The porphyrias are a group of metabolic disorders of haem biosynthesis that result in the accumulation of porphyrins or their precursors(Reference Nordmann and Puy1, Reference Badminton and Elder2). Although people affected by porphyria can remain asymptomatic for a long period of time or even for a lifetime, there are some agents that can precipitate acute attacks and the appearance of clinical symptoms(Reference Gordon3). Variegate porphyria (VP), an autosomal dominant type of hepatic porphyria, is the result of decreased protoporphyrinogen oxidase (PPOX) activity, the penultimate enzyme of haem biosynthesis. It is characterised clinically by skin lesions and acute attacks that can occur separately or together(Reference Nordmann and Puy1). The chronic accumulation of haem precursors in erythrocytes, liver and other cell types can induce cellular damage due to their ability to produce free radicals and to activate oxygen, inducing oxidative stress(Reference Monteiro, Abdalla and Faljoni-Alario4–Reference Thunell, Andersson and Carlmark7).
Antioxidant defenses and oxidative stress have been studied in some types of porphyria, but not in VP. Decreased plasma antioxidant vitamins' levels and increased oxidative damage markers have been described in porphyria cutanea tarda patients(Reference Rocchi, Stella and Cassanelli8, Reference Rocchi, Casalgrandi and Masini9). In contrast, no differences have been found in the levels of antioxidant vitamins or oxidative damage markers in acute intermittent porphyria patients(Reference Rocchi, Ventura and Ronzoni10). Therefore, it is necessary to describe the levels of antioxidant defenses and oxidative damage markers in VP patients in order to characterise this porphyria as a pro-oxidative disease. The determination of these parameters in plasma is a good marker of the systemic situation, but studying concrete cell types should be useful to further understand the impact of the disease in the normal function of cells. Neutrophils are phagocytic leukocytes normally found in the blood stream, but they can migrate to tissues following inflammation signals. In this instance, in situations of inflammation, blood neutrophil counts are increased. When neutrophils are activated in response to immune stimulation they get primed to the oxidative burst, in which large amounts of reactive oxygen species (ROS) are produced. The toxicity of ROS produced by neutrophils could damage the neutrophil itself and adjacent tissues contributing to the oxidative stress situation(Reference Miller and Britigan11, Reference Peake and Suzuki12). In order to cope with this high production of ROS, neutrophils contain antioxidant enzymes, such as superoxide dismutases (SOD), catalase (CAT) and glutathione peroxidase, which are known to be regulated by inflammatory cytokines, oxygen tension and ROS in human neutrophils(Reference Pietarinen-Runtti, Lakari and Raivio13). We have previously described that exercise induces an acute phase immune response in neutrophils, by increasing their number in circulation, getting them primed to the oxidative burst and decreasing their antioxidant enzyme activities, possibly due to enzyme release into plasma(Reference Sureda, Ferrer and Tauler14). In addition to the possible oxidative stress induced by the accumulation of haem precursors in the neutrophil, the lower PPOX activity in VP patients could compromise haem biosynthesis per se and the level and function of haem proteins.
It has been described that nutritional interventions with vitamin E and other antioxidants such as vitamin C or melatonin decrease the urinary levels of porphyrins or their precursors in patients affected by different types of porphyria(Reference Kirshenbaum and Singal15–Reference Qi, Reiter and Tan19). However, other treatments with antioxidants have failed to obtain beneficial results(Reference Percy, Naidoo and Joubert20, Reference Thunell, Andersson and Harper21). The failure to note a clinical response to antioxidant therapy may be due to factors dependent upon dosage of, or interaction between, the antioxidant compounds given, or on restricted bioavailability of the antioxidants at critical anatomical sites, and does not invalidate the model of acute porphyria as a hyperoxidative condition per se (Reference Thunell, Andersson and Harper21). Either way, dietary supplementation with antioxidant nutrients has been shown to decrease the levels of oxidative damage markers in lipids, proteins and DNA and to activate antioxidant enzymes after physical activity(Reference Rokitzki, Logemann and Sagredos22–Reference Sumida, Doi and Sakurai25). We have previously described that the prolonged consumption of high doses of vitamins C and E induces an increase in the activity of antioxidant enzymes such as SOD and CAT in neutrophils of healthy sportsmen(Reference Tauler, Aguiló and Fuentespina26). The effect of this kind of nutritional intervention in the antioxidant defenses and oxidative damage markers of VP patients is unknown. Therefore, we hypothesised that women affected by VP would present evidence of a systemic situation of oxidative stress and inflammation, and that neutrophils from VP patients would be in an activated state. Dietary supplementation with antioxidant nutrients such as vitamins E and C should lead to ameliorate the antioxidant status of women affected by VP by decreasing oxidative damage and/or enhancing the endogenous antioxidant defenses. Our aim was to analyse the influence of VP on the antioxidant defenses and markers of oxidative damage and inflammation in plasma and neutrophils from VP patients, and the effects of a dietary supplementation with vitamins E and C on these parameters.
