INTRODUCTION
Rift Valley fever virus (RVFV) is a zoonotic mosquito-borne member of the Phlebovirus genus in the Bunyaviridae family of viruses that was first isolated in Kenya in the early 1930s [Reference Bishop1, Reference Daubney, Hudson and Granham2]. The virus was subsequently recognized as the aetiological agent of Rift Valley fever (RVF) disease, which is associated with abortions and perinatal mortality in livestock, and acute mild febrile illness in humans [Reference Daubney, Hudson and Granham2] with occasional cases (<8%) of encephalitis, retinitis, and generalized haemorrhagic syndrome [Reference Meegan, Bailey and Monath3]. Large epidemics of RVFV follow cyclical heavy rainfalls and flooding, particularly in areas of low rainfall [Reference Anyamba, Linthicum and Tucker4]. The most severe outbreaks of RVF occurred in Egypt (1977) affecting about 200 000 people and resulting in over 600 deaths, and in Kenya and Somalia (1997–1998) affecting over 89 000 people and resulting in more than 450 deaths [Reference Meegan5, Reference Woods6]. During epidemics, the virus devastates livestock, including cattle, sheep, goats, and camels, with mortality rates reaching 30% in adult animals and up to 100% in young animals [Reference Madani7–Reference Gerdes9]. Abortions occur in up to 100% of pregnant cattle, sheep, and goats [Reference Nichol, Knipe and Howley8, Reference Gerdes9]. The virus causes periodic epidemics in both Eastern Africa (Kenya, Somalia and Tanzania) as well as other African countries including Zimbabwe, South Africa, Egypt, Mauritania, Senegal, The Gambia, and Madagascar [Reference Meegan5, Reference Gerdes9, Reference Zeller10–Reference El12]. Of these countries, severe outbreaks of RVFV involving both humans and livestock have been most frequent in Kenya. In 2000, the virus was introduced into the Arabian Peninsula following a severe outbreak in Saudi Arabia and Yemen associated with importation of livestock from East Africa in 2000–2001 [Reference Al-Afaleq13–Reference Bird15].
An intriguing aspect of RVF epidemiology in Kenya is the periodicity of the outbreaks, between which inter-epidemic periods (IEPs) occur with low or no activity. Retrospective analysis of available livestock data collected through passive surveillance at the Kenya Ministry of Livestock and Fisheries Development starting from 1975 indicates that the increased numbers of RVFV cases were reported in 1990, 1997–1998, and recently in 2006–2007. In contrast, no cases were recorded between 1991 and 1996, and only one case was recorded between 2000 and mid-2006 [16]. Cryptic maintenance and transmission cycles of RVFV have been postulated but the exact mechanism remains poorly understood. The prevailing hypothesis is that RVFV is maintained in the eggs of floodwater Aedes mosquitoes belonging to the subgenera Neomelaniconion and Aedemorphus, which breed in isolated grassland depressions called dambos [Reference Linthicum, Davies and Kairo17, Reference Turell, Linthicum and Beaman18]. When the dambos flood during heavy rainy seasons, transovarially infected Aedes mosquitoes hatch and the subsequent infected adult mosquitoes transmit the virus to domestic animals including sheep, goats, cattle, and camels. The dambos and other flooded areas also serve as a habitat for Culex mosquito species, which also utilize the habitat after the floodwater Aedes have rapidly disappeared [Reference Turell, Linthicum and Beaman18–Reference Linthicum20]. The domestic animals amplify the virus to high titres and provide a source of infection for the Culex and other species that are capable of transmitting the virus beyond the dambo habitat to additional livestock and humans.
