In the general population, considerable epidemiological evidences have proven that dietary fibre is related to the decreased risk of various chronic diseases such as CVD, type 2 diabetes, hypertension, major cancer(Reference Bradbury, Appleby and Key1) and chronic kidney disease (CKD)(Reference Streppel, Arends and Veer2–Reference Cho, Qi and Fahey6). A higher fibre intake is also independently associated with all-cause and CVD mortality in the general population, and among patients with CVD, diabetes mellitus (DM), and cancer, as shown from prospective cohort studies and meta-analysis(Reference Tanaka, Yoshimura and Kamada7–Reference Yao, Fang and Xu9).
For patients with CKD, the predictive role of dietary fibre in mortality has been reported from the Uppsala Longitudinal Study of Adult Men (ULSAM)(Reference Xu, Huang and Riserus10) and National Health and Nutrition Examination Survey III (NHANES III) cohorts(Reference Krishnamurthy, Wei and Baird11). There are still limited data on the relationship between dietary fibre and mortality in dialysis population, in spite of the recent observational studies indicating that fibre supplementation could improve the lipid profile and oxidative status and decrease the systemic inflammatory state in patients on dialysis(Reference Sutton, Ovington and Engel12, Reference Xie, Ge and Huang13). The current guidelines also make little or no reference to dietary fibre intake for patients with CKD due to the lack of evidence(Reference Toigo, Aparicio and Attman14–Reference Wheeler and Kasiske16). Recently, a meta-analysis in CKD assessed the benefits of whole dietary pattern in the decreased risk of mortality rather than the nutrient components of the diet(Reference Kelly, Palmer and Wai17). The so-called healthy dietary pattern, enriched with fruit and vegetables, fish, legumes, cereals, whole grains and fibre, cannot recommend the target for a specific nutrient to meet patients’ needs.
Therefore, in the present study, we aimed to evaluate the association between dietary fibre intake measured at base-line or in a time-averaged manner and the all-cause and CVD mortality through a prospective peritoneal dialysis (PD) cohort. The prognostic value of dietary fibre would also be explored among patients categorised as CVD or non-CVD, DM or non-DM.
Subjects and methods
Subjects and follow-up
This is a prospective cohort with data retrospectively analysed, and the present study was carried out at the PD centre of Peking University First Hospital. Between 1 October 2002 and 31 August 2014, all incident PD patients were screened. Patients were excluded if they refused to complete the baseline test, denied the diagnosis of end-stage renal disease or could not be regularly followed. All patients were followed until death, transfer to haemodialysis (HD), renal transplantation, loss to follow-up or 30 June 2018 (the end of study). All patients were treated with continuous ambulatory PD and visited a physician at least once every 3 months. All patients began the PD programme within 1 month after catheter implantation and were given lactate-buffered glucose dialysate with a twin-bag connection system (Baxter Healthcare). The present study was approved by the Medical Ethics Committee of Peking University. Written informed consent was obtained from each patient.
Data collection
Demographic and clinical data including age, sex, BMI, the presence of CVD and DM were collected within the week preceding PD catheter implantation. Baseline values included all measurements of blood pressure, biochemistry, dialysis adequacy, dietary and nutrition parameters in the first 3 months. Baseline values of dietary nutrients were calculated in the first 6 months. All the above measurements during the study period were prospectively collected and averaged for each 6-month interval to calculate the time-averaged values.
Dietary variables
During the follow-up, patients completed 3-d dietary records before they visited the dietician. A dedicated dietician checked the diary using food models. The dietary records would be invalid if they were recorded in less than 3 d or did not get checked successfully by the dietitian. Food models were used to estimate the actual amount of foods recorded in the diet diary. Daily fibre, protein, energy, carbohydrate and fat were calculated using a computer software program (PD information Management System, Peritoneal Dialysis Center, Peking University). Oral nutrition supplements including nutritionally complete food product such as Ensure and nutritionally incomplete food product such as protein powder were also recorded to calculate the total amount of protein and energy intake. The daily energy intake includes intake from dietary and dialysate sources.
Biochemical, dialysis adequacy and nutrition variables
Biochemistry data including Hb, serum albumin (Alb), lipid spectrum, glucose, uric, urea, creatinine, Ca, phosphate, intact parathyroid hormone (iPTH) and so on were examined using an automatic Hitachi chemistry analyzer (Hitachi Chemical). Serum high-sensitive C-reactive protein (hs-CRP) was measured by immune rate nephelometric analysis. Dialysis adequacy, residual renal function (RRF) and glucose absorption were measured by collecting 24-h urine and dialysate. Dialysis adequacy was defined as total urea clearance and total creatinine clearance. Residual renal function was estimated using the average renal clearance of urea and creatinine. Glucose absorption via dialysate was calculated by subtracting glucose amounts in drained dialysate from that in instilled dialysate, expressed as g glucose/d and then the dialysate energy absorption was calculated as kJ of energy/d.
