The incidence of oesophageal adenocarcinoma has risen in recent decades in several developed countries of the world( Reference Botterweck, Schouten and Volovics 1 – Reference Coleman, Bhat and Murray 5 ). Barrett's oesophagus, a premalignant metaplastic condition of the distal oesophagus, is a precursor of oesophageal adenocarcinoma( Reference Ruol, Parenti and Zaninotto 6 ). Chronic gastro-oesophageal reflux, male sex, Caucasian ethnicity, age, obesity and cigarette smoking are risk factors for the development of Barrett's oesophagus( Reference Winberg, Lindblad and Lagergren 7 ). The majority of these risk factors are difficult or impossible to modify. The identification of modifiable environmental risk factors of Barrett's disease, including dietary factors, may help in the formulation of prevention strategies for oesophageal adenocarcinoma.
Few studies have focused on dietary factors in relation to Barrett's oesophagus risk( Reference De Ceglie, Fisher and Filiberti 8 ), and they have suggested an inverse association with vegetable and fruit consumption. However, no prospective cohort study has investigated this relationship. Case–control studies are prone to bias due to the possibility that individuals with preclinical symptoms may voluntarily, or on a physician's advice, change their diet.
Green vegetables are a major source of dietary nitrate intake. Nitrate may have several beneficial health effects mediated through reactive N intermediates, including antibacterial effects and effects on gastric mucosal integrity( Reference Lundberg, Weitzberg and Cole 9 ). Nitrate content may explain part of the potentially protective effect of vegetables in various health conditions. On the other hand, nitrate is also involved in the endogenous formation of N-nitroso compounds through reduction by anaerobic bacteria to nitrite and subsequent formation of nitrosating agents( Reference Tricker 10 ). The N-nitrosation process can be inhibited by antioxidants such as vitamin C. Epidemiological studies have not shown an association of nitrate intake( Reference Cross, Freedman and Ren 11 , Reference Keszei, Goldbohm and Schouten 12 ), or nitrate from plant sources( Reference Ward, Heineman and Markin 13 ), with oesophageal adenocarcinoma. No study has evaluated the association between nitrate intake and Barrett's oesophagus risk.
The aim of the present study was to investigate the association between vegetable and fruit consumption, as well as nitrate intake, and Barrett's oesophagus risk in a large prospective cohort of Dutch men and women.
Subjects and methods
Population and follow-up
The Netherlands Cohort Study was started in September 1986. At baseline, 58 279 men and 62 573 women aged 55–69 years were recruited, and a self-administered questionnaire was completed by the study participants( Reference van den Brandt, Goldbohm and van 't Veer 14 ). Ethical approval to conduct the study was obtained from the institutional review boards of the University Hospital Maastricht and TNO Nutrition and Food Research. The cohort was followed for 16·3 years until 31 December 2002 for incident Barrett's disease cases by computerised record linkage to the nationwide network and registry of histo- and cytopathology in The Netherlands (PALGA)( Reference Steevens, Schouten and Driessen 15 ). After manual check for false-positive linkages, excerpts of all pathology records of cases were reviewed independently by a pathologist (A. L. C. D.) and a pathologist in training (C. J. R. H.) who were blinded to exposure. Of 974 cases, 106 were excluded due to uncertain diagnosis, seventy-six due to prevalent cancer or Barrett's disease at baseline, fifty-eight due to oesophageal or gastric cancer diagnosed before or within half a year of Barrett's disease diagnosis, and 148 cases because of incomplete or inconsistent dietary data or missing data on confounders. Histology was not completely specified in the excerpts available from the central PALGA database for 250 cases. Full pathology reports of these cases were retrieved from the local pathology laboratories and were reviewed by the pathologists to identify the type of metaplasia. A total of 603 cases were available for analysis, of which 433 cases had specialised intestinal metaplasia. A case–cohort design was employed and hence a random subcohort (n 5000) was selected at baseline. Due to prevalent cancer or Barrett's disease at baseline and due to inconsistent or missing data, 230 and 1053 subcohort members were excluded from further analysis, respectively.