The involvement of wildife species during epidemics and the existence of sylvatic cycles involving wildlife and mosquitoes in maintenance and perpetuation of the virus during IEPs have never been investigated. The wildlife-mosquito cycling of RVFV could maintain the virus at low levels and might be difficult to detect if the wildlife reservoirs undergo mild or asymptomatic infections. When flooding occurs, the proliferation of the competent mosquito vectors results in the transmission of the virus possibly because more livestock animals are infected and have higher viraemias. This is followed by transmission from livestock to humans. It has been suggested that mosquitoes can transmit the virus to wild animals, particularly buffalo which can develop low viraemia with high survival, and possibly low abortion rates [Reference Linthicum, Davies and Kairo17]. This wildlife-mosquito cycling may involve low-level livestock infections since limited data in countries where RVFV outbreaks occur suggest that between 2·5% and 23% of livestock may have been infected by RVFV during an IEP [Reference Dohm21, Reference Swanepoel22]. Available data on the prevalence of RVFV antibodies in wildlife are limited and conflicting. A study of 281 black and white rhinos taken from Kenya, Namibia and South Africa between 1987 and 1997 found no antibodies by enzyme-linked immunosorbent assay (ELISA) against RVFV, whereas another study reported high levels of RVFV antibodies in black and white rhino, buffalo, and waterbuck taken from Zimbabwe [Reference Chevalier23, Reference Fischer-Tenhagen24]. In this study, we investigated the presence of RVFV-neutralizing antibodies in diverse species of wildlife in Kenya collected from various regions, including regions where RVFV outbreaks have been documented in the past, during the 1999–2005 IEP. We also tested a limited number of wildlife specimens collected during the recent severe outbreak of 2006–2007 [Reference Anderson and Rowe25, 26].
MATERIALS AND METHODS
Wildlife sera
A total of 1008 sera from 16 different species of Kenyan wildlife were tested for the presence of RVFV-specific antibodies. Of these, 759 serum samples were collected by the Kenya Wildlife Service (KWS) during the inter-country Rinderpest Surveillance Programme, or in the course of routine investigation of wildlife diseases or animal rescues between 1999 and 2005 (Table 1). An additional 249 were collected between January and February 2007 during the most recent RVFV outbreak involving livestock and humans. Only sera that were properly labelled with animal species, location, and year of collection were included in the study. Age was recorded for about 50% of the animals. The sera were tested at the Special Pathogens Unit of the National Institute for Communicable Diseases, Sandringham, South Africa by the virus neutralization test. Buffalo sera were also tested by indirect enzyme-linked immunosorbent assay (I-ELISA). This study was approved by the Kenya Medical Research Ethics Committee. Permission from the Convention of International Trade in Endangered Species of fauna and flora (CITES) to test sera from CITES-listed species (black rhino, African elephant, lion, and leopard) was obtained from KWS as well as an export permit from the Director of Veterinary Services.
Table 1. Location (district and province) of wildlife specimen collection

Virus neutralization test (VNT)
The VNT was conducted according to a previously described method [27] with minor modifications. Virus stocks consisted of the supernatant fluids from Vero cell cultures infected with the AR 20 368 isolate of RVFV [Reference Swanepoel28] stored in 0·5 ml volumes at −70°C. Sera were inactivated at 56°C for 30 min and serial twofold dilutions were prepared in 50 μl volumes of culture medium in flat-bottomed 96-well tissue-culture microplates (Nunc, Roskilde, Denmark). Equal volumes of the virus suspension containing a 100 TCID50 were added to each well and the serum virus mixtures incubated at 36°C for 60 min before seeding with 2×105 Vero cells per well in 100 μl Eagle's Essential Medium (Cambrex, East Rutherford, NJ, USA) containing 8% fetal calf serum (Delta Bioproducts, Johannesburg, South Africa), 100 IU penicillin, 100 μg streptomycin and 0·25 μg fungizone per ml of tissue culture medium. Four replicates per dilution were used in challenge virus titrations and the end-points were estimated by the method of Kärber [Reference Kärber29]. The sera were tested in duplicate and the titres were recorded as the reciprocals of the highest serum dilution that inhibited ⩾75% of viral cytopathic effect. A serum sample was considered positive when it had a titre of ⩾1:10.
