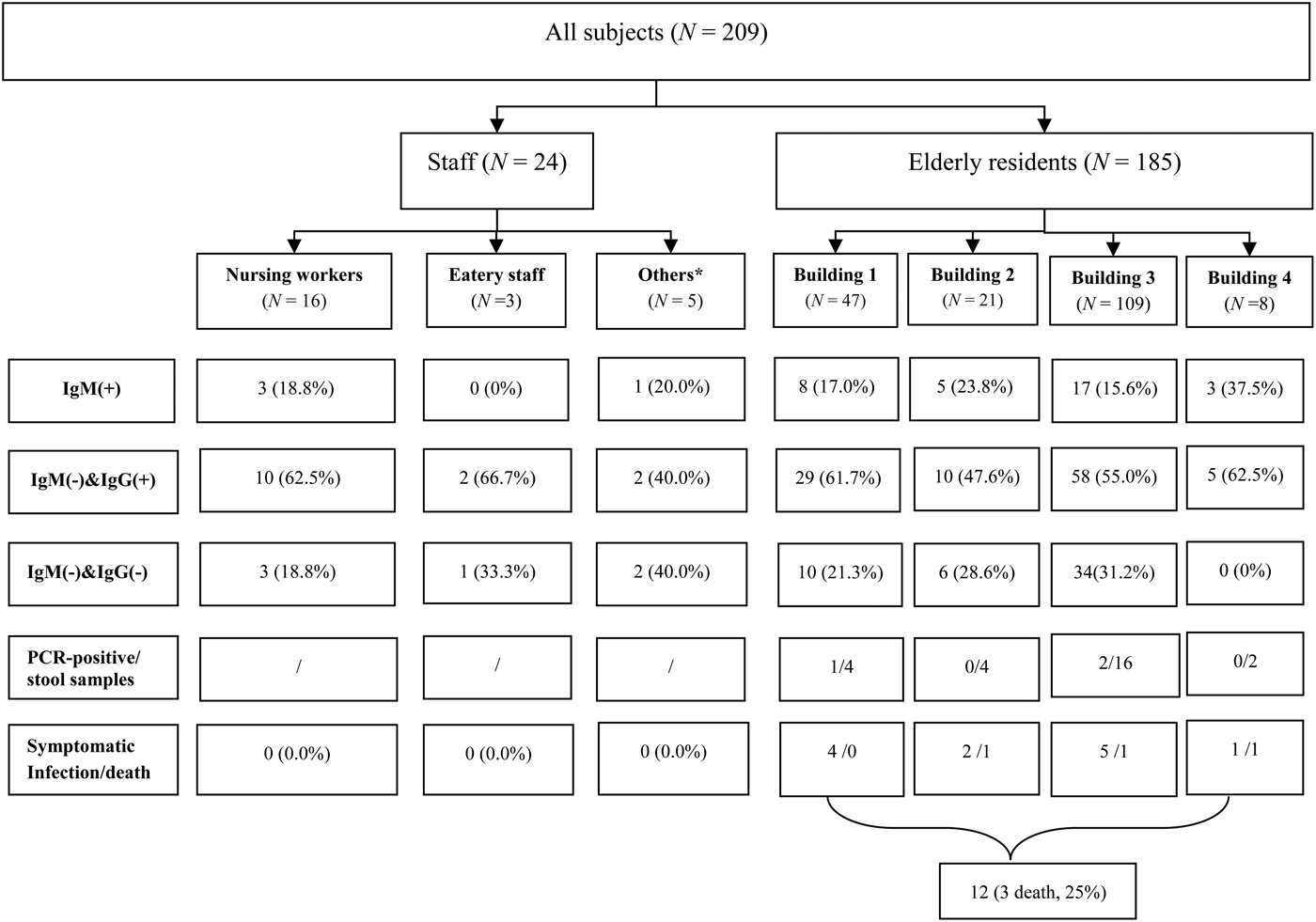
Fig. 2. Profiles of the subjects. * Others included administrative staff and security guards.
Specific attack rates were also calculated. For occupation, the attack rates reached 17·8% (33/185) in senior residents and 16·7% (4/24) in staff members. For sex, the attack rates reached 18·3% (17/93) for males and 17·0% (20/116) for females. For building residence, the attack rates reached 18·3% (8/47) in building 1, 23·8% (5/21) in building 2, 15·6% (17/109) in building 3, and 37·5% (3/8) in building 4 (Fig. 2). There was no statistical difference in different occupation (χ 2 = 0·000, P = 1·000), sex (χ 2 = 0·038, P = 0·845) and building residence (χ 2 = 2·457, P = 0·504).