Assessment of determinants
Vegetable and fruit intake
Food and beverage intake during the year preceding the Netherlands Cohort Study baseline was assessed using a 150-item semi-quantitative FFQ. The participants were queried about vegetable consumption frequency in summer and that in winter separately( Reference Steevens, Schouten and Goldbohm 16 ). The participants could choose one of six categories ranging from ‘never or less than once per month’ to ‘three to seven times per week’. The subjects were asked about usual serving sizes only for string beans and cooked endive; the mean of these values served as an indicator to derive the serving sizes of all the cooked vegetables using a vegetable-specific factor calculated based on the results of a pilot study, which showed an intra-individual correlation between serving sizes of different vegetables. Vegetables that were eaten regularly, but were not specifically queried about in the questionnaire, could be entered in an open-ended question along with consumption frequency and amount consumed on each occasion. For most of the vegetables, the questionnaire explicitly specified whether the vegetable was eaten raw or cooked.
The participants were queried about fruit consumption frequency and amount of fruits consumed using categories ranging from ‘never or less than once per month’ to ‘six or seven times per week’.
Data were key-entered and processed in a standardised manner, blinded to case/subcohort status. Mean daily intakes were calculated from frequencies and serving sizes( Reference Steevens, Schouten and Goldbohm 16 ).
The FFQ was validated against a 9 d diet record( Reference Goldbohm, van den Brandt and Brants 17 ). The Spearman correlation coefficients were 0·38 for total vegetable consumption and 0·60 for total fruit consumption. The FFQ appeared to slightly overestimate vegetable consumption, on average, while underestimating fruit consumption when compared with the diet records.
Nitrate intake from diet
Food composition values for nitrate were derived from the databank on contaminants in food from the State Institute for Quality Control of Agricultural Products (RIKILT). Estimations were based on the mean nitrate contents between 1985 and 1989. Distinction between summer and winter was made while calculating nitrate intake from some vegetables (i.e. endive (raw/cooked) and lettuce), and information on nitrate losses during preparation (washing, cutting or cooking) was considered. For several vegetables, experimental data were available regarding nitrate losses during preparation( Reference van de Worp 18 , Reference Driessen 19 ). Nitrate loss percentages used to construct the nitrate table were 16, 31, 42, 20 and 49 % for endive, spinach, chicory, cabbage and potatoes, respectively. For other vegetables consumed after cooking, nitrate losses were estimated to be 40 %. For lettuce, a 20 % loss was estimated.
Nitrate intake from drinking water
Information on nitrate content in drinking water from all the pumping stations in The Netherlands in 1986 (Vereniging van Exploitanten van Waterleiding bedrijven in Nederland (VEWIN) 1989) was used to determine the nitrate concentration in drinking water for each home address by postal code. To calculate nitrate intake from water, we used information from the questionnaire about the amount of water, coffee, tea and soup consumed. Total nitrate intake was calculated by summing dietary nitrate intake and nitrate intake from water. In the subcohort, the median proportion of dietary nitrate to total nitrate was 99 %, and the median proportion of intake from vegetables was 90 %. The major source of nitrate intake among vegetables was leafy vegetables in line with studies of nitrate intake in the Dutch population( Reference van Loon and van Klaveren 20 ).
Vitamin C intake
Daily vitamin C intake was calculated from the FFQ data using the Dutch food composition table.
Assessment of potential confounders
Information on education (primary school, lower vocational, high school or higher vocational/university), cigarette smoking status (never-smoker, ex-smoker or current smoker), smoking history (number of cigarettes smoked and duration of smoking), total energy intake (kJ/d), BMI (kg/m2), non-occupational physical activity ( < 30, 30–60, 60–90 or >90 min/d), alcohol intake (g/d), and long-term (more than half year) use of non-steroidal anti-inflammatory drugs and lower oesophageal sphincter-relaxing medications (nitroglycerins, aminophyllines, β-blockers, anticholinergics, nifedipine and benzodiazepines) was obtained from the baseline questionnaire.