I-ELISA for detection of RVF-specific IgG
RVF-specific IgG in buffalo were detected using a modification of an I-ELISA based on the recombinant nucleocapsid protein (rNp) of RVFV that has been reported to have high analytical accuracy for the detection of IgG antibody in experimentally infected and vaccinated sheep [Reference Paweska30]. The procedure has recently been validated for use with both human and buffalo sera [Reference Jansen van Vuren31]. The test has not been validated for other wildlife species. It involved production of rNp antigen and the assay procedure was carried out as described previously with minor modification [Reference Paweska30]. Maxisorb immunoplates (Nunc) were coated with stock antigen, diluted 1:2000 in carbonate-bicarbonate buffer (pH 9·6) and incubated overnight at 4°C. After washing three times with a wash buffer consisting of phosphate-buffered saline (PBS; pH 7·2) and 0·1% Tween-20, the plates were blocked with 200 μl of 10% fat-free milk powder in PBS and incubated in a moist chamber for 1 h at 37°C and then washed as described previously. Control and test sera were diluted 1:400 in PBS containing 2% milk powder (diluting buffer) and 100 μl was added to the plates. Each test serum was assayed in duplicate and each internal control was tested in quadruplicate. After incubation in a moist chamber for 1 h at 37°C, plates were washed three times with the washing buffer and 100 μl of a 1:5000 dilution of the horseradish peroxidase-conjugated Protein G (Zymed Laboratories Inc., San Francisco, CA, USA) was added. Plates were incubated for 1 h at 37°C, washed three times, and 100 μl of 2,2′-azino di-ethyl-benzothiazoline-sulfonic acid substrate was added to each well. Plates were then incubated in the dark at room temperature for 30 min. The reactions were stopped by the addition of 100 μl of 1% sodium dodecyl sulphate and the optical densities were measured at 405 nm. The results were expressed as percentages of the high-positive control serum. The sensitivity of the indirect rNp ELISA is 98·7% whereas the specificity is 99·4% [Reference Paweska32]. For the purpose of interpreting the I-ELISA test, the VNT test was regarded the gold standard of serological testing with perfect sensitivity and specificity.
RESULTS
Prevalence of RVFV-neutralizing antibodies
A total of 759 serum specimens from 16 different species of wildlife were collected from 19 districts across six of the eight provinces in Kenya (Table 1) during the 1999–2005 IEP. All specimens were tested for RVFV-neutralizing antibodies (Table 2). By far, the largest number of specimens tested were from African buffalo (Syncerus caffer; n=350) followed by giraffe (Giraffa leo; n=116) and zebra (Equus burchelli; n=102). Of those tested, some specimens from seven animal species were positive for RVFV-neutralizing antibodies including African buffalo, black rhino (Diceros bicornis), Thomson's gazelle (Gazella thomsonii), lesser kudu (Tragelaphus strepsiceros), impala (Aepyceros melampus), African elephant (Loxodonta africana), and waterbuck (Kobus ellipsiprymnus) (see Table 2). The six species that had ⩽2 animals with detectable neutralizing antibodies (with neutralizing titres ⩽1:10) for RVFV were lion (Panthera leo), giraffe (Giraffa camelopardalis), plains zebra (Equus burchelli), kongoni (Alcelaphus buselaphus coxii), eland (Taurotragus oryx), and warthog (Phacochoerus aethiopicus) (see Table 2). The highest prevalence of RVFV antibodies were observed in ruminant wildlife including Thomson's gazelle (87·5%), impala (62·5%), lesser kudu (50%), black rhino (32·6%), waterbuck (20%), and buffalo (15·6%). Analysis of the serum-neutralizing titres in various animal species revealed slightly higher mean serum-neutralizing titres in buffalo compared to other animal species. For instance, of the 37 buffalo positive for RVFV-neutralizing antibodies, 26 (70·2%) had titres ⩾1:80, and 13/37 (35·1%) had titres ⩾1:320 (Fig. 1). In contrast 7/14 (50%) of the positive black rhinos had titres ⩾1:80 and 2/14 (14·5%) had titres ⩾1:320; while only 3/28 (10%) of the other species had titres ⩾1:320.

