Vitamin D plays an important role in bone mineralisation and other metabolic processes in the human body such as Ca and phosphate homeostasis and skeletal growth( Reference Tsiaras and Weinstock 1 , Reference Haroon and Regan 2 ). Vitamin D deficiency, for example, causes rickets in children, leading to skeletal abnormalities, short stature, delayed development or failure to thrive( Reference Holick 3 ). In adults, low values of vitamin D are associated with osteomalacia, osteopenia, osteoporosis and subsequent risk of fractures( Reference Tsiaras and Weinstock 1 ). In addition to beneficial effects on musculoskeletal health, observational studies have suggested that low 25-hydroxyvitamin D (25(OH)D) values are associated with an increased risk for several extraskeletal diseases including cancer, infections, autoimmune diseases and CVD( Reference Pilz, Kienreich and Tomaschitz 4 ). In light of the global ageing population( Reference Mithal, Wahl and Bonjour 5 ), an almost fourfold increase in osteoporotic hip fractures since 1990( 6 ) and the possible risk of other chronic diseases, patterns of low 25(OH)D levels are of substantial public health interest.
Vitamin D status is traditionally measured through assays of 25(OH)D, the major circulating form of vitamin D( Reference Holick 7 ). Although 25(OH)D levels below 25 nmol/l have been associated with disorders of bone metabolism( Reference van Schoor and Lips 8 ) and are used to indicate severe vitamin D deficiency, the threshold for defining adequate stores of vitamin D in humans has not been established clearly( Reference Thacher and Clarke 9 ). The Institute of Medicine has suggested, for example, that approximately 97·5 % of the population across all age groups meet their requirements for vitamin D, having serum 25(OH)D values higher than 50 nmol/l( Reference Ross, Manson and Abrams 10 ). However, others consider 25(OH)D values of 75 nmol/l or higher to be adequate( Reference Holick 11 , Reference Holick, Binkley and Bischoff-Ferrari 12 ).
Given the absence of uniformly accepted definitions, previous reviews have reported substantial variations in the prevalence of vitamin D deficiency across countries throughout the world, with estimates ranging from 2 to 90 % depending on the cut-off value and study population selected( Reference van Schoor and Lips 8 , Reference Arabi, El Rassi and El-Hajj Fuleihan 13 – Reference McKenna 16 ). Insights from these earlier studies are limited, however, due to a focus on specific geographical regions, age or risk groups. Moreover, use of a binary approach to define the presence of vitamin D deficiency in some studies might have also obscured important relationships with chronic disease that might exist across a broader spectrum of values.
To provide a basis for future efforts to limit the health consequences of vitamin D deficiency and insufficiency worldwide, we conducted a systematic literature review of studies performed worldwide using continuous values for 25(OH)D to enable comparisons across studies and between different subgroups within the population. The specific objective of the present study, therefore, was to assess vitamin D status across a range of values at the population level and within key population subgroups defined by age, sex and region.
Methods
Literature search
We searched the Medline and EMBASE databases for original articles on vitamin D status in the general population. Keywords were chosen from the Medical Subject Headings terms and the EMTREE thesaurus, respectively, using the following search strategy: (vitamin D/D3 OR 25-hydroxyvitamin D/D3 OR 25(OH)D/D3 OR calcidiol) AND (epidemiologic studies OR population-based OR population OR survey OR representative OR cross-sectional OR observational) NOT (dihydroxycholecalciferols OR case reports OR case–control studies OR clinical trials OR reviews) AND humans. Search terms for vitamin D included the controlled term ‘vitamin D’ (including calcifediol and 25-hydroxycholecalciferol) and several free-text terms taking different notations of 25(OH)D into account.