Definition of outcome event
The outcomes were defined as cardiovascular and all-cause death. Cardiovascular death is caused due to myocardial infarction, congestive heart failure, cerebral bleeding, cerebral infarction, arrhythmia and peripheral arterial disease(Reference Smith, Jackson and Pearson18).
In all analyses, we censored follow-up at transferring to HD, renal transplantation, loss to follow-up or the end of the study (30 June 2018).
Statistical analysis
The mortality rate in PD cohorts over an average follow-up of 45 months was estimated as 40 % based on our previous research(Reference Dong, Li and Xu19, Reference Dong, Li and Yang20). The standard deviation of fibre intake in HD patients was 5 g/d(Reference Fassett, Robertson and Geraghty21, Reference Kalantar-Zadeh, Kopple and Deepak22) and the expected hazard radio of fibre intake in PD patients was estimated to be 0·95(Reference Krishnamurthy, Wei and Baird11), according to previous research. We estimated that with a sample of 421 participants, the study would have 90 % statistical power at a significance level of 0·05 for a two-sided test. In order to achieve the greatest statistical power, we included all individuals in the cohort. Statistical analyses were performed using the SPSS software package version 24.0 (SPSS). Parametric data are presented as means and standard deviations. Non-parametric data are presented as median values and interquartile ranges. Categorical variables are expressed as percentages or ratios. Baseline fibre intake and time-averaged fibre intake were categorised by tertile based on the distribution among the study population. One-way ANOVA, Kruskal–Wallis or the χ 2 test was used to compare the differences in variables between groups. Differences in the survival curves between the three groups were evaluated using the log rank test. Changes in blood pressure, renal function and laboratory data over time were also compared among groups using a mixed model analysis of variance, with bootstrap covariance accounting for correlated measures within a subject adjusted for baseline characteristics, age, sex, and BMI. Recognised confounders combined with the baseline and time-averaged fibre intakes were evaluated by the Cox proportional regression model to determine the risk of CVD and all-cause mortality. When the baseline fibre intake was examined, the covariates included age, sex, BMI, baseline mean arterial pressure, Alb, Hb, hs-CRP, TAG, iPTH, RRF, total