Fig. 1. Number of animals (%) positive for RVFV-neutralizing antibodies in sera collected from nine species of wild animals in Kenya. Animals testing positive included buffalos (· · ·◆· · ·, n=237), black rhinos (––■––, n=43), and a variety of animals (– –▲– –, kudus, waterbucks, elephants, Thomson's gazelles, and impalas; n=120). The cut-off of 1:10 serum dilution was used to minimize non-specific serum toxicity.
Table 2. Prevalence of RVFV-neutralizing antibodies in Kenyan wildlife
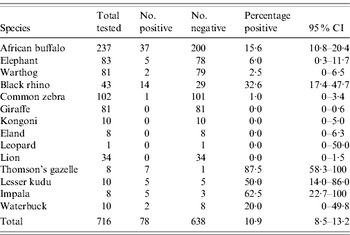
RVFV, Rift Valley fever virus; CI, confidence interval.
In addition to the VNT, 342 buffalo sera collected from various regions of the country were tested using an I-ELISA technique. The I-ELISA test results correlated with the VNT (R=0·86, Pearson's correlation coefficient), with most VNT-positive animals being also positive by I-ELISA. However, I-ELISA detected 49 out of 237 (18·5%) positive animals whereas VNT on the same specimens detected 30 (11·3%) positive animals (Table 3).
Table 3. Location (district) of buffalo tested for RVFVFootnote *
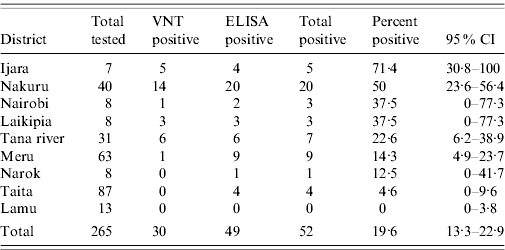
RVFV, Rift Valley fever virus VNT, Virus neutralization test; ELISA, enzyme-linked immunosorbent assay; CI, Confidence interval.
* Only results for all districts where five or more buffalos were tested are shown.
Location of RVF-positive wildlife
Location (district) of wildlife species that tested positive for RVFV (excluding giraffe, zebra, lion, kongoni, and warthog) were identified. At least five wildlife samples were tested from 13 of the 72 districts in the country (based on 2006 district demarcations), located within five of the eight provinces. Since buffalo had the largest sample size and were well represented throughout the locations sampled, the species was used for the mapping. Seven districts that had at least two positive buffalos were identified, with the highest prevalence in Ijara District in Northeastern Province and Nakuru and Laikipia Districts in Rift Valley Province (Table 3, Fig. 2). Nakuru district had 8/14 (57·1%) buffalo and 8/28 of the other wild species (28·6%) with VNT titres ⩾1:320. This was followed by Laikipia District with 6/16 (37·5%) wildlife species (including two of three buffalo) that had such high titres of RVFV-neutralizing antibodies. Interestingly, the two districts with highest prevalence in these studies were not reported to be as heavily affected in the RVFV outbreaks of 1997–1998 and 2006–2007 that involved both livestock and humans.
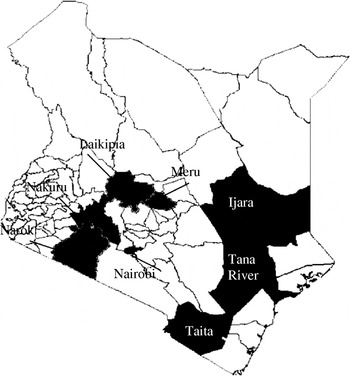
Fig. 2. Map of Kenya showing the seven districts (shaded black) where RVFV-positive buffalos were identified.