Experimental methods
Subjects and study design
The study was performed with twelve women affected by VP and twelve pair-matched healthy control women. All the subjects were recruited through the Balearic Association of Porphyria, which holds a register of all the porphyric patients in the Balearic Islands. The subjects included in the VP group had been previously diagnosed as porphyric by specialised doctors on the basis of different parameters such as plasma fluorescence peak at 626 nm, levels of excreted urinary and faecal porphyrins and clinical manifestations such as abdominal pain during porphyric attacks(Reference Ferrer, Tauler and Sureda27). The diagnosis was additionally confirmed by the determination of PPOX levels in erythrocytes(Reference Ferrer, Tauler and Sureda27). The control women were also recruited through the Balearic Association of Porphyria and they were pair matched in age with the women included in the porphyric group. All the subjects participating in the study had no additional diagnosed pathologies, except for VP in the porphyric group. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Ethical Committee of Clinical Investigation of the Balearic Islands on 30 March 2004 (ID: 306/04). Written informed consent was obtained from all subjects/patients.
The subjects participated in a double-blind crossover study. Each participant drank, for 6 months, 500 ml/d of an almond-based beverage enriched with vitamin E (10 mg/100 ml) and vitamin C (30 mg/100 ml) or an identical beverage, but not enriched with antioxidant vitamins. The almond beverage drink was a carbohydrate-electrolyte solution with almond and orange juice in its recipe. It contained 34·7272 kJ (8·3 kcal)/100 ml, 203 kJ/100 ml, 1·9 % lipids, 6·8 % total sugars, 1·0 % proteins, 1·8 mg/100 ml Ca, 4·2 mg/100 ml Mg, 12·8 mg/100 ml Na, 33·7 mg/100 ml K, 69·7 mg/100 ml Fe and 44·5 mg/100 ml Zn. Non-enriched almond beverage just contained a trace of vitamins E and C due to the loss of antioxidant vitamins during the manufacture. The enriched beverage contained 10 mg/100 ml of α-tocopherol acetate and 30 mg/100 ml of ascorbate. The almond-based beverage was manufactured by Liquats Vegetals S.L. (Viladrau, Gerona, Spain). Three months after finishing the first supplementation period, the groups were crossed over and another supplementation was performed for 6 months. Therefore, each subject was supplemented with both placebo and supplemented beverages. Blood samples were obtained before the beginning and at the end of every supplementation period.
Venous blood samples were obtained from the antecubital vein of control and porphyric women in resting conditions after overnight fasting. Plasma, neutrophil and erythrocyte fractions were purified from whole blood following an adaptation of the method described by Boyum(Reference Boyum28, Reference Ferrer, Sureda and Batle29).
Anthropometric data and dietary intake
Different anthropometric indices were calculated: BMI (mass (kg)/squared height (m)); waist–hip index (waist perimeter (cm)/hip perimeter (cm)). Height was determined using a mobile anthropometer (KaWe 44 444; Asperg, Germany) to the nearest millimetre, with the participant's head in the Frankfurt plane. Body mass was determined to the nearest 100 g using a digital scale (Tefal, sc 9210, Rumilly, France). Participants were weighed barefoot while wearing light underwear, which was accounted for by subtracting 300 g from the measured weight. Waist and hip perimeters were measured to the nearest 0·1 cm with the participant's right arm relaxed, using a non-stretchable measuring tape (KaWe 43 972; Asperg, Germany).