Statistical analyses
The characteristics of cases and subcohort members are described using percentages, means, standard deviations, medians and interquartile ranges. Incidence rate ratios and 95 % CI for Barrett's oesophagus were estimated using Cox proportional hazards models comparing quintiles of intakes and using continuous variables. Analyses were carried out using Prentice's method of weighting for case–cohort designs( Reference Barlow, Ichikawa and Rosner 21 ). Men and women were analysed separately. Standard errors were calculated using a robust variance estimator( Reference Lin and Wei 22 ). Results were similar while using time on study and age as the time scale; hence, only results with the former are presented. Tests of linear trend in the incidence rate ratios were carried out by fitting models with the median values of each exposure quintile as a continuous variable. The proportional hazards assumption was assessed using the scaled Schoenfeld residuals( Reference Schoenfeld 23 ) and by introducing time–covariate interactions into the models and examining estimates and testing their significance using the Wald test. Substantial deviation from the assumption was not detected for any of the exposure variables. Effect modification by sex and vitamin C intake was tested using cross-product terms. All the models were adjusted for age, and multivariable models were additionally adjusted for smoking status (current: yes/no), number of cigarettes smoked per d, duration of smoking (years), total energy intake (kJ/d), alcohol intake (g/d), BMI categories (quintiles), levels of education (four categories), non-occupational physical activity (four categories) and long-term use of lower oesophageal sphincter-relaxing medications (yes/no). Models for nitrate intake were also adjusted for vegetable intake (g/d). Primary analysis was carried out using only cases with intestinal metaplasia (n 433). Long-term use of reflux medications and vitamin C supplement use were also considered in multivariable models, but were not included in the final reported models as they had only minor effects on the estimates. Additional analyses were carried out restricted to individuals who reported having similar vegetable and fruit intakes 5 years before baseline. Furthermore, we carried out an analysis by excluding the first 2 years of follow-up.
Based on a method proposed by Cai & Zeng( Reference Cai and Zeng 24 ) and using the available number of cases and subcohort members, we estimated that an 80 % power could be achieved to show hazard ratio (HR) of 0·55 and 0·5 between quintiles of exposure with a two-sided type 1 error of 0·05 among men and women, respectively.
Results
The baseline characteristics of 241 male and 192 female cases and 3717 subcohort members are given in Table 1. Cases were more often men, were less likely to be current smokers at baseline, and were somewhat more likely to have used lower oesophageal sphincter-relaxing medications and non-steroidal anti-inflammatory drugs. The median intake of total vegetables was 179 g/d (interquartile range 138–229 g/d) in the subcohort and was higher in women than in men (Kruskal–Wallis χ2(df = 1): 7·3, P= 0·007). The intakes of different groups of vegetables were comparable between cases and subcohort members (Table 1). Vegetables consumed in the largest amount in the subcohort were tomatoes, onions, string beans and cauliflower (median 19, 17, 17 and 13 g/d, respectively). Median fruit consumption in the subcohort was 157 g/d (interquartile range 94–234 g/d) with a higher intake in women (Kruskal–Wallis χ2(df = 1): 153, P= 0·0001).
Table 1 Characteristics of Barrett's disease cases and subcohort members in the Netherlands Cohort Study on diet and cancer, 1986–2002 (Median values and interquartile ranges (IQR))
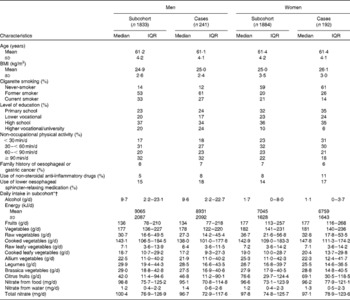
* Vegetables included string/French beans, cauliflower, lettuce, carrots (raw/cooked), endive (raw/cooked), Brussels sprouts, sauerkraut, tomatoes, onions, spinach, beetroot, kale, cabbage, leek, mushrooms, broad beans, sweet peppers, rhubarb and gherkins.