The index case, a 79-year-old man with a history of colon cancer surgery 8 years ago and chronic bronchitis for >30 years, had onset of illness on 13 January 2015, with anorexia, hypodynamia and dark brown urine. After consulting the First People's Hospital of Jiashan on 18 January, he was treated as an inpatient. His condition rapidly worsened and he died of multiple organ failure on 21 January. Case 2, a 65-year-old man who had hypertension for 5 years, poliomyelitis for >60 years and hepatic cirrhosis caused by schistosomiasis for 40 years, developed abdominal bloating, anorexia, nausea, hypodynamia and dark brown urine on 20 January 2015. He was hospitalized on 26 January and died of acute hepatic failure on 9 February.
The epidemic curve of 12 clinical cases showed a unimodal distribution and declined rapidly after measures were taken. The measures taken included isolation and treatment of cases, improvement in drinking water standards and toilet facilities, and enhancing food safety during the food processes (Fig. 3). Considering the likelihood of water pollution, corresponding meteorological data were included in the analysis. Of note, it rained for 2 weeks about 1·5 months prior to the outbreak. On 30 November 2014, there was heavy rain with cumulative rainfall over 33·1 mm.

Fig. 3. The epidemic curve and rainfall amount. The epidemic curve (blue areas) represents cases. The start date of the histogram is 13 January and the time interval differentiated from the graph's horizontal axis is 5 days.
Clinical manifestations and clinical laboratory results
The average age of the 12 symptomatic patients was 76·8 years. Symptoms of illness included jaundice (100%), anorexia (58%), dark brown urine (58%), hypodynamia (42%), abdominal distension (33%) and nausea and vomiting (17%). Laboratory abnormalities included raised ALT (92%), aspartate transaminase (92%), and total bilirubin (100%).
Case-control study
There were 37 cases (17 males, 20 females) and 52 controls (14 males, 38 females) enrolled in the case-control study. The investigation was completed for all the cases and controls without any missing items. They are comparable in sociodemographic characteristics [sex (P = 0·063), age (P = 0·215)]. Univariate analysis indicated that two factors, gargling using tap water (P < 0·05) and washing dishes in tap water (P < 0·05), were statistically significant between the two groups (Table 2). Furthermore, a statistically significant positive interaction between gargling using tap water and washing dishes in tap water was discovered by multiplicative interaction for risk ratios (P < 0·05) (Table 3).
Table 2. Univariate analyses for identification of risk factors for HEV infection
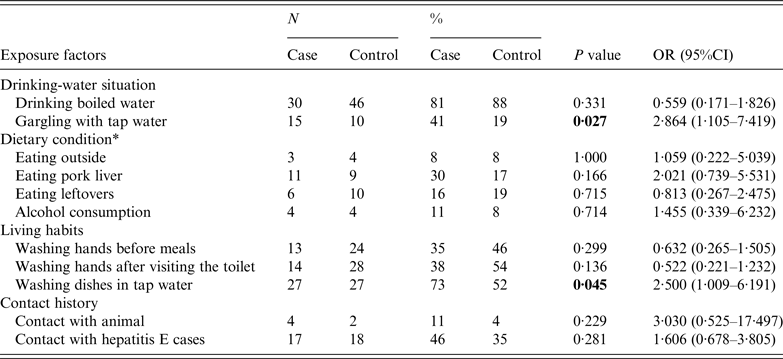
OR, Odds ratio; CI, confidence interval.
* Dietary condition included whether a certain food was eaten in the 2 months before the index case was identified.
Table 3. The interaction effect between two statistically significant factors related to water, by multiplicative interaction for risk ratios

OR, Odds ratio; CI, confidence interval.
* The interaction on the odds ratio scale is then measured by: OR11/(OR10 × OR01) = 6·857/(1·948 × 1·714) = 2·05, which is >1, so the interaction is positive (if <1 then negative).
Laboratory investigation
Evaluation of water quality testing results
As shown in Table 4, a water sample from the water well in the nursing home contained high levels of bacteria.