Dietary habits were assessed using a 3 d dietary record questionnaire completed at the beginning of the study. A qualified dietitian verified and quantified the food records. All food items consumed were transformed into nutrients using a special computerised program based on the European and Spanish food composition tables(Reference Tauler, Ferrer and Romaguera30).
Serum clinical analysis
γ-Glutamyl transpeptidase, lactate dehydrogenase, creatine kinase, C-reactive protein (CRP), uric acid and bilirubin were determined by standard procedures using commercial clinical kits in an autoanalyser system (Technicon DAX System, Tarrytown, NY, USA). Transferrin was measured by immunoprecipitation using the ILAB 600 (Clinical Chemistry System, Izasa, Barcelona, Spain).
Enzymatic determinations
All activities were determined in neutrophils and erythrocytes with a Shimadzu UV-2100 spectrophotometer at 37°C. CAT activity was measured by the spectrophotometric method of Aebi(Reference Aebi31) based on the decomposition of H2O2. Glutathione peroxidase activity was measured using an adaptation of the spectrophotometric method of Flohé & Gunzler(Reference Flohé and Gunzler32) using H2O2 as substrate. Glutathione reductase (GR) activity was measured by a modification of the Goldberg & Spooner spectrophotometric method(Reference Goldberg, Spooner and Bergmeyer33). SOD activity was measured by an adaptation of the method of McCord & Fridovich(Reference McCord and Fridovich34).The xanthine/xanthine oxidase system was used to generate the superoxide anion. This anion produced the reduction in cytochrome c, which was monitored at 550 nm. The SOD in the sample removed the superoxide anion and produced an inhibition of the cytochrome c reduction.
Vitamin determinations
Vitamin E was determined in plasma and neutrophils. The extraction of liposoluble vitamins was carried out using n-hexane after deproteinisation with ethanol containing 0·2 % butylated hydroxytoluene. Vitamin E was determined by HPLC in the n-hexane extract after drying under a nitrogen current and redissolving in ethanol. The mobile phase consisted of 550:370:80 acetonitrile–tetrahydrofuran–H2O. The HPLC was a Shimadzu with a diode array detector and the column was a Nova Pak, C18, 3·9 × 150 mm. α-tocopherol was determined at 290 nm.
Ascorbate was determined in plasma and neutrophils by an HPLC method with electrochemical detection(Reference Tsao and Salimi35) after deproteinisation with meta-phosphoric acid. The mobile phase consisted of 0·05 m sodium phosphate, 0·05 m sodium acetate, 189 μm dodecyltrimethylammonium chloride and 36·6 μm tetraoctylammonium bromide in 25:75 methanol–water, pH 4·8. The HPLC system was a Shimadzu with a Waters, Inc. electrochemical detector and a Nova Pak, C18, 3·9 × 300 mm column. The potential of the chromatographic detector was set at 0·7 V v. an Ag/AgCl reference electrode.
Malondialdehyde determination
Malondialdehyde (MDA) as a marker of lipid peroxidation was analysed in plasma and neutrophils by a colorimetric assay kit (Calbiochem, San Diego, CA, USA). The method used is specific for MDA determination.
Protein carbonyl derivate determination
Protein carbonyl derivates were measured in plasma and neutrophils by an adaptation of the method of Levine et al. (Reference Levine, Williams and Stadtman36) using the precipitates of deproteinised samples. Precipitates were resuspended with 2,4-dinitrophenylhydrazine 10 mm, and incubated for 60 min at 37°C. Then samples were precipitated with 20 % TCA, and centrifuged for 10 min at 1000 g at 4°C. The precipitate was washed twice with ethanol–ethyl acetate (1:1) to remove free 2,4-dinitrophenylhydrazine. About 6 m guanidine in 2 mm phosphate buffer, pH 2·3, was added to the precipitate, and samples were incubated for 40 min at 37°C. Finally, samples were centrifuged for 5 min at 3000 g at 4°C to clarify the supernatant and absorbance was measured at 360 nm. The molar absorption of 22 000 M− 1 cm− 1 was used to quantify the protein carbonyl levels. Samples were analysed against a blank of guanidine solution.