protein intake and total energy intake; when the time-averaged fibre intake was examined, the covariates included age, sex, time-averaged BMI, mean arterial pressure, Alb, Hb, hs-CRP, TAG, iPTH, RRF, total protein intake and total energy intake. We reported the multivariable-adjusted hazard ratios (HR) with 95 % CI. The calculation of time-averaged biochemistry, nutrition and dialysis parameters in the models was made using the half-yearly measurements. And we chose the 3-year period of observation here to calculate the total time-averaged values. All statistical tests were two tailed, and the significance level was set at P < 0·05.
Results
Subject demographics and follow-up
We followed 881 incident PD patients (434 men and 447 women), mean age of 57·7 (sd 14·8) years for 45·0 (interquartile range 21·5, 74·0) months; 42·1 % had DM, and CVD was present in 42·6 % (Table 1).
Table 1. Baseline clinical characteristics of peritoneal dialysis (PD) patients (n 881)
(Mean values and standard deviations; numbers of patients and percentages; medians with upper and lower quartiles)
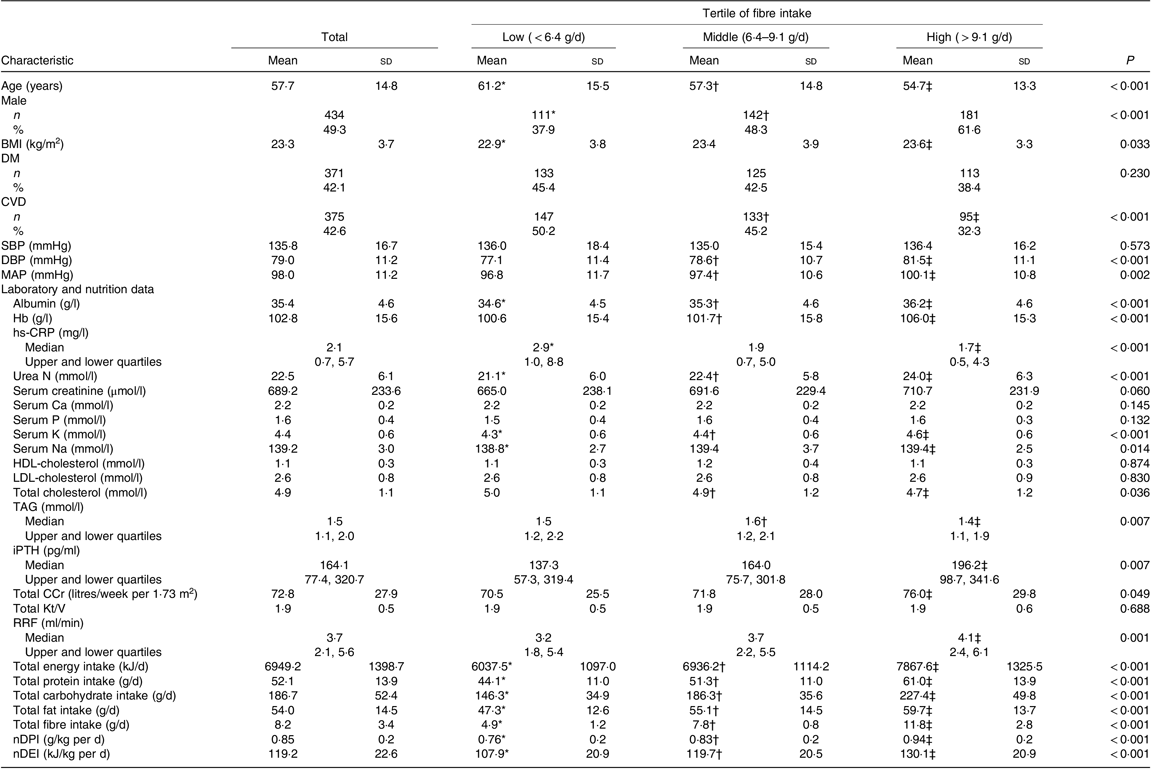
DM, diabetes mellitus; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; hs-CRP, high-sensitive C-reactive protein; iPTH, intact parathyroid hormone; Total CCr, total creatinine clearance; Total Kt/V, total urea clearance; RRF, residual renal function; nDPI, normalised protein intake; nDEI, normalised energy intake.
* P < 0·05 low-tertile group compared with the middle-tertile group.
† P < 0·05 middle-tertile group compared with the high-tertile group.
‡ P < 0·05 high-tertile group compared with the low-tertile group.
At the end of the study, 151 patients were still being maintained on PD, 434 had died, 164 were transferred to HD, 114 had undergone renal transplantation and eighteen were lost to follow-up (Fig. 1). A total of 178 (41·0 %) of 434 of all deaths were due to cardiovascular causes, and 107 (24·7 %) of 434 were due to infection (Table 3). According to the time-averaged fibre intake, patients in the lower tertile had a much shorter follow-up time and higher mortality rate (P < 0·001). Along with an increased intake of fibre, patients were less prone to be transferred to HD due to socio-economic causes and more likely to receive the renal transplantation. The main characteristics of ceased and alive patients are presented in Table 2.
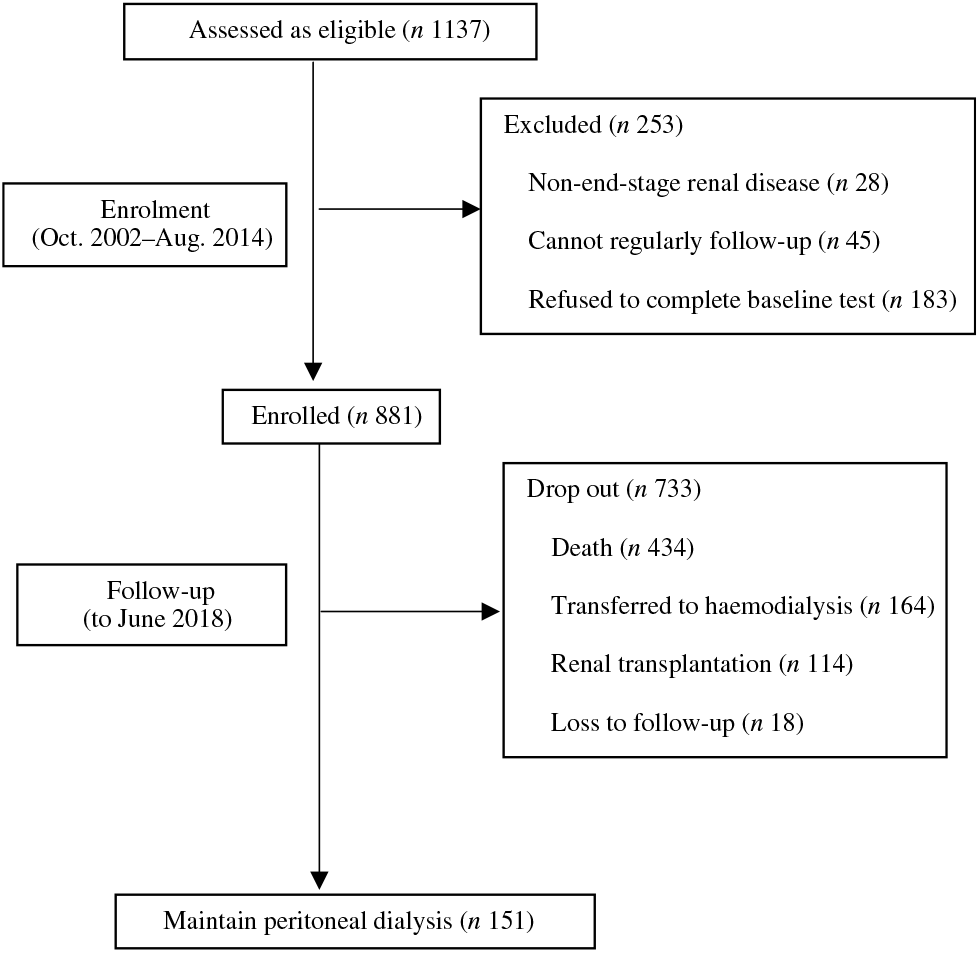
Fig. 1. Flow chart of the study.