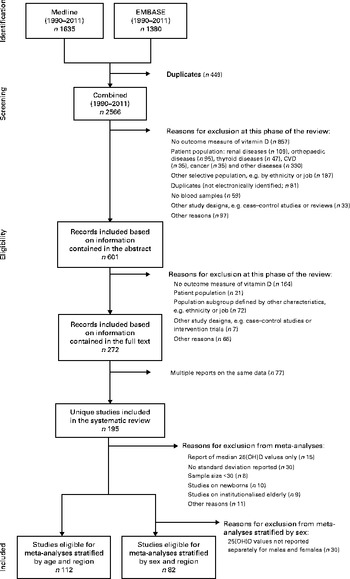
Articles published in English between 1 January 1990 and 28 February 2011 (date of the final screen) were considered. We excluded articles published before 1990 because of a general shift in lifestyle, particularly in industrialised nations (e.g. spending less time outdoors), that might have affected mean population-level 25(OH)D values( Reference Nair and Maseeh 17 ). The final screen produced 2566 hits from both databases after excluding 449 exact duplicates identified using EndNote X6 (Thomson Reuters). Wherever possible, the methods used in the present review follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement( Reference Moher, Liberati and Tetzlaff 18 ).
Study selection
Studies were included in the present review if they met the following criteria defined a priori: (1) outcomes – report of mean or median plasma level for 25(OH)D; (2) study participants – randomly selected samples from the general population as well as subgroups defined by age, sex and specific areas within a country; (3) study designs – cross-sectional studies or baseline data from population-based cohorts. Studies were excluded if vitamin D status was estimated (e.g. through self-reported nutritional intake) or if data were available only on vitamin D2. We also did not consider studies using a binary indicator for vitamin D deficiency or insufficiency as the sole outcome measure, given differing thresholds used in the literature to define either state( Reference Mithal, Wahl and Bonjour 5 ). Furthermore, clinical samples or studies restricted to subgroups with specific characteristics (e.g. ethnicity, job and skin colour) were excluded, as they were not randomly selected from the general population.
All studies were independently screened and evaluated for selection by two of the authors (R. H. and A. F.). Inter-rater agreement was good to moderate, and disagreements were discussed and resolved by consensus in each case (abstract selection: κ = 0·719; full-text selection: κ = 0·544). Following the application of exclusion criteria to information contained in the study abstract, we reduced the 2566 screened records to 601 (Fig. 1); application of these criteria following review of each full-text article reduced the pool of potentially eligible articles to 272. Given the presence of multiple reports based on the same data, our final analytical sample comprised 195 unique studies. In several instances, multiple articles from single studies were retained for analysis as they provided separate 25(OH)D values for subgroups with the characteristics of interest (age, sex and region).
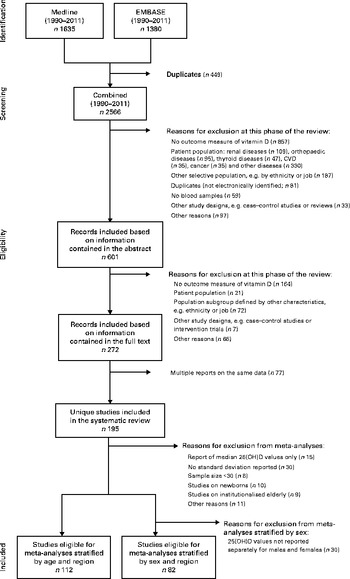
Fig. 1 Flow chart of the study selection (1990–2011). 25(OH)D, 25-Hydroxyvitamin D.
Data extraction, data elements and quality assessment
Each study was evaluated using a standardised data extraction form. In each case, we assessed a wide range of variables including vitamin D values, assays used and study characteristics as well as characteristics of the study population and method of recruitment. Data from most studies were represented in the dataset by a single entry for the total study population. Multiple subentries for a single study were included if data were presented by age, sex or region. All 25(OH)D values were expressed in nmol/l, following conversion from ng/ml (multiplied by a factor of 2·496) as necessary.
Based on the WHO recommendations, we classified geographical regions as follows: Latin America; North America; Europe; Asia/Pacific; Middle East/Africa( 19 ). To determine age-related differences, we defined four age groups: newborns/infants (0–1 years); children/adolescents (>1–17 years); adults (>17–65 years); elderly (>65 years). In instances where details about age were not provided, we created a separate category (‘other’). Where possible, we also distinguished elderly living in nursing homes (institutionalised elderly) from those living in the community.