Chemiluminescence assay
ROS production was measured in neutrophils. Opsonised zymosan was used as a neutrophil stimulant. Zymosan A (SigmaSt. Louis, MO, USA) was suspended in Hank's buffered salt solution at a concentration of 1 mg/ml and incubated with 10 % human serum at 37°C for 30 min to opsonise the zymosan, followed by centrifugation at 750 g for 10 min at 4°C. The precipitate was washed twice in Hank's balanced salt solution and finally resuspended in Hank's balanced salt solution at 1 mg/ml.
Chemiluminescence assay was performed by an adaptation of the method by Edwards(Reference Edwards37). Luminol is a lumigenic probe, which can be oxidised by H2O2 and HOCl. Thus, in activated neutrophils, luminol chemiluminescence measures the combined activities of the NADPH oxidase plus myeloperoxidase. Opsonised zymosan suspension (100 μl) was added to a ninety-six-well microplate containing 50 μl neutrophil suspension and 50 μl luminol solution (2 mm in PBS, pH 7·4). Chemiluminescence was measured at 37°C for 90 min in FLx800 Microplate Fluorescence Reader (Bio-tek Instruments, Inc., Winooski, VT, USA). Each sample was determined in duplicate.
Statistical analysis
Statistical analysis was carried out using a statistical package for social sciences (SPSS 13.0 for Windows (SPSS Institute, Chicago, IL, USA)). Results are expressed as means with their standard errors and P < 0·05 was considered statistically significant. The statistical significance of the data was assessed by Student's t test for unpaired data. To test the effects of supplementation and disease, a two-way ANOVA was performed. The statistical factors analysed were (D) disease and (S) supplementation. When significant effects were found, one-way ANOVA was used to determine the differences between the groups involved.
Results
No differences were observed between control and VP women concerning age (51·7 (sem 2·9) and 57·7 (sem 2·7) years, respectively) and anthropometric parameters such as BMI (28·8 (sem 2·1) and 27·2 (sem 0·4) kg/m2, respectively) or waist–hip index (0·82 (sem 0·02) and 0·76 (sem 0·03), respectively). The composition of the habitual diet did not differ either between control and VP women in the basis of total energy consumed (8179·72 (sem 435·136) kJ (1955 (sem 104) kcal) and 8598·12 (sem 573·208) kJ (2055 (sem 137) kcal), respectively), energy from proteins (18·2 (sem 4·4) and 20·2 (sem 3·1) %, respectively), energy from carbohydrates (42·3 (sem 3·4) and 43·5 (sem 2·4) %, respectively), energy from fats (42·8 (sem 3·7) and 38·7 (sem 2·5) %, respectively), vitamin C intake (127 (sem 28) and 99·1 (sem 11·8) mg, respectively) or vitamin E intake (11·3 (sem 1·3) and 11·5 (sem 1·3) mg, respectively). The dietary supplementation raised the antioxidant vitamin intake to about 60 mg vitamin E and 250 mg vitamin C.