Table 2. Baseline clinical characteristics of patients who ceased and maintained peritoneal dialysis (PD) (n 881)
(Mean values and standard deviations; numbers of patients and percentages; medians with upper and lower quartiles)
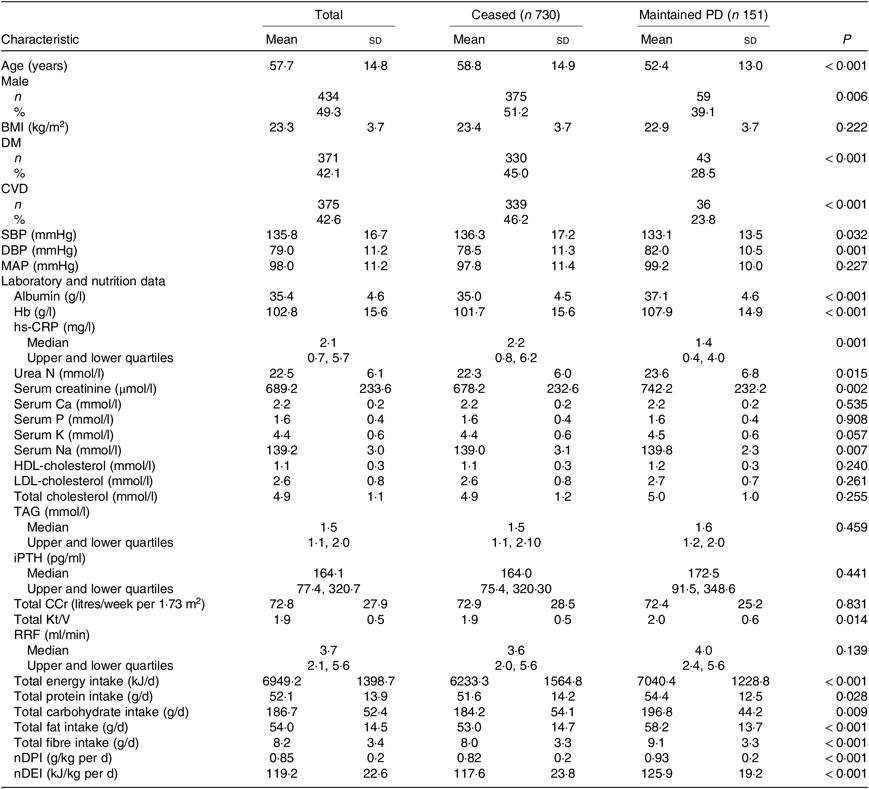
DM, diabetes mellitus; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; hs-CRP, high-sensitive C-reactive protein; iPTH, intact parathyroid hormone; Total CCr, total creatinine clearance; Total Kt/V, total urea clearance; RRF, residual renal function; nDPI, normalised protein intake; nDEI, normalised energy intake.
Table 3. Outcomes among peritoneal dialysis (PD) patients (n 881)
(Medians and interquartile ranges; number of events and event rate/100 person-years)
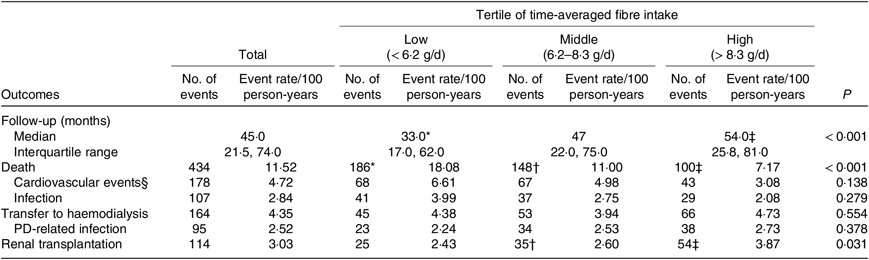
* P < 0·05 low-tertile group compared with middle-tertile group.
† P < 0·05 middle-tertile group compared with high-tertile group.
‡ P < 0·05 high-tertile group compared with low-tertile group.
§ Cardiovascular events include cardiovascular events, cerebrovascular events and sudden death.