Table 4. Analyses of water sources for faecal indicator bacteria and hepatitis E virus (HEV) in the nursing home
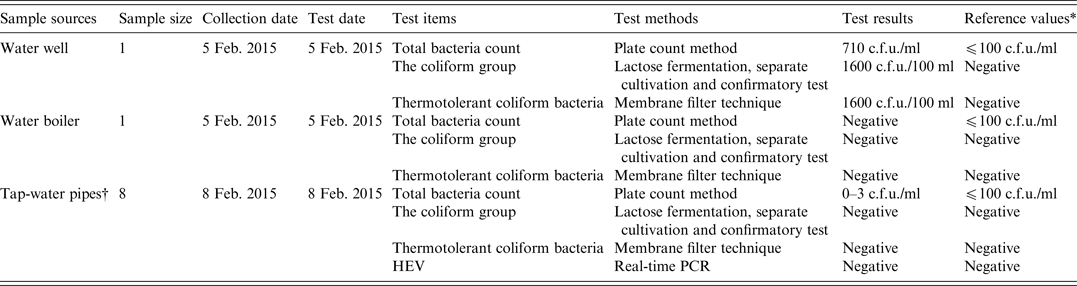
* The reference values were derived from standards for drinking water quality issued by the Ministry of Health of China.
† The water samples were collected from four different tap-water pipes. Of these, three were located in the rooms of different cases, while one was located in the refectory of the nursing home. Two samples were collected from each tap-water pipe.
Phylogenetic analysis of three HEV genome sequences
Phylogenetic trees were constructed based on the partial HEV ORF2 genome sequences (400 bp). The phylogenetic trees showed that these different HEV strains were divided to four genotype. Three Jiaxing strains isolated in this study [JX18 (Genbank accession no.: KT337475), JX23 (Genbank accession no.: KT337476), JX25 (Genbank accession no.: KT337477)] belonged to HEV genotype 4, subgenotype d (Fig. 4). The homology analysis indicated that the identity of the nucleotide sequence with three HEV isolates was high (99·5–100%). The sequences of three Jiaxing strains displayed high similarity to the AJ272108·1/China/human and AJ428854·1/China/swine sequences, with levels of 94·6% and 93·7%, respectively.

Fig. 4. Phylogenetic trees were constructed based on the partial HEV ORF2 genome sequences (400 bp) using MEGA 5·1. Three Jiaxing strains were isolated in this study [JX18 (Genbank accession no.: KT337475), JX23 (Genbank accession no.: KT337476), JX25 (Genbank accession no.: KT337477)].
Other important observations
-
• The elderly entered the nursing home without medical examination or screening for HEV infection.
-
• None of the individuals in our study had received HE vaccine.
-
• Without a carefully guarded entrance, people have free access to the nursing home.
-
• Ganyao town received 33·1 mm of rainfall on 30 November 2014 and 43·7 mm of rainfall on 31 October 2014.
-
• Water sanitation evaluation: (1) The water temperature of the water boiler in building 1 was set to ⩽90 °C, and was probably as low as 60–70 °C at peak water periods. (2) The septic tank was placed beside the drinking-water system, which had been used for many years and was badly rusted. The water pipe joints over the septic tank were rusted and loose. Visible leakage was present in the water pipe joints (Fig. 5). (3) Faeces were released directly into the septic tanks without safe excreta disposal. (4) There was an entertainment room in building 1 with several mahjong tables. The elderly having activities in this room often shared their drinking glasses. (5) The sanitation condition was relatively better in building 3 which had an independent water supply system and a water boiler on each floor. Of note, the elderly living here had activities in the courtyard during daytime but often obtained water from the water boiler in building 1. (6) The tableware was cleaned only by tap water without disinfection. The cupboard for storage of dishes was dirty and the dishes had not been sterilized before use.
-
• Food sanitation evaluation: (1) All three refectory staff had health certificates. (2) Daily menus were made one day in advance. All individuals living or working in the nursing home were provided with standard meals every day. (3) According to the daily menu, meat dishes mainly included chicken, duck and fish. All food is well cooked by boiling or frying. Food containing pork livers had been supplied three times in 2014, the details are as follow: salted sour cabbage-fried pork livers on 11 November, shallot-fried pork livers on 27 November, and garlic-fried pork livers on 13 December. (4) The ingredients for the food were bought from one of the markets in Ganyao town. There were no changes in the manner by which meat and other food products from pigs were cooked, no changes in the supplier of pig-derived products, and no changes to the origin of the pigs from which products were supplied to the nursing home. (5) None of the residents in the surrounding area had acute hepatitis symptoms and they might have used a similar source of meat as the nursing home. (6) No positive detection results were found in the pig faecal and serum samples that were collected from surrounding pig farms, which supplied pig products to the nursing home.