The porphyria disease did not affect the circulating activities of the enzymes γ-glutamyl transpeptidase, lactate dehydrogenase or creatine kinase, shown in Table 1. The levels of the inflammation marker CRP were, however, increased in women affected by VP when compared with controls. Porphyric patients presented higher MDA levels, while carbonylated proteins were maintained in porphyric patients. The higher levels of oxidative damage in plasma of VP patients could be a consequence of decreased circulating antioxidant defenses in addition to the likely increased production of free radicals as a consequence of the disease. However, the plasma levels of uric acid, bilirubin, transferrin, ascorbate and α-tocopherol did not differ between VP and control women (data not shown). Neutrophils produce increased levels of ROS when activated, so their activity can contribute to oxidative stress in the whole organism. We then studied whether the porphyric condition affected the oxidative status of neutrophils. Antioxidant defenses and oxidative damage markers in neutrophils are shown in Table 2. VP significantly reduced the activity of the antioxidant enzymes CAT and GR in neutrophils, while glutathione peroxidase and SOD activities were maintained. Intracellular levels of antioxidant vitamins ascorbate and α-tocopherol in neutrophils were not affected by the porphyric condition. Women affected by VP showed increased protein oxidative damage levels in neutrophils, as shown by the increased carbonylated proteins. Neutrophil priming to the oxidative burst was analysed by ROS production after neutrophil stimulation. As shown in Table 2, neutrophils from VP patients were more primed to the oxidative burst, as shown by the greater ROS production after neutrophil stimulation with zymosan.
Table 1 Effects of variegate porphyria on serum and plasma damage markers
(Mean values with their standard errors)
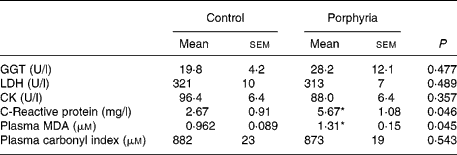
GGT, γ-glutamyl transpeptidase; LDH, lactate dehydrogenase; CK, creatine kinase; MDA, malondialdehyde.
Statistical analysis: Student's t test for unpaired data.
* Mean values were significantly different between porphyria and control groups (P < 0·05).
Table 2 Effects of variegate porphyria on neutrophil antioxidant defenses and oxidative damage
(Mean values with their standard errors)

GPx, glutathione peroxidase; nkat, nanokatal; pkat, picokatal; GR, glutathione reductase; SOD, superoxide dismutases; MDA, malondialdehyde; ROS, reactive oxygen species; RLU, relative luminescence units.
Statistical analysis: Student's t test for unpaired data.
* Mean values were significantly different between porphyria and control groups (P < 0·05).
With the evidence that VP induces a situation of oxidative damage in neutrophils and plasma, we tested the effects of a functional beverage enriched with vitamins C and E on the antioxidant status of porphyric and control subjects. The effects of this nutritional intervention on the antioxidant defenses and the appearance of oxidative damage in plasma and neutrophils are shown in Table 3. Supplementation with the beverage enriched with vitamins C and E for 6 months significantly attenuated the values of plasma markers for lipid oxidative damage, as the plasma MDA decreased about 30 % both in the control and in the porphyric women. However, carbonylated proteins were not affected by the supplementation. In neutrophils, an effect of supplementation on the cellular levels of α-tocopherol was observed. Subjects consuming the antioxidant-enriched beverage presented higher α-tocopherol levels in neutrophils when compared with subjects consuming the placebo beverage. Ascorbate, MDA and carbonylated protein levels were not affected in neutrophils by supplementation or by disease after the nutritional intervention. The antioxidant enzyme activities in neutrophils were not affected by supplementation (Table 4). The differences between porphyric and control subjects observed in basal conditions concerning CAT and GR activities were not evidenced after the supplementation period. Table 4 also shows the activities of antioxidant enzymes in erythrocytes after the dietary intervention with antioxidant vitamins. An effect of supplementation was observed on CAT and GR activities. Subjects consuming the beverage enriched with vitamins C and E presented higher CAT and GR activities in erythrocytes. The difference between the non-enriched and the enriched groups were significant only in the control group, while in the porphyric groups, no significant differences were evidenced.
Table 3 Effects of dietary supplementation with vitamins E and C on plasma and neutrophil antioxidant defenses and oxidative damage markers of variegate porphyria (VP) patients
(Mean values with their standard errors)
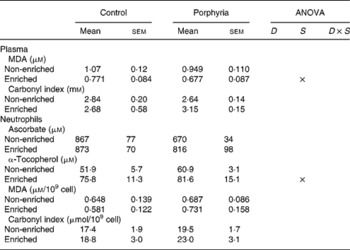
D, significant effect of disease; S, significant effect of supplementation; D × S, significant interaction between the two ANOVA factors; MDA, malondialdehyde.