We assessed study quality using data reported in each study on representativeness, validity and reliability. A study was considered representative if (1) this feature of the study was explicitly addressed in the corresponding full-text article or (2) any statement made by the authors suggested that the actual sample reflected the target population. A study was classified as non-representative if the corresponding full-text article contained information about an existing selection bias, which might also occur in a randomly selected sample (e.g. overestimation of females). Measurement validity was evaluated using information about the 25(OH)D measure (e.g. participation of the laboratory in the International Vitamin D Quality Assessment Scheme)( Reference Carter 20 ). Finally, a study was classified as reliable if the intra- and inter-assay coefficients of variation were below 10 and 15 %, respectively. In instances where details about representativeness, validity or reliability were not provided, we created a separate category (‘unknown’) for each quality criterion.
Statistical analyses
Descriptive statistics were calculated for baseline characteristics of all the included studies. If mean 25(OH)D values were not reported in an article, we used median values (9·2 % of the studies) in our descriptive analyses.
Meta-analyses were performed for subgroups stratified by age, sex and geographical region using random-effects models. Studies reporting median 25(OH)D values (n 15) or mean values without a corresponding standard deviation (n 30) were not included in this phase of the analyses (Fig. 1). In addition, our focus in the meta-analyses was limited to studies/subgroups with sample sizes greater than 30, given concerns about the precision of estimates. Studies on newborns (n 10) and institutionalised elderly (n 9) were also not included in the meta-analyses. For analyses stratified by sex, we also excluded studies that did not report separate 25(OH)D values for males and females (n 30).
Heterogeneity between the studies was assessed by visual inspection of forest plots and calculation of I 2 statistics. Because we found substantial heterogeneity across the studies, we decided to further explore potential explanatory factors. Therefore, we conducted heterogeneity analyses within each subgroup by accounting for a range of characteristics other than age and sex, which included season, assay type, distance from the equator( Reference Mithal, Wahl and Bonjour 5 ) and components of study quality. Studies were grouped by study characteristics (e.g. season and assay type) to assess whether heterogeneity was reduced as indicated by the I 2 statistics and the inspection of forest plots.
Supplementary analyses explored patterns of vitamin D status within specific subgroups (e.g. institutionalised elderly) and for selected associations reported in previous work. The purpose of these exploratory analyses was to support further research in this area by generating hypotheses that might be tested more thoroughly in future studies. All statistical analyses were conducted using STATA version 12.1 (StataCorp).
Results
Description of studies
Studies included in the present review (Table 1) contained data on a total of 168 389 participants from forty-four countries. The sample size of individual studies ranged from 11 to 18 462 participants with a median of 316 (interquartile range 117–861). While the majority of studies contained data on males and females, nine studies (4·7 %) restricted their focus to males, while fifty-four studies (28·0 %) contained data on only females. The overall proportions of males and females were 33·3 and 66·7 %, respectively, and the mean age of the participants was 51·7 (sd 24·3) years. Most studies were conducted in Europe (45·1 %), followed by the Asia/Pacific region (23·8 %) and North America (19·7 %). In terms of the country in which studies were conducted, most were carried out in the USA (n 28), followed by Iran (n 12), New Zealand (n 11) and Canada (n 10).
Table 1 Characteristics and main results from single studies on 25-hydroxyvitamin D (25(OH)D)*

NA, not available; O, others; A, adults; E, elderly; C, children and adolescents; I, newborns/infants.
* Data from three studies not indicating geographical region have been excluded( Reference Breen, Laing and Hall 221 – Reference Sadideen and Swaminathan 223 ); data from a single study( Reference Andersen, Molgaard and Skovgaard 40 ) providing country-specific data on four nations in Europe are represented separately. In some cases, 25(OH)D mean values were available by age, sex or region only. For some studies, multiple reports have been published, which are not listed in this table( Reference Ginde, Liu and Camargo 23 , Reference Nakamura, Nashimoto and Hori 27 , Reference Ginde, Sullivan and Mansbach 30 , Reference Boonen, Cheng and Nijs 224 – Reference Visser, Deeg and Puts 297 ).
† 25(OH)D mean values for men.
‡ 25(OH)D mean values for women.
§ 25(OH)D median values.
∥ 25(OH)D mean values for institutionalised elderly.
The assays reported to measure 25(OH)D values included RIA (55·9 %), competitive protein-binding assays (14·0 %) and other methods such as chemiluminescence immunoassay and HPLC.