† Fruits included apples, pears, strawberries, oranges and fresh orange juice, grapes, mandarins, bananas, grapefruits and grapefruit juice, raisins, and other dried fruits.
Total nitrate intake strongly correlated with vegetable intake in the subcohort (r 0·83) and median intake was comparable between cases and subcohort members (Table 1).
Among men, total vegetable intake was inversely associated with the risk of Barrett's oesophagus with intestinal metaplasia (Table 2), but a clear linear trend was not observed. A strong inverse association with a clear trend was observed with raw leafy vegetable consumption. The multivariable-adjusted model with continuous exposure variables estimated a 45 % decrease in risk per 25 g/d increase in raw leafy vegetable intake. An inverse association was also found for total raw vegetable and Brassica vegetable intake. No association was found for cooked vegetable and fruit intake.
Table 2 Hazard ratios (HR) for Barrett's oesophagus with specialised intestinal metaplasia in men by vegetable and fruit intake in the Netherlands Cohort Study on diet and cancer, 1986–2002 (Hazard ratios and 95 % confidence intervals)
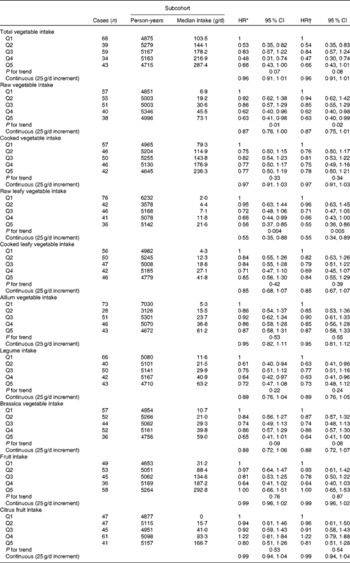
Q, quintile.
* Adjusted for age (years); calculated using Cox proportional hazards model.
† Adjusted for age (years), smoking status (current v. non-current smoker), duration of cigarette smoking (years), number of cigarettes smoked per d, total energy intake (kJ/d), BMI (quintiles), alcohol intake (g/d), levels of education (four categories), non-occupational physical activity (four categories) and use of lower oesophageal sphincter-relaxing medications (yes/no); calculated using Cox proportional hazards model.
Associations between vegetable and fruit intake and Barrett's oesophagus risk among women are summarised in Table 3. The strongest inverse association was observed for Brassica vegetables, but significant inverse associations were not detected. HR estimates for cooked leafy vegetables suggested positive associations, but the estimate for the highest category was not significant. HR estimates were generally higher than those found among men, and interaction analyses with continuous variables suggested effect modification by sex for leafy vegetables (Wald: P= 0·04 and P= 0·07 for raw and cooked leafy vegetables, respectively), but not for other vegetable groups.
Table 3 Hazard ratios (HR) for Barrett's oesophagus with specialised intestinal metaplasia in women by vegetable and fruit intake in the Netherlands Cohort Study on diet and cancer, 1986–2002 (Hazard ratios and 95 % confidence intervals)
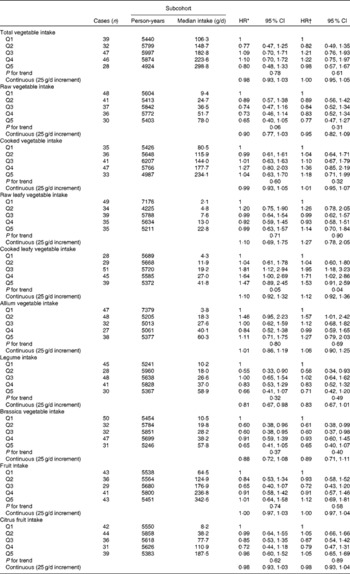
Q, quintile.
* Adjusted for age (years); calculated using Cox proportional hazards model.
† Adjusted for age (years), smoking status (current v. non-current smoker), duration of cigarette smoking (years), number of cigarettes smoked per d, total energy intake (kJ/d), BMI (quintiles), alcohol intake (g/d), levels of education (four categories), non-occupational physical activity (four categories) and use of lower oesophageal sphincter-relaxing medications (yes/no); calculated using Cox proportional hazards model.