Twelve women affected by VP and twelve pair-matched healthy control women participated in a double-blind crossover study. Each participant consumed an almond-based beverage (non-enriched) or the same beverage containing 10 mg/100 ml of vitamin E and 30 mg/100 ml of vitamin C (enriched). Statistical analysis: two-way ANOVA.
Table 4 Effects of dietary supplementation with vitamins E and C on neutrophil and erythrocyte antioxidant enzyme activities of variegate porphyria (VP) patients
(Mean values with their standard errors)

D, significant effect of disease; S, significant effect of supplementation; D × S, significant interaction between the two ANOVA factors; GPx, glutathione peroxidase; GR, glutathione reductase; SOD, superoxide dismutases.
Twelve women affected by VP and twelve pair-matched healthy control women participated in a double-blind crossover study. Each participant consumed an almond-based beverage (non-enriched) or the same beverage containing 10 mg/100 ml of vitamin E and 30 mg/100 ml of vitamin C (enriched). Statistical analysis: two-way ANOVA.
† Mean values were significantly different significant differences between non-enriched and enriched beverage groups (P < 0·05).
Discussion
VP is the result of decreased PPOX activity, the penultimate enzyme of haem biosynthesis. This disease is characterised by an accumulation of some haem precursors in liver and erythrocytes and by a low rate of haem biosynthesis as a result of the genetic error in PPOX(Reference Nordmann and Puy1). It has been shown that the accumulation of the haem precursors constitutes an endogenous source of ROS, triggering oxidative damage to cellular components(Reference Monteiro, Abdalla and Faljoni-Alario4, Reference Pereira, Curi and Kokubun6). We studied different damage markers in plasma, which are good indices of cellular damage and global oxidative stress. γ-Glutamyl transpeptidase, lactate dehydrogenase and creatine kinase activities are markers of whole-body stress(Reference Lee, Blomhoff and Jacobs38) and of muscle inflammation and damage(Reference Mastaloudis, Traber and Carstensen39). Neither of these markers was affected by VP. However, CRP circulating levels were higher in VP patients than in control subjects. CRP is a key inflammatory factor produced by the liver in response to inflammation and its concentration in plasma can increase several fold, so CRP levels are widely used as a marker of chronic inflammation(Reference Puglisi and Fernandez40). The enhanced CRP levels in VP patients may be an indicator of a situation of greater systemic inflammation in these subjects when compared with healthy subjects. The presence of greater levels of a lipid oxidative damage marker such as MDA in plasma also evidences a situation of plasma oxidative stress in women affected by VP. This condition of oxidative stress could be a consequence of reduced antioxidant defenses in plasma or a greater production of ROS. However, none of the antioxidant substances measured in plasma showed differences between porphyric and control subjects. However, the increased MDA levels could be an indicator of a situation of oxidative stress in plasma, which could be a direct consequence of the increased haem intermediates(Reference Monteiro, Abdalla and Augusto5, Reference Pereira, Curi and Kokubun6) or the slightly higher situation of chronic inflammation shown in the porphyric subjects.