In terms of study quality, more than half of the studies (50·2 %) were classified as non-representative of the target population and 14·9 % qualified as representative according to the criteria defined previously. Evidence of representativeness could not be established in 34·9 % of the studies due to missing information. Information on assay reliability was provided in 61·0 % of the studies with 52·8 % classified as providing reliable 25(OH)D measurements. Assay validity was reported in a minority of studies (9·7 %).
Global vitamin D status
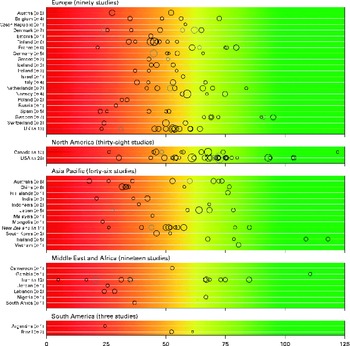
There was a significant variability in the estimates of 25(OH)D values across the studies with mean and median values ranging from 4·9 to 136·2 nmol/l and 20·7 to 91·0 nmol/l, respectively. We found that 88·1 % of the samples presented in the present review had mean 25(OH)D values below 75 nmol/l, 37·3 % had mean values below 50 nmol/l and 6·7 % had mean values below 25 nmol/l. Fig. 2 provides an overview of the distribution of country- and study-specific mean 25(OH)D values, stratified by region. In addition, a visualisation of the available data on a global map can be found elsewhere( Reference Wahl, Cooper and Ebeling 21 ).
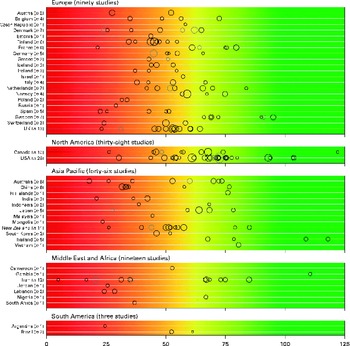
Fig. 2 Mean/median 25-hydroxyvitamin D (25(OH)D) values, by geographical region and country. Note: medians (![]() ) are shown where mean values (○) are not reported; Study size is indicated by circle size. The background colour scheme is intended to reflect the current uncertainty around the definition of thresholds for deficient, insufficient and adequate 25(OH)D levels. Mean/median values falling within the intensely red zone are most consistent with severe vitamin D deficiency; those in the green zone reflect adequate vitamin D levels. Values within the yellow zone are those thought to be indicative of insufficiency. Data from three studies not indicating geographical region have been excluded(
Reference Breen, Laing and Hall
221
–
Reference Sadideen and Swaminathan
223
); data from a single study(
Reference Andersen, Molgaard and Skovgaard
40
) providing country-specific data on four nations in Europe are represented separately. One study(
Reference Chailurkit, Rajatanavin and Teerarungsikul
195
) reported a mean 25(OH)D value of 136·2 nmol/l and therefore is not presented in the figure due to graphical reasons.
) are shown where mean values (○) are not reported; Study size is indicated by circle size. The background colour scheme is intended to reflect the current uncertainty around the definition of thresholds for deficient, insufficient and adequate 25(OH)D levels. Mean/median values falling within the intensely red zone are most consistent with severe vitamin D deficiency; those in the green zone reflect adequate vitamin D levels. Values within the yellow zone are those thought to be indicative of insufficiency. Data from three studies not indicating geographical region have been excluded(
Reference Breen, Laing and Hall
221
–
Reference Sadideen and Swaminathan
223
); data from a single study(
Reference Andersen, Molgaard and Skovgaard
40
) providing country-specific data on four nations in Europe are represented separately. One study(
Reference Chailurkit, Rajatanavin and Teerarungsikul
195
) reported a mean 25(OH)D value of 136·2 nmol/l and therefore is not presented in the figure due to graphical reasons.
Vitamin D status by age, sex and region
Due to a limited number of studies being identified from Latin America, it was not possible to perform meta-analyses for this region. Depending on the stratifying variable, I 2 values ranged from 84·5 to 99·7 %, indicating substantial heterogeneity between the studies.