Wildlife tested for RVFV during a severe outbreak
The most recent outbreak of RVF in Kenya started in early December 2006 and ended in late March 2007 resulting in over 600 reported human cases and more than 150 deaths [Reference Anderson and Rowe25, 26]. The KWS collected 249 sera from African buffalo, giraffe, eland, warthog, waterbuck and wildebeest from six districts between January and February 2007 and tested for RVFV-neutralizing antibodies by VNT. The results are summarized in Table 4. In Garissa and Ijara Districts, 11/27 animals tested were positive including 4/18 warthogs (mean neutralizing titre 1:10), and all five gerenuk (mean neutralizing titres >1:80). Garissa and Ijara Districts were also severely affected by the RVFV outbreak of 2006–2007 reporting 176 severe human cases with 59 deaths, and 131 cases with 27 deaths, respectively. The five positive warthogs were among eight collected from a village of Shanta Abaq in the Shanta Abaq Division of Garissa District (GPS locator UTM 0051633-0053557), which was also a centre of a serious livestock and human outbreak that reported 16 severe human cases with three deaths and two cases with unknown outcome. In addition, the five positive gerenuk were collected from the village of Bour Algi in the Dadaab Division of Garissa District (GPS locator UTM 0576077-0577786), another site of a livestock and human outbreak that reported eight severe human cases with three deaths and one case of unknown outcome. In contrast, Laikipia, Nakuru, Narok, and Homa Bay Districts did not report any human cases except one traveller reported from Nakuru (Table 4). Eleven of 12 waterbucks tested from Nakuru, Narok, and Homa Bay Districts had mean neutralizing titres ⩾1:80.
Table 4. Detection of RVFV-neutralizing antibodies in wildlife during the 2006 outbreak
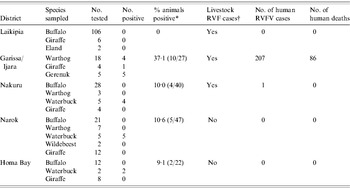
RVF, Rift Valley fever; RVFV, Rift Valley fever virus.
* Percent calculated from all species tested in the district as indicated in parentheses.
† Specimens from at least 10 livestock (cattle, sheep, or goats) were tested from each of the districts. Number of affected livestock farms could not be determined due to lack of systematic sampling.
DISCUSSION
Ever since RVFV was isolated, a central question that has not been adequately investigated has been the epidemiology of the virus during IEPs. A number of hypotheses have been advanced, including the endemicity of the virus in indigenous forests where it circulates between mosquitoes and vertebrates [Reference Paweska32]. A previous study found antibodies against RVFV in rhinos from Zimbabwe, whereas another study failed to demonstrate the viral antibodies in rhinos from Kenya, Namibia, and South Africa [Reference Chevalier23, Reference Fischer-Tenhagen24]. In this study, high prevalence (>15%) of neutralizing antibodies against RVFV were demonstrated in buffalo, lesser kudu, Thomson's gazelle, impala, black rhino, and waterbuck suggesting that these animal may be permissive to RVFV infection. In contrast a significant number of lions, zebras, giraffes, and warthogs from the same regions did not have detectable RVFV-neutralizing antibodies, indicating that they may either not be permissive for viral replication or the vector involved in viral transmission may not prefer these species for feeding. Buffalo, which provided the largest number of specimens (n=342) had both a high prevalence of anti-RVFV antibodies (16·95%) and also significantly higher titres of viral-neutralizing antibodies compared to other animal species. The presence of neutralizing antibodies in wildlife does not preclude these animal species from being reservoirs of RVFV in a sylvatic cycle.