To further corroborate that women affected by VP present an oxidative stress condition, we studied antioxidant defenses and oxidative damage markers in neutrophils. The increased capabilities to produce ROS in neutrophils are related with the pathogenesis of some degenerative diseases such as Alzheimer and Parkinson(Reference Gatto, Riobo and Carreras41, Reference Vitte, Michel and Bongrand42). Women affected by VP present lower neutrophil activities of both CAT and GR, together with higher ROS production after neutrophil stimulation with opsonised zymosan than control women. Together with the increased values for CRP levels, these results reinforce the situation of chronic inflammation present in VP patients. Neutrophils are cells that respond to stimulation by altering their antioxidant enzyme activities. Neutrophils adapt to oxidative stress (i.e. induced by exercise) by reducing antioxidant enzyme activities(Reference Sureda, Ferrer and Tauler14, Reference Ferrer, Tauler and Sureda43). After intense exercise, neutrophils respond in an acute phase-like immune response, by increasing their number in circulation, decreasing their antioxidant enzyme activities and getting primed to the oxidative burst(Reference Sureda, Ferrer and Tauler14). In these subjects, neutrophils are in a more activated state with decreased antioxidant enzyme activities and increased susceptibility to activate the oxidative burst. Furthermore, we found evidence of protein oxidative damage in neutrophils of VP women. δ-aminolevulinic acid has been shown to directly oxidise proteins(Reference Princ, Juknat and Amitrano44, Reference Rocha, Dutra and Bandy45); thus, the increased levels of oxidised groups in the proteins from VP patients could be a direct effect of haem precursors. The decreased CAT activity shown by women affected by VP can therefore be attributed to three different mechanisms: (1) as CAT has haem as a prosthetic group, the limitation in haem synthesis attributed to the genetic defect in PPOX could lead to limitations in the synthesis of active CAT, thus reducing total enzyme activity; (2) the increased levels of δ-aminolevulinic acid or other ROS, as a consequence of the accumulation of haem precursors in VP patients, could directly oxidise CAT protein causing oxidative damage and reducing enzyme activity; or (3) the activation state of neutrophils as a consequence of the disease-induced CAT release into plasma as pointed out previously(Reference Sureda, Ferrer and Tauler14). Proposals (2) and (3) can also be applied to the observed decrease in GR.
Once evidenced that women affected by VP show greater oxidative stress than control healthy women, we tested the effects of dietary supplementation with an almond-based beverage enriched with vitamins C and E. The oxidative stress situation associated to VP is ameliorated after the consumption of the antioxidant-enriched almond-based beverage. Dietary supplementation with the enriched drink induced lower levels of plasma MDA. The intracellular neutrophil levels of α-tocopherol were increased in the supplemented groups, although no effects of supplementation were observed on oxidative damage markers or antioxidant enzyme activities in neutrophils. The differences in antioxidant enzyme activities and carbonylated proteins between porphyric and control subjects described in basal conditions were not evidenced after the supplementation period in the placebo or in the supplemented groups, thus indicating that the almond beverage used as the vehicle for supplementation could have had beneficial effects on these parameters. There are some antioxidant compounds in the almonds, such as phytic acid, phenolic compounds and others, which could contribute to the overall antioxidant effects of the almond-based beverage(Reference Venkatachalam and Sathe46). However, the lack of a negative control group without the beverage intake makes it difficult to conclude.
Dietary supplementation with vitamins C and E also induced the activity of CAT and GR in erythrocytes. As erythrocytes cannot express genes or synthesise new proteins, these results indicate that changes in GR and SOD activities can be attributed to direct effects on the protein. GR and SOD can be directly activated by transcriptional events(Reference Aguilo, Tauler and Fuentespina47, Reference Tauler, Aguilo and Guix48). In addition, we have previously evidenced in an in vitro experiment that GR activity increases when measured in the presence of CAT(Reference Tauler, Aguilo and Guix48). It is therefore evident that the activity of antioxidant enzymes can be directly modified by the levels of ROS. The higher amounts of antioxidants could protect the catalytic site or the stability of GR and CAT. Furthermore, a synergistic effect between CAT and GR could be involved in the activation of these enzymes.
The main findings of the present study are that women affected by VP present a chronic situation of inflammation and plasma oxidative damage. This situation of inflammation is reinforced with the presence of more activated neutrophils in VP women, with decreased CAT and GR activities and higher ROS production and protein oxidative damage. Dietary supplementation with vitamin E (50 mg/d) and vitamin C (150 mg/d) for 6 months decreases plasma oxidative damage and enhances the erythrocyte activities of CAT and GR.
Acknowledgements
The present work was supported by a grant from the Spanish Ministry of Science and Education (DPS2008-07033-C03-03 and AGL2007-62 806/ALI) and FEDER funds. M. D. F. was funded by a grant from the Spanish Ministry of Science and Education. The authors state that there are no conflicts of interest. All authors have contributed to experimental design and collection and analysis of data. We are grateful to Ms Magdalena Ordinas, Asociación Balear de Porfíria, who collaborated in the family studies.