Fig. 5. A brief diagram of the water supply and sewage of the nursing home.
DISCUSSION
To the best of our knowledge, this is the first study to report the clinical, epidemiological, and molecular characterization of an outbreak of genotype 4 HE in China. After consideration of many potential risk factors, we found that this outbreak was most likely caused by tap water contamination due to the leaking septic tank.
HEV genotype 4 is believed to be transmitted mainly through consumption of infected animal meat or products [Reference Kmush, Nelson and Labrique27]. However, food seem not to be a risk factor in this outbreak. First, similar types of food were supplied to the residents and staff by the nursing home refectory each dinner, and few of the residents were recorded eating outside. Second, seafood was not included in the daily menus, and pork products were well cooked and rarely eaten. Other meat and vegetables were also well cooked by either boiling or frying. Third, there was no significant difference in morbidity between the group exposed to pork livers and the unexposed group (P = 0·166). Finally, there were no changes in the dietary and cooking habits. All pig-derived products were from the same suppliers. None of the residents in the surrounding areas had symptoms of acute hepatitis despite the fact that they obtained meats from a similar source to the nursing home. Moreover, all of the 111 serum samples were HEV-IgM negative, indicative of no recent infection of HEV, and no positive detection results were found in pig faecal and serum samples.
The evidence from this investigation suggests that the tap-water contamination might be the major risk factor for this outbreak. First, the cases were limited only to the nursing home during the whole epidemic period. Second, the results of the case-control study indicated that gargling with tap water and washing dishes in tap water were two independent risk factors and the interaction effect related to the water were also found between them. Third, the characteristics of the epidemic curves suggested that this outbreak was a point-source exposure, namely a transient common-source exposure. Finally, by integrating information on meteorological and spot hygiene, transient tap-water pollution after heavy rain was possible.
Faecal contamination of water can occur either at the source, such as rivers, streams, ponds or shallow wells, or during transport or storage. Several outbreaks have been linked with flooding following heavy rains, which washes faecal matter into surface water sources such as rivers and ponds [Reference Viswanathan28]. Leaky water pipes that pass through soil contaminated with human faeces may also allow for contamination of water during periods of low water flow due to the negative pressure within the pipes has been reported [Reference Sailaja29]. Similarly, in our study, we hypothesized that leaky water pipes passing through septic tanks may be contaminated with human faeces after storm water and flooding. Detailed link analysis on water pollution supported the following conclusions: First, this nursing home was characterized by obsolete health facilities and existing hidden dangers without safe excreta disposal. Second, the rusted tap-water pipeline was built close to a drainage pipe in a septic tank, with obvious visible leakage at the pipe joint. Third, according to the onset time and incubation period (2–8 weeks) of HEV, the median exposure time was estimated to be during October and December 2014. It rained heavily for a few days in Ganyao town between 31 November 2014 and 14 January 2015. The elderly and staff members recalled, ‘when it rains, sewage overflows from the septic-tank’. Heavy rain caused the water pipeline to be immersed directly in the septic tank. The similar phenomenon could still be observed when the investigation was carried out in February. Therefore, it is likely that HEV-infected sewage and faeces contaminated the water network. HEV remains viable after heating to 56 °C for 1 h and incubation at 71 °C for 20 min are required to fully inactivate the virus [Reference Emerson, Arankalle and Purcell30, Reference Barnaud31]. However, the temperature of the water boiler in building 1 did not always meet the standard for inactivating HEV. Finally, as described the history of HE epidemics earlier, the HEV infectious source probably existed in the nursing home.
All other possible factors such as infected animal transmission and person-to-person transmission were also taken into consideration in our study. According to previous studies, the HEV strains that infect mammals such as pigs, rats, and rabbits are causative agents of zoonotic infection in humans. The major animal reservoir is the pig, in which there is a very high prevalence of asymptomatic infection in many countries. In our study, most cases reported no history of contact with animals, and the minority who had contact with animals such as rats and dogs were all IgM negative. Person-to-person transmission has been reported in northern Uganda [Reference Teshale32]. In our study, 13 cases had a history of contact with HEV-infected individuals and four medical staff members who were in charge of taking care of these elderly patients were also infected. However, no statistically significant difference was found in the infection rate between those who had a case contact history and those who had no exposure to the HEV-infected individuals.