Dietary fibre intake and clinical characteristics at baseline
The baseline fibre intake was 8·2 (sd 3·4) g/d in our cohort. The baseline characteristics of the study population tertiled by baseline fibre intake are given in Table 1. Significant differences were found between groups with regard to age; sex; BMI; the prevalence of CVD; diastolic blood pressure (DBP) and mean arterial pressure; Alb; Hb; hs-CRP; urea N; K; Na; total cholesterol; TAG; iPTH; RRF; total creatinine clearance; dietary nutrients including energy, protein, carbohydrate, fat, fibre, normalised daily protein and energy intake levels (P < 0·001, 0·01 or 0·05). Patients in the middle or/and high tertile were more likely to be male, younger, have higher BMI and low prevalence of CVD than those in the low tertile. The levels of DBP, mean arterial pressure, Alb, Hb, K, Na, iPTH and all dietary nutrients in the middle or/and high tertile were also significantly higher than those in the low tertile. In addition, patients in the middle or/and high tertile had better RRF and serum lipid spectrum and lower inflammation level.
Dietary fibre intake and the change in trend of blood pressure and biochemistry data during follow-up
The change in trend of blood pressure and all laboratory measurements was explored according to the tertile of the time-averaged dietary fibre intake adjusted for age, sex and BMI. The trends of DBP, serum Alb, Hb and hs-CRP were significantly different between tertiles (P < 0·001). The high or/and middle fibre intake group had increased DBP values compared with low-fibre intake group during the first 24-month or increased Hb values during the first 12-month observation period (P < 0·05) (Fig. 2(b) and (c)). The serum Alb values increased with increased intake of fibre at each time point during the whole period (P < 0·05) (Fig. 2(d)). Besides, hs-CRP values in low-fibre intake group were significantly higher than those in the high-fibre intake group during the whole period and also were higher than those in the middle group after 6 months (P < 0·05) (Fig. 2(f)).
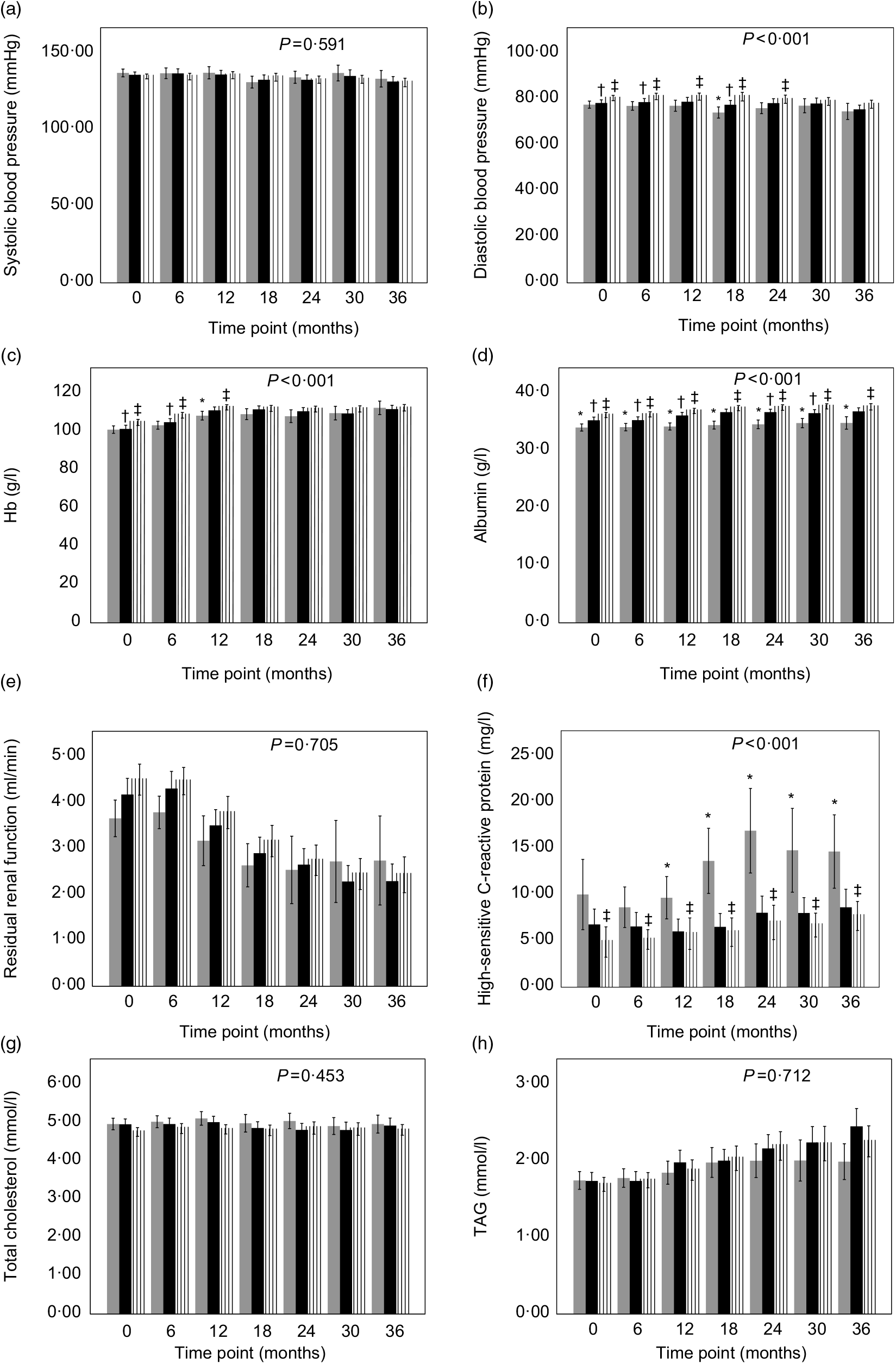
Fig. 2. Blood pressure, renal function and laboratory data of peritoneal dialysis patients with different tertiles of time-averaged fibre intake (![]() , low tertile;
, low tertile; ![]() , middle tertile;
, middle tertile; ![]() , high tertile) observed for a period of 36 months. Values are means, and lower and upper bar boundaries are 95 % confidence intervals. P value comparing the three groups over time was obtained from the linear mixed model with bootstrap covariance accounting for correlated measures within a subject adjusted for baseline characteristics, age, sex and BMI. * P < 0·05 low-tertile group compared with the middle-tertile group at the same time point. † P < 0·05 middle-tertile group compared with the high-tertile group at the same time point. ‡ P < 0·05 high-tertile group compared with the low-tertile group at the same time point.