Associations between nitrate intake and Barrett's oesophagus risk in men and women are summarised in Tables 4 and 5, respectively. The highest v. the lowest category of total nitrate and nitrate intake from food was inversely associated with Barrett's oesophagus risk among men after adjustment for confounders including vegetables. However, the estimate for nitrate intake from food in the multivariable model was not significant. Nitrate intake from water was positively associated with Barrett's oesophagus risk. Among women, estimates for the highest category suggested moderately strong positive associations for all the three nitrate variables. Test for interaction with sex was significant for age-adjusted and multivariable models with categorical exposure variables for total nitrate intake (P= 0·04). Interaction between nitrate intake and vitamin C intake was not significant (P= 0·10 and P= 0·99 in men and women, respectively).
Table 4 Hazard ratios (HR) for Barrett's oesophagus with specialised intestinal metaplasia in men by nitrate intake from diet and water in the Netherlands Cohort Study on diet and cancer, 1986–2002 (Hazard ratios and 95 % confidence intervals)

Q, quintile.
* Adjusted for age (years); calculated using Cox proportional hazards model.
† Adjusted for age (years), smoking status (current v. non-current smoker), duration of cigarette smoking (years), number of cigarettes smoked per d, total energy intake (kJ/d), vegetable intake (g/d), fruit intake (g/d), BMI (quintiles), alcohol intake (g/d), levels of education (four categories), non-occupational physical activity (four categories) and use of lower oesophageal sphincter-relaxing medications (yes/no); calculated using Cox proportional hazards model.
‡ Total nitrate is the sum of nitrate intake from food and nitrate intake from water.
Table 5 Hazard ratios (HR) for Barrett's oesophagus with specialised intestinal metaplasia in women by nitrate intake from diet and water in the Netherlands Cohort Study on diet and cancer, 1986–2002 (Hazard ratios and 95 % confidence intervals)
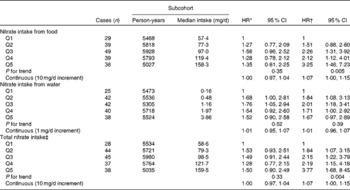
Q, quintile.
* Adjusted for age (years); calculated using Cox proportional hazards model.
† Adjusted for age (years), smoking status (current v. non-current smoker), duration of cigarette smoking (years), number of cigarettes smoked per d, total energy intake (kJ/d), vegetable intake (g/d), fruit intake (g/d), BMI (quintiles), alcohol intake (g/d), levels of education (four categories), non-occupational physical activity (four categories) and use of lower oesophageal sphincter-relaxing medications (yes/no); calculated using Cox proportional hazards model.
‡ Total nitrate is the sum of nitrate intake from food and nitrate intake from water.
Analysis restricted to individuals who reported having similar vegetable and fruit intakes 5 years earlier was based on 104 male and 89 female cases and 1632 subcohort members. An association with total vegetable intake was no longer apparent among men (HR for the highest v. the lowest category: 0·81, 95 % CI 0·40, 1·65). The HR for the highest category of raw vegetable intake compared with the lowest category was 0·38 (95 % CI 0·18, 0·80) in men and 0·94 (95 % CI 0·45, 1·95) in women (P for interaction = 0·79). Analyses for nitrate intake were similar to the primary analysis with somewhat attenuated HR estimates (data not shown). Analyses excluding the first 2 years of follow-up did not substantially change the estimates for vegetable/fruit intake and nitrate intake (data not shown).
Discussion
This is the first prospective cohort study to examine the association between vegetable and fruit consumption, as well as nitrate intake, and Barrett's oesophagus risk. The results indicate an inverse association between total vegetable intake and Barrett's oesophagus risk among men. Raw vegetable intake, especially raw leafy vegetable intake, was inversely associated with Barrett's oesophagus risk in men and the highest category of Brassica vegetable intake also suggested a protective effect. Estimates among women, in general, do not suggest an inverse association. Fruit consumption was not associated with Barrett's disease risk in men or in women. In men, an inverse association with nitrate intake was observed, but not with nitrate intake from water sources. In women, nitrate intake was positively associated with Barrett's disease risk. Effects were not different across the levels of vitamin C intake.