No significant age- or sex-related differences in 25(OH)D values were observed in the sample of eligible studies worldwide (data not shown). However, we observed differences by region with values being significantly higher in North America than in Europe or the Middle East/Africa region (Figs. 3–6). In an analysis stratified by age and region, we did not find age-related differences for Europe and North America (Table 2). However, in the Asia/Pacific region, children/adolescents were found to have significantly lower 25(OH)D values than adults and elderly. In contrast, children/adolescents from the Middle East/Africa region had significantly higher values than the other two age groups. No significant sex-related differences were observed in any of the regions (Figs. 3–6). However, reports of 25(OH)D values in women tended to be lower, especially in the Asia/Pacific and Middle East/Africa regions.

Fig. 3 Forest plot for Europe stratified by sex. ES, effect estimator. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)

Fig. 4 Forest plot for North America stratified by sex. ES, effect estimator. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)

Fig. 5 Forest plot for the Asia/Pacific region stratified by sex. ES, effect estimator. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)

Fig. 6 Forest plot for the Middle East/Africa region stratified by sex. ES, effect estimator. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Table 2 Effect estimators (ES) from the meta-analyses stratified by age and region* (ES and 95 % confidence intervals)
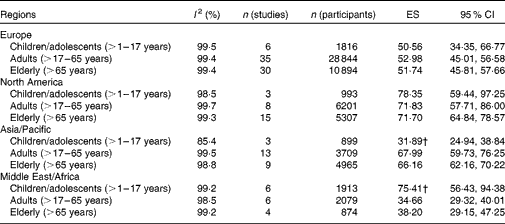
* Meta-analyses were not conducted for studies carried out in Latin America due to the limited number of eligible studies.
† Values were significantly different from those of the other age groups.
Heterogeneity analyses
The substantial heterogeneity that we observed within the different geographical regions could not be explained by the characteristics of the study population or features of study quality. Grouping studies by age category and sex, assay type, season, distance from the equator or representativeness, for example, did not significantly reduce heterogeneity across the studies in our sample, as measured by the I 2 statistics.
Exploratory analyses
We found that mean 25(OH)D values for institutionalised elderly were lower than those for non-institutionalised elderly, especially in Europe and the Asia/Pacific region. Moreover, in specific subgroups in single countries within Europe, we observed differences, with Swedish elderly having higher 25(OH)D mean values than the elderly in other European countries. In addition, we found that newborns had lower 25(OH)D values than the other three age groups in several countries worldwide.
Discussion
Summary of the main findings
The published evidence on vitamin D status at the population level, as assessed by mean or median 25(OH)D values, is characterised by a high degree of variability across studies, countries and regions. Although no age- or sex-related significant differences in 25(OH)D values were observed across the sample of studies that we reviewed, we did observe differences by region with values being significantly higher in North America than in Europe or the Middle East/Africa region. In stratified analyses, significant age-related differences were observed in the Asia/Pacific and Middle East/Africa regions, but not elsewhere. However, exploratory analyses suggested that newborns and institutionalised elderly were more likely to have lower reported 25(OH)D values in several regions worldwide. We found substantial heterogeneity between the studies in our sample from each geographical region that could not be explained in a detailed analysis.
Interpretation and comparison with previous studies
In contrast to previous reviews( Reference Mithal, Wahl and Bonjour 5 , Reference Arabi, El Rassi and El-Hajj Fuleihan 13 , Reference Hagenau, Vest and Gissel 14 ), we could not find differences in 25(OH)D values for children/adolescents, adults and elderly. However, in analyses stratified by geographical region, significant age-related differences could be observed for the Asia/Pacific region, with children/adolescents having lower 25(OH)D values than older groups. This might be primarily due to the low 25(OH)D values found for Chinese children/adolescents as reported in previous work( Reference Arabi, El Rassi and El-Hajj Fuleihan 13 ), who were observed to have low dietary Ca intake and limited sunlight exposure as possible reasons. In contrast, in the Middle East/Africa region, children/adolescents were found to have significantly higher 25(OH)D values than adults and elderly, a finding consistent with at least one previous study( Reference van Schoor and Lips 8 ). One potential explanation for this pattern in the Middle East/Africa region could be that children/adolescents from this region generally spend more time outdoors compared with the other age groups (e.g. indoor working by the adult population)( Reference Gharaibeh and Stoecker 22 ). However, others have also found age-related differences in other regions( Reference Mithal, Wahl and Bonjour 5 , Reference Arabi, El Rassi and El-Hajj Fuleihan 13 , Reference Hagenau, Vest and Gissel 14 ), which could not be confirmed in the present meta-analyses. A reduction in differences and thus greater similarities across age groups might be attributable to lifestyle changes over the course of time in which younger individuals from industrialised countries spend more time indoors watching television, using computers and playing video games compared with older adults( Reference Ginde, Liu and Camargo 23 ).