After adjusting for other risk factors, water-borne transmission was believed to be the most probable cause in our study suggesting the existence of other transmission routes for genotype 4d HE. In Italy, five cases were identified in 2011 and the genetic analysis showed high identity with HEV4d swine hb-3 and T1 isolates from China (Genbank accession nos.: GU361882 and AJ272108). This outbreak was not linked to infection by imported foods or persons travelling from endemic areas and, due to a lack of hard evidence, the cause is unclear to date [Reference Garbuglia20]. In China, an outbreak of genotype 4d HE in a nursing home in 2012 was reported in Jiangsu Province, in which 30 cases were identified and the isolates shared high identity with HEV4d T1. Contamination of public tableware in the refectory by HEV infection in faeces or water may have resulted in this outbreak, but the evidence is inadequate [Reference Xu33].
Contrary to previous reports in the literature, our study integrated with more investigation such as laboratory detection and hygienic investigation. Two important findings were reported in our study. First, HEV1 and HEV2 mainly infect humans via contaminated water, while HEV3 and HEV4 mainly infect humans or animals via infected and undercooked pork or meat products [Reference Kamar19]. Other than HEV4 outbreaks previously reported, the transmission mechanism in our study is similar to that of HEV1 and HEV2. As several other latent infections have been discovered in this county, we speculated that one or more residents or staff members in the nursing home may be infected with HEV occasionally. Further, the HE outbreak occurred when the drinking-water system was transiently polluted by HEV-infected sewage and faeces on rainy days. Second, compared to other HE outbreaks which also occurred in nursing homes in China [Reference Xu33, Reference LI34], the symptoms were more serious and the case-fatality rate (8·1%, 3/37) was higher in our study. Except for age and underlying health problem, virulence variation is believed to be an important reason for this high mortality. Next, we will explore the virulence variation for further study.
There were several limitations to our study. First, the long incubation period and self-limiting nature of HE infection made it difficult for the elderly to recall possible risk factors. For the same reason, the possibly contaminated water and food which had been consumed over a lengthy period could not be collected. Second, according to ‘Diagnosis and treatment criteria for hepatitis E’ issued by the China Association of Infection [35], a confirmed HE case is defined as having any of the acute HEV infection markers: HEV RNA, anti-HEV IgM and positive seroconversion. But the detection indexes in our study were qualitative with no measurement of specific antibody titres. Consequently, subjects who had achieved seroconversion (⩾fourfold rise in titre) could not be distinguished from those who were seropositive but without a significant change in antibody titres, which may lead to underestimation of the number of cases. Third, the serum samples were collected from all residents and staff members in the nursing home for serology detection rather than RNA detection. This is one limitation of our study which may lead to false negatives, especially for immunocompromised patients who may not mount a good antibody response to HEV after infection [Reference Nanda36, Reference Gerolami, Moal and Colson37]. Finally, no positive results were obtained from water samples using the PCR method. One possible reason was that the transmission and circulating period of the viruses may have occurred weeks ago when human cases were infected during the outbreak [Reference Guerrero-Latorre, Hundesa and Girones38]. On the other hand, detection of water samples was conducted by the local CDC without equipment for water enrichment. After detection, the samples were discarded, hence further confirmation was not carried out.
In summary, we have summarized the clinical, epidemiological, and molecular characterization of an outbreak of genotype 4 HE in China; the information will be useful for the prevention and control of HE. A total of 37 cases were identified and the majority of severe cases involved elderly men. Based on epidemiological evidence, we assume that tap-water pollution in the nursing home is the most probable cause. The outbreak was well controlled by isolating sources of infection, solid waste management and hygiene promotion in emergencies, and ensuring chlorination of drinking water at the household level, and by repairing the nursing home small-scale piped water distribution systems and wastewater treatment, [3]. The last case was identified on 18 March and no new case had been found following two long latent periods of HE (about 4 months) indicating that the outbreak was effectively controlled. More studies on molecular mechanisms will be applied to this outbreak and further comparison between sporadic and outbreak cases will soon be done in Zhejiang Province.
ACKNOWLEDGEMENTS
We thank all the assistants for support we received during this case-control study, with special thanks to the hospital president, Mingquan Wu and all the investigators from Ganyao town health centre, Jiashan County Center for Disease Control and Prevention and Jiaxing City Centers for Disease Control and Prevention.
This work was financially supported by a grant from the Medical Research Programme of Zhejiang Province (2016KYB057). None of the funders had any role in the study design and the information collection, data analysis and interpretation, or in the writing of the article and the decision to submit it for publication. The researchers confirm their independence from the funders and sponsors.
DECLARATION OF INTEREST
None