, high tertile) observed for a period of 36 months. Values are means, and lower and upper bar boundaries are 95 % confidence intervals. P value comparing the three groups over time was obtained from the linear mixed model with bootstrap covariance accounting for correlated measures within a subject adjusted for baseline characteristics, age, sex and BMI. * P < 0·05 low-tertile group compared with the middle-tertile group at the same time point. † P < 0·05 middle-tertile group compared with the high-tertile group at the same time point. ‡ P < 0·05 high-tertile group compared with the low-tertile group at the same time point.
No differences were observed between groups with regard to the change in trend of systolic blood pressure, RRF, TAG and cholesterol between groups (Fig. 2(a), (e), (g) and (h)). In addition, other laboratory measurements such as serum Ca, P, K, Na and iPTH did not show significant differences during the follow-up between groups (data not shown).
Predictive value of fibre intake for all-cause and CVD mortality
The relationship between baseline or time-averaged fibre intake and outcomes was analysed. The baseline and time-averaged fibre intake were significantly associated with lower all-cause mortality in the unadjusted analysis (HR 0·82 (95 % CI 0·78, 0·85), P < 0·001 and HR 0·87 (95 % CI 0·84, 0·90), P < 0·001). By multivariate Cox regression analysis, with an increase of 1 g/d in baseline and time-averaged dietary fibre intake, the all-cause mortality showed a trend to decrease by 4 % (P = 0·053) and 6 % (P = 0·065), respectively, not achieving significant differences after multivariate adjustment. For subgroups analyses, an increase of 1 g/d in time-averaged fibre intake was significantly associated with a 13 % decrease in all-cause mortality (HR 0·87 (95 % CI 0·76, 0·98), P = 0·022) among non-diabetic patients (Table 4).
Table 4. Prognostic value of dietary fibre for all-cause mortality
(Hazard ratios (HR) and 95 % confidence intervals)
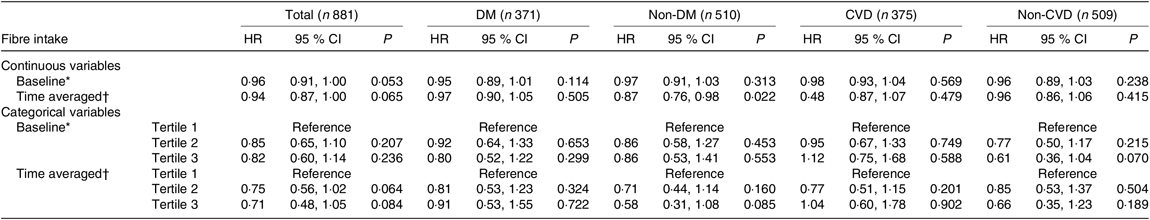
DM, diabetes mellitus; MAP, mean arterial pressure; hs-CRP, high-sensitive C-reactive protein; iPTH, intact parathyroid hormone; RRF, residual renal function.
* Adjusted for age, sex, BMI, MAP, Hb, albumin, hs-CRP, TAG, iPTH, RRF, total protein intake, total energy intake.
† Adjusted for age, sex, time-averaged BMI, time-averaged MAP, time-averaged Hb, time-averaged albumin, time-averaged hs-CRP, time-averaged TAG, time-averaged iPTH, time-averaged RRF, time-averaged total protein intake, time-averaged total energy intake.
When our subjects were categorised by baseline or time-averaged dietary fibre, we did not find that high-tertile group showing any protective effects on the all-cause mortality compared with the low-tertile group. A trend of benefits for high tertile of fibre intake in all-cause mortality were found among patients without CVD, but not achieving significant differences.