Epidemiological studies have suggested an inverse association between vegetable and fruit intake and oesophageal adenocarcinoma, and stronger inverse associations have been reported with the intake of green leafy, raw and cruciferous vegetables in several studies( Reference Kubo, Corley and Jensen 25 ). Few studies have examined the association between vegetable and fruit intake and Barrett's disease, a precursor lesion for adenocarcinoma. Case–control studies have observed an inverse association with vegetable and/or fruit intake( Reference Anderson, Watson and Murphy 26 – Reference Pohl, Wrobel and Bojarski 29 ), a ‘health-conscious’ diet including fruits and vegetables( Reference Kubo, Levin and Block 30 ), and fibre intake( Reference Kubo, Block and Quesenberry 31 , Reference Mulholland, Cantwell and Anderson 32 ). A case–control study carried out in the USA has evaluated specific subtypes of vegetables and found an inverse association with dark green vegetables( Reference Jiao, Kramer and Rugge 33 ). The present results suggest that a possible protective effect could be due to raw leafy vegetable and cruciferous vegetable consumption, in line with previous findings for oesophageal adenocarcinoma( Reference Kubo, Corley and Jensen 25 ) and Barrett's oesophagus( Reference Jiao, Kramer and Rugge 33 ).
Vegetables are a major source of nitrate in human diet. Nitrate intake might be responsible for some of the effects of raw leafy vegetables found in the present study. Absorbed nitrate is excreted by the salivary glands in high concentrations and is reduced to nitrite by anaerobic bacteria( Reference Lundberg, Weitzberg and Cole 9 ). High concentrations of NO are generated from nitrite at the gastro-oesophageal junction under acidic conditions( Reference Iijima, Henry and Moriya 34 ), and high concentrations of NO can also be found within the columnar lined oesophagus of patients with Barrett's oesophagus during acid reflux( Reference Suzuki, Iijima and Scobie 35 ). It has thus been suggested that high doses of NO in the oesophagus may be important in the development of Barrett's oesophagus( Reference Iijima and Shimosegawa 36 ), possibly through its effect on oesophageal tissue damage( Reference Ishiyama, Iijima and Asanuma 37 ). It has also been shown that dietary nitrate may induce N-nitrosation in juxtaluminal compartments of the upper gastrointestinal tract of healthy subjects and patients with Barrett's oesophagus via the generation of nitric oxide( Reference Winter, Paterson and Scobie 38 ). In the absence of vitamin C and under acidic conditions, nitrite is converted to nitrous acid and nitrosating agents that can react with secondary amines to form N-nitrosamines( Reference Mirvish 39 ). On the other hand, ingested nitrate also has several beneficial effects through the formation of NO( Reference Gilchrist, Winyard and Benjamin 40 ), including protection of gastric mucosa from damage( Reference Kitagawa, Takeda and Kohei 41 , Reference Miyoshi, Kasahara and Park 42 ) and from gastrointestinal infections( Reference Dykhuizen, Frazer and Duncan 43 , Reference Dykhuizen, Fraser and McKenzie 44 ). We are not aware of any previous epidemiological studies that have evaluated the association between nitrate intake and Barrett's oesophagus risk. Studies assessing the risk of oesophageal adenocarcinoma did not show associations with nitrate intake from diet and/or drinking water( Reference Cross, Freedman and Ren 11 – Reference Ward, Heineman and Markin 13 ). Although the present results cannot be unambiguously explained by the possible positive and negative effects of nitrate in the gastro-oesophageal junction, our findings provide additional support to the hypothesis that increased nitrate intake may increase or decrease the risk of health outcomes in different populations( Reference Gilchrist, Winyard and Benjamin 40 ).
