Cachexia is a complex metabolic syndrome characterised by ongoing loss of body weight and skeletal muscle mass, which cannot be fully reversed by conventional nutritional support(Reference Muscaritoli, Anker and Argiles1). The pathophysiology of cachexia encompasses a negative protein and energy balance, driven by a variable combination of reduced food intake and abnormal metabolism. Cachexia is frequently observed in patients with cancer, and is associated with progressive functional impairment, intolerance to anticancer treatment and shorter survival(Reference Muscaritoli, Anker and Argiles1–Reference Davis and Dickerson3).
The severity of cachexia in patients with cancer varies from non-symptomatic inflammatory derangements and minimal weight and muscle loss in the early stage to severe muscle wasting and low performance status in patients not responding to anticancer treatment(Reference Fearon, Strasser and Anker4).
In order to define and stage cachexia, a number of frameworks in patients with chronic diseases(Reference Muscaritoli, Anker and Argiles1, Reference Evans, Morley and Argiles5) and cancer(Reference Fearon, Strasser and Anker4, Reference Bozzetti and Mariani6–Reference Argiles, Lopez-Soriano and Toledo8) have been described. Recently, an international expert group proposed a conceptual framework for cancer cachexia, with a classification for three stages of clinical relevance: pre-cachexia, cachexia and refractory cachexia(Reference Fearon, Strasser and Anker4). Overall, existing instruments use slightly different nutritional and inflammatory parameters and cut-off points to define pre-cachexia and cachexia.
Despite the growing understanding of the pathophysiology and staging of cachexia, assessment of cachexia in clinical practice is limited. These studies clearly showed the occurrence of weight loss and features of cachexia in patients with cancer.
In patients with lung cancer, high prevalences of involuntary weight loss have been reported(Reference Ross, Ashley and Norton9–Reference Laviano and Meguid11). Lung cancer is frequently associated with cachexia. Weight loss in patients with lung cancer was associated with systemic inflammation, loss of muscle mass, an increased acute-phase response, decreased levels of the anabolic hormone insulin-like growth factor-I(Reference Simons, Schols and Buurman12) and hypermetabolism(Reference Simons, Schols and Buurman12, Reference Staal-van den Brekel, Dentener and Schols13). Weight loss was also associated with reduced quality of life(Reference Ovesen, Hannibal and Mortensen14), response to chemotherapy(Reference Mohan, Singh and Kumar15) and survival(Reference Ross, Ashley and Norton9, Reference Martin, Santolaria and Batista16) in patients with lung cancer.
The staging of cachexia in patients with lung cancer has not been described, but could help clinicians to decide on early interventions or cachexia treatment. Up to now, the validity and usefulness of cachexia instruments in patients with cancer is unknown, and the recognition and nutritional management of cancer cachexia remains unsatisfactory(Reference Muscaritoli, Anker and Argiles1).
Comprehensive data of cancer populations could give more insight into the pathophysiology of (pre)cachexia, and could be used to apply cachexia frameworks and to investigate the outcomes and differences between frameworks. Therefore, we aimed to retrospectively study the presence of (pre)cachexia at diagnosis of stage III non-small-cell lung cancer (NSCLC), using recently described consensus-based frameworks(Reference Muscaritoli, Anker and Argiles1, Reference Fearon, Strasser and Anker4, Reference Evans, Morley and Argiles5), and to explore the prognostic value of pre-cachexia and cachexia. Second, we explored quality of life, and nutritional and inflammatory parameters associated with (pre)cachexia. We hypothesise that (pre)cachexia is present in this locally advanced patient population and that cachexia is associated with a decreased quality of life and shorter survival.
Materials and methods
Patients
Between March 2005 and October 2007, forty patients with histologically or cytologically proven stage III NSCLC, aged 18–80 years and having a life expectancy of at least 3 months, were included at the start of concurrent chemoradiotherapy. Patients were excluded if they had undergone surgery, chemotherapy or radiotherapy during the previous month; if they had oedema, ascites or severe co-morbidities; or if they used high-dose corticosteroids or fish oil supplements.
Data used for the present retrospective analysis were collected at the inclusion for a prospective double-blind randomised controlled trial that has been carried out at our centre from 2005 to 2008. Out of fifty-five enrolled patients, four patients did not meet the inclusion criteria, nine patients refused to participate and two had disease progression (Fig. S1, available online). We used the baseline and survival data of forty patients, irrespective of the intervention in the trial. After carrying out baseline measurements, patients were randomly assigned to receive two cans per d of either a protein- and energy-dense oral nutritional supplement containing n-3 PUFA or an isoenergetic control oral nutritional supplement during 5 weeks of chemoradiotherapy(Reference Meij van der, Langius and Smit17).
Throughout chemoradiotherapy, the dietitian monitored dietary intake and provided dietary counselling. Tube feeding was indicated in the case of an (expected) oral intake of < 75 % of energy requirements for more than 3 d, combined with the inability to increase energy intake by oral food or sip feeds.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human patients were approved by the Medical Ethics Committee of the VU University Medical Center, Amsterdam, The Netherlands. Written informed consent was obtained from all patients.
Baseline measurements
At baseline, before the start of chemoradiotherapy, weight loss, BMI, fat-free mass (FFM), energy expenditure, anorexia, inflammation, muscle strength, quality of life and physical activity were assessed.
Weight loss and BMI
Pre-illness weight, unintentional weight loss in the last month and during the last 6 months and height were recorded. Body weight, without shoes and wearing light clothing, was measured on a compact digital flat scale (SECA 888) to the nearest 0·2 kg. BMI was calculated by dividing body weight (kg) by the