As for CVD mortality, only the baseline fibre intake showed a protective effect (HR 0·94 (95 % CI 0·89, 0·99), P = 0·019) in the unadjusted analysis. Based on multivariate Cox regression analysis, no obvious association was found between dietary fibre intake as continuous or categorical variables and CVD mortality, either in the whole cohort or in other subgroups (Table 5).
Table 5. Prognostic value of dietary fibre for CVD mortality
(Hazard ratios (HR) and 95 % confidence intervals)
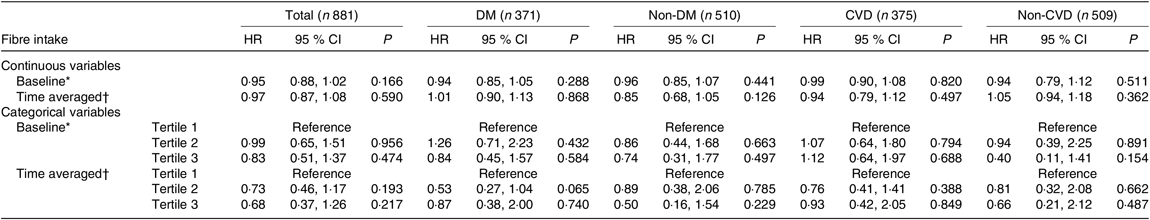
DM, diabetes mellitus; MAP, mean arterial pressure; hs-CRP, high-sensitive C-reactive protein; iPTH, intact parathyroid hormone; RRF, residual renal function.
* Adjusted for age, sex, BMI, MAP, Hb, albumin, hs-CRP, TAG, iPTH, RRF, total protein intake, total energy intake.
† Adjusted for age, sex, time-averaged BMI, time-averaged MAP, time-averaged Hb, time-averaged albumin, time-averaged hs-CRP, time-averaged TAG, time-averaged iPTH, time-averaged RRF, time-averaged total protein intake, time-averaged total energy intake.
Discussion
In this long-term prospective cohort study, participants with higher fibre intake were more likely to be younger and male. They tended to have better nutritional status, higher blood pressure and lower inflammatory status at baseline and afterward. Both baseline and time-averaged fibre intake showed protective effects against all-cause mortality by univariate analysis. Despite that this benefit was weakened after multivariate adjustment for the whole cohort, an independent association between fibre intake and all-cause mortality was still found among non-diabetic patients on PD.
An inverse association between dietary fibre intake and all-cause mortality is considered plausible. Some clinical clues should be recognised from the present study. Patients with higher fibre intake were likely to eat more food enriched with all dietary nutrients and thus maintain high values of serum Alb and Hb during the study period. Since serum Alb is significantly predictive of clinical outcomes(Reference Friedman and Fadem23), an increase of approximate 2 g/l in serum Alb in the high tertile might have substantial contribution to the benefits of dietary fibre intake on mortality, as shown in our data. In addition, serum CRP levels decreased along with the increased dietary fibre across the tertiles during the whole period. Similarly, the NHANES III study reported that the odds of elevated serum CRP levels decreased by 38 % in subjects with CKD for each 10-g/d increase in total fibre intake(Reference Krishnamurthy, Wei and Baird11). The ULSAM cohort study also showed that the odds of having CRP > 3 mg/l were lower in the quartiles that consumed more fibre(Reference Xu, Huang and Riserus10). The causes underlying the relationship between dietary fibre and inflammation have been explored recently. A high-fibre diet could alter gut bacterial metabolism and decrease the generation and absorption of some toxins that trigger systemic inflammation(Reference Evenepoel, Meijers and Bammens24). A high-fibre diet also has been proposed to be associated with a lower glycaemic load of rapidly digestible and absorbable dietary carbohydrates(Reference Liu, Manson and Buring25, Reference Qi, van Dam and Liu26) and higher plasma levels of the anti-inflammatory protein adiponectin(Reference Qi, Rimm and Liu27). In addition, fibres may provide a number of health benefits on the kidney by selectively stimulating favourable growth or activity of a limited number of indigenous bacteria to alter the composition of the intestinal flora and promote toxin discharge, improving the lipid metabolism(Reference Slavin28). Since dietary fibre could exert multifactorial effects for CKD and PD patients, we should draw more attention to observe the potential benefits of increasing fibre intake via dietary or supplements in this population.
Previous studies in the general population suggested that dietary fibre is predictive of lower blood pressure(Reference Khan, Jovanovski and Ho29), which might be due to the improved postprandial glucose excursions, insulin resistance(Reference Wong and Jenkins30) and endothelial dysfunction(Reference Montero31). Our data indicated a positive association of fibre intake and DBP at baseline and during follow-up. This paradoxical phenomenon might be explained as follows. Our subjects in the high-tertile fibre group had a significantly higher nutrient intake. We cannot exclude the possibility that other nutrients, such as Na, phosphate and water, exerted harmful effects on blood pressure(Reference Sanghavi and Vassalotti32, Reference Chan, Stamler and Griep33), offsetting the benefits of dietary fibre. Besides, dietary fibre has many types, such as chitosan, mannans and pectins, which may exert different effects on the blood pressure(Reference Evans, Greenwood and Threapleton34, Reference Wood, Fernandez and Sharman35), and an overall analysis of the total dietary fibre intake cannot determine the effects in detail.