Identifying wildlife hosts and the vectors that may be involved in a RVFV maintenance during an IEP could provide valuable information for the development of effective surveillance and potentially provide an early warning system that may warn of the potential for severe outbreaks in livestock and humans. From the results of this study, we can assume that lions, giraffes, zebras and warthogs, which had no detectable RVFV antibodies, may not support RVFV replication. Of the ruminant wildlife with RVFV-neutralizing antibodies, it is unlikely that the endangered black rhinos with their dwindling numbers in the Kenyan national parks have played a significant role in maintaining the virus in the recent years. Clearly, demonstrating RVFV antibodies in wildlife does not prove a vector–vertebrate maintenance cycle. This could instead be explained by livestock-to- wildlife transmission either during outbreaks or during IEPs since viraemia in livestock is high and sustained. However, it is important to note that some of the positive wild animals with high prevalence of viral antibodies were small ruminants born after the 1997–1998 outbreak, suggesting that there was some virus replication activity during the 1999–2006 IEP. Other data collected from livestock (sheep and goats) from Kenya born during the same IEP have shown RVFV antibodies in 4–5% of the sheep and goats (M. Rostal et al., unpublished observations). In addition RVFV antibody prevalence studies performed in Senegal and Nigeria in Africa have demonstrated between 2·4% and 23% prevalence in small ruminants [Reference Swanepoel22, Reference Swanepoel, Coetzer, Coetze and Tustin33].
It is unlikely that RVFV causes a severe disease in wildlife similar to that observed in livestock. This is because there were no reports of abortions, haemorrhagic disease, or massive deaths in wildlife in the Northeastern and Rift Valley provinces of Kenya during the 2006–2007 RVF outbreaks despite increased public education and surveillance by the KWS. The more likely scenario is that RVFV causes a mild subclinical disease in the wildlife ruminants with perhaps prolonged but low viraemia that enables transmission of the virus from wildlife to wildlife and occasionally wildlife to livestock. The vector involved in this cycle, which has not been identified, would be different from the flood-associated Aedes spp. involved in outbreaks. Entomological data indicates that mosquitoes other than flood-associated Aedes are capable of transmitting RVFV under experimental conditions [Reference Turell, Linthicum and Beaman18, Reference Zeller34, Reference Jupp35], supporting the possibility of a more prevalent mosquito being the vector for the maintenance cycle. However, this also creates a challenge in identifying the actual vector involved in the natural maintenance cycle. In this scenario, the subsequent emergence of large number of Aedes mosquitoes associated with the heavy flooding provide competent vectors capable of biting both wildlife and livestock hosts to transmit the virus to livestock and cause a noticeable outbreak.
The limited data from wildlife sampled during the 2006–2007 outbreak are inconclusive. Whereas on the one hand it shows a high prevalence (>80%) of neutralizing antibodies against RVFV in species such as gerenuk and waterbuck, on the other low levels of neutralizing antibodies were also detected in warthogs from a region that had heavy livestock and human involvement. These findings may suggest widespread and indiscriminate transmission of the virus during outbreaks (bystander effect) perhaps also involving mechanical vectors. Additional research is needed to determine the role played by various wildlife species in the transmission cycle of RVFV, including duration and level of viraemia in these species and their attractiveness to competent vector species.
ACKNOWLEDGEMENTS
We thank the many Kenya Wildlife Service staff that were involved in the collection and processing of all the wildlife specimens. We thank Heather Burke, and Evelyne Mulama at the CDC – Kenya for administrative support, and Drs Kathryn Diehl, Marguerite Pappaioanou, Srinand Sreevatsan, and Stephan Singleton from University of Minnesota for helpful advice. Funding for the project was provided by the Centers for Disease Control and Prevention and the Special Pathogens Unit of the National Institute of Communicable Diseases, South African. Melinda Rostal was funded by the Morris Animal Foundation, the Judd Foundation, Dr J. Arthur Meyers Endowment for International Experience in Public Health, College of Veterinary Medicine Travel Grant and the University of Minnesota's College of Veterinary Medicine Summer Scholars programme.
DECLARATION OF INTEREST
None.