In contrast to previous reviews, we were also unable to find significant sex-related differences( Reference van Schoor and Lips 8 , Reference Arabi, El Rassi and El-Hajj Fuleihan 13 , Reference McKenna 16 ). On examining our data by region, however, we observed that females tended to have lower 25(OH)D values, especially in the Middle East/Africa and Asia/Pacific regions. Some have suggested that this finding may be related to cultural factors such as differences in clothing styles that may impede vitamin D conversion in the skin( Reference Batieha, Khader and Jaddou 24 ).
The highest mean 25(OH)D values were generally observed in North America, a finding that might be explained by the routine fortification of several foods (e.g. milk, juice and cereals) in the USA( Reference Prentice 25 ). The absence of significant differences between studies conducted in North America and those carried out in the Asia/Pacific region, however, may have been influenced by relatively high values found in Thailand, a country located near the equator with significant year-round sunlight exposure and higher daytime temperatures, resulting in the use of lighter-weight clothes, which afford less UV protection( Reference Chailurkit, Kruavit and Rajatanavin 26 ). Studies conducted in Japan and other Asian countries may have further contributed to somewhat higher regional values, resulting from diets rich in vitamin D foods such as oily fish( Reference Nakamura, Nashimoto and Hori 27 ).
Previous reviews( Reference Mithal, Wahl and Bonjour 5 , Reference van Schoor and Lips 8 , Reference Lips 15 ) have reported an apparent north–south gradient for 25(OH)D in Europe, with Scandinavian countries showing generally higher values than the Southern European countries. This finding is thought to result, in part, from population-based differences in skin pigmentation, diets rich in oily fish, the common use of cod-liver oil and a higher degree of vitamin D supplementation in Scandinavian countries( Reference Hagenau, Vest and Gissel 14 , Reference Lips 15 ). Although we did not find such a gradient in the present review, we observed generally higher 25(OH)D values in Swedish elderly than in those from other European countries. Some have suggested that this finding can be explained by the routine fortification of oil and low-fat milk products with vitamin D in Sweden( Reference Gerdhem, Ringsberg and Obrant 28 ).
In accordance with other reviews( Reference Mithal, Wahl and Bonjour 5 , Reference van Schoor and Lips 8 , Reference Lips 15 ), our exploratory analyses also suggested that institutionalised elderly in Europe and the Asia/Pacific region had lower mean 25(OH)D values than the elderly living in the community. It is possible that such a finding may result from less time spent outdoors due to poorer health status( Reference Theiler, Stahelin and Tyndall 29 ), although similar findings in other groups of institutionalised individuals could be expected elsewhere. Further investigations of the patterns of vitamin D deficiency and insufficiency are needed in this vulnerable subgroup. Another interesting finding from our exploratory analyses was that newborns/infants were reported to have lower 25(OH)D values than the members of other age groups in several countries worldwide. Because newborn vitamin D status is mainly determined by maternal vitamin D status( Reference Ginde, Sullivan and Mansbach 30 ), this finding may be explained by generally inadequate vitamin D levels in pregnant women as suggested in previous work( Reference Dror 31 ). Future research in these groups is needed to confirm these findings and test interventions aimed at interrupting this putative mechanism.