In the present study, the amounts of time-averaged dietary fibre intake were markedly low, that is, 4·6, 6·8 and 10·1 g in the three tertiles, respectively, which are relatively lower than 10·2–12·4 g/d of fibre in HD patients(Reference Kalantar-Zadeh, Kopple and Deepak22, Reference Khoueiry, Waked and Goldman36), and 19·8 g/d of fibre in non-dialysed patients with CKD(Reference Fassett, Robertson and Geraghty37). All the above-mentioned fibre intake levels among CKD patients are lower than the target set for healthy individuals(38–40). Indeed, patients in the late stage of CKD, with the loss of renal function, were requested to decrease the intake of dietary K, phosphate and fluid. Their dietary pattern may lead to a simultaneous reduction in dietary fibre due to the restriction on vegetables, fruit and nuts. Unfortunately, current guidelines provide inconsistent references for dietary fibre intake for CKD patients(Reference Sabatino, Regolisti and Gandolfini41). The Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guidelines, European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines and Kidney Health Australia – Caring for Australasians with Renal Impairment (KHA-CARI) guidelines emphasise the inclusion of fibre-rich foods in the diet or a balanced diet rich in fruit and vegetables but do not recommend an exact amount for fibre(Reference Toigo, Aparicio and Attman14–Reference Wheeler and Kasiske16). The Kidney Disease Outcomes Quality Initiative (KDOQI) Guidelines on dyslipidaemia recommend 20–30 g/d of fibre(42). In view of limited data in the field, it is necessary to call for robust evidence regarding the appropriate quantity of dietary fibre in non-dialysed and dialysed CKD populations.
The present study had several strengths. To the best of our knowledge, it is the first study to explore whether the baseline and time-averaged fibre intake can predict all-cause mortality in patients on PD. We collected repeat measurements for dietary nutrients rather than a single-point assessment. The patients were thoroughly examined using time-averaged parameters for their dietary record, biochemical and nutrition data, giving us a unique chance to test our hypothesis. Oral nutrition supplements were also taken into account when evaluating the dietary nutrients. Furthermore, the study was performed in a large PD cohort with a relatively long follow-up and sufficient endpoints. We adjusted for total protein and energy intake when analysing the correlation between fibre and outcomes to minimise the confounding influences of these nutrients.
In spite of those strengths, the results of the present study should be interpreted in caution. First, an observational design cannot establish the cause–effect relationship between dietary intake and outcomes such as survival, serum Alb and hs-CRP. We cannot exclude that the inverse association of dietary fibre intake and all-cause and CVD mortality is confounded by physical activity or other healthy habits. More biological properties of dietary fibre, including its effect on insulin sensitivity, oxidative stress, inflammation and protein-bound uremic toxins, need to be investigated to explain the observed phenomenon in the dialysis population(Reference Lattimer and Haub43–Reference Rossi, Johnson and Xu45). Moreover, dietary fibre was not distinguished by soluble and insoluble fibre. It was not determined whether different sources and types of fibre exert varied effects on clinical outcome. Last, the present study was conducted in a single centre, which limits the generalisability of our data.
Our study revealed that higher dietary fibre intake appeared to have a protective effect on all-cause mortality in non-diabetic PD patients, which suggests that PD patients should be encouraged to consume a fibre-rich diet. Following the observation that PD patients with higher fibre intake concomitantly have better nutrition status and lower inflammation, more potential mechanisms for the benefits of dietary fibre in traditional and non-traditional risk factors need to be explored. Interventional studies are warranted to determine the efficacy and feasibility of fibre intake by dietary or oral supplements in PD patients. Targets for dietary fibre intake should also be determined for patients with CKD.
Acknowledgements
The authors express their appreciation to the patients and staff of the Peritoneal Dialysis Center of Peking University First Hospital, for their continuing contribution to the present study. This work is supported in part by Capital Characteristic Clinic Research Grant from Beijing Science & Technology Committee (Z111107058811110), New Century Excellent Talents from Education Department of China, Clinic Research Award from ISN GO R&P Committee.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
The contributions of the authors are as follows. Research idea and study design: J. D.; data acquisition: Z. L., Y. C., H. L.; statistical analysis: X. X., Z. L.; manuscript drafting or revision: X. X., J. D.; supervision or mentorship: J. D. Each author accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. J. D. takes responsibility that the present study has been reported honestly, accurately and transparently; that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained. All authors read and approved the final manuscript.
There were no conflicts of interest.