Previous epidemiological studies did not evaluate the possible differences between men and women regarding the association of Barrett's disease risk with vegetable intake( Reference Anderson, Watson and Murphy 26 – Reference Thompson, Beresford and Kirk 28 ). The present results suggest a differential effect of raw vegetables in men and women, which might be partly explained by the sex differences in the occurrence of Barrett's oesophagus and differences in oesophageal pathophysiology. Barrett's disease occurs more often in men with a male to female ratio of 2:1 estimated in a meta-analysis( Reference Cook, Wild and Forman 45 ), and male sex is an independent risk factor for oesophagitis( Reference El-Serag and Johanson 46 , Reference Ford, Forman and Reynolds 47 ). Studies comparing men and women with reflux symptoms suggest sex-specific differences in oesophageal acid exposure, presence of defective oesophageal sphincter and hiatus hernia( Reference Banki, Demeester and Mason 48 ). Animal studies have suggested that female sex hormones might have an effect on parietal cell mass and decrease basal acid secretion( Reference Adeniyi 49 ), and in rat models of reflux oesophagitis, oesophageal damage has been shown to be more prominent in male rats than in female rats in the presence of NO administration( Reference Masaka, Iijima and Endo 50 ). These sex-specific differences support the hypothesis that dietary factors might have differential effects on the progression from reflux disease to Barrett's oesophagus. However, if these experimental results translate to effects on human risks, they would suggest more protective effects of sex hormones in women rather than in men.
Differential bias could also partly explain our dissimilar findings in men and women. Since the absence of Barrett's disease in the cohort of the present study cannot be verified, false-negative cases might have occurred. A previous study investigating the presence of Barrett's oesophagus among asymptomatic individuals has found a prevalence of 3 % in men and 1 % among women( Reference Fan and Snyder 51 ). We cannot rule out the possibility that different false-negative proportions among men and women could have led to differential bias in the present study.
Other limitations include the lack of information on gastro-oesophageal reflux and Helicobacter pylori infection, which has been shown to be inversely associated with Barrett's oesophagus( Reference Thrift, Pandeya and Smith 52 ). Additionally, it is difficult to measure vegetable and fruit intake in large epidemiological studies. Low correlations were observed for vegetable intake in the validation of the FFQ( Reference Goldbohm, van den Brandt and Brants 17 ). We would expect to find attenuations of HR estimates, and it is unlikely that differential bias across cases and non-cases would have been introduced. Similarly, attenuation of estimates might have resulted from the lack of repeated exposure measurements over time.
The prospective design, the large number of cases and the availability of full pathology reports to identify intestinal metaplasia are the most important strengths of the present study.
In conclusion, results obtained for this large prospective cohort are consistent with an inverse association between vegetable intake, especially green leafy vegetable intake, and Barrett's oesophagus risk. These findings add to the limited number of published epidemiological research on the relationship between vegetable intake and Barrett's oesophagus risk. However, the possibility that the beneficial effects of vegetables might be stronger among men should be evaluated further.
Acknowledgements
The authors thank PALGA and the pathologist for supplying the pathology reports of Barrett's disease cases. They also thank Dr A. Volovics and Dr A. Kester for their statistical advice; S. van de Crommert, H. Brants, J. Nelissen, C. de Zwart, M. Moll, W. van Dijk, M. Jansen, Ellen Dutman and A. Pisters for their assistance; and H. van Montfort, T. van Moergastel, L. van den Bosch and R. Schmeitz for their programming assistance.
The present study was supported by a grant from Maag Lever Darm Stichting (MLDS) (WO 09-53). MLDS had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: R. A. G., L. J. S., Y. C. A. K. and P. A. vd B. designed the research; R. A. G., L. J. S. and P. A. vd B. were involved in the coordination of the study; A. P. K., A. L. C. D., C. J. R. H. and L. J. S. conducted the research; A. P. K. analysed the data and wrote the article; P. A. vd B. had primary responsibility for the final content. All authors read and approved the final manuscript.
None of the authors has a conflict of interest to declare.