Strengths and limitations
To our knowledge, the present systematic review, conducted in accordance with the PRISMA statement( Reference Moher, Liberati and Tetzlaff 18 ), is among the first to focus on patterns of vitamin D status worldwide and in key population subgroups. We purposefully sought to identify studies with randomly selected samples from the general population to reduce sources of bias, which may otherwise obscure the public health importance of vitamin D status across the world. Use of continuous 25(OH)D values in our analyses is another important strength of the present study, given the inconsistent application of thresholds to indicate 25(OH)D deficiency, insufficiency and adequacy. A systematic search strategy based on two of the largest biomedical literature databases also reduced the probability of missing relevant articles. Besides the detailed data on 25(OH)D values among important subgroups by age, sex and region, the present review adds to the current understanding of vitamin D status in both developed and developing countries worldwide. We used the random-effects model to account for the substantial heterogeneity that we observed across the studies. Between-study heterogeneity is common in systematic reviews, especially in observational epidemiology where unobserved characteristics at both the study and individual levels affect the outcomes of interest. The random-effects model adjusts for this heterogeneity by incorporating a between-study component of variance in the weights used for calculating the summary estimate( Reference DerSimonian and Laird 32 ).
It is important to consider the findings of the present review in the context of several potential limitations. First, we cannot fully exclude publication bias as studies reporting vitamin D deficiency might have been more likely to be published than those reporting mean or median levels within the normal range. Second, language bias may have affected the results, as we limited the present review to articles written in English. This may have accounted, for example, for the relative under-representation of studies conducted in Latin America in our sample. Efforts to identify and review studies published in languages other than English are needed in the future to gain a clear understanding of the full scope of vitamin D deficiency worldwide. Third, our strict inclusion criteria (e.g. inclusion of studies with randomly selected samples) might also explain the limited number of studies identified from some regions. However, previous reviews using more liberal inclusion criteria have also identified a limited number of studies conducted in these regions( Reference van Schoor and Lips 8 , Reference McKenna 16 ). Fourth, recruitment strategies in the studies that we sampled may have focused to an extent on healthier populations, resulting in an overestimation of the prevalence of adequate vitamin D levels and a consequent minimisation of observable differences between the sexes or age-related subgroups. Fifth, we observed substantial heterogeneity between the studies in our sample that could not be explained by variables such as age, sex, season, distance from the equator, assay type or representativeness. Other unmeasured factors influencing vitamin D status (e.g. dietary intake, clothing style, time spent outdoors and use of sunscreen) may have contributed to the heterogeneity of results. Differences across the studies in study quality, adjustment for potential confounders and the definition of some characteristics or factors such as season may have contributed substantially to the heterogeneity that we observed. Finally, the precision of the estimates of vitamin D status in the subgroups of interest in the present review was probably affected by their relative under-representation in studies conducted in many regions of the world. High-quality population-based studies that assess and report all relevant data on 25(OH)D levels and central covariates including lifestyle factors to enable comparison of 25(OH)D values in the future, at least for population subgroups within the same country, have to be conducted.
Conclusion
Although we found a high degree of variability in reports of vitamin D status at the population level, more than one-third of the studies in the present systematic review reported mean 25(OH)D values below 50 nmol/l. Given the substantial heterogeneity of published evidence to date, further research on worldwide patterns of vitamin D deficiency at the population level and within key subgroups is needed to inform public health policy development to reduce risk for potential health consequences of an inadequate vitamin D status. The present review further suggests the importance of developing and implementing research designs that minimise potential sources of bias and consequently strengthen our understanding on vitamin D status in key subgroups worldwide.
Acknowledgements
We thank Elisabeth Stöcklin and Manfred Eggersdorfer from DSM Nutritional Products Limited, Judy Stenmark from the International Osteoporosis Foundation and David Litaker from the Mannheim Institute of Public Health for their intellectual input, and Bernd Genser and Marc Jarczok (Mannheim Institute of Public Health) for their statistical support.
The present study was funded by an unrestricted educational grant from DSM Nutritional Products Limited, a bulk supplier of vitamins. A. F., F. R. and P. W. are employed by DSM Nutritional Products Limited.
The authors' contributions were as follows: J. H., P. W. and K. H. defined the scope of the project, wrote the paper and had primary responsibility for the final content; A. F. and R. H. performed the literature search; A. F., J. H. and T. R. extracted the data; J. H. conducted the statistical analyses; F. R. was responsible for the visual presentation of the data; D. A. W. contributed to the study conception and design; D. D. P. carefully revised the content of the manuscript. All authors contributed to the interpretation of data and read and approved the final manuscript.
All authors declare that they have no conflicts of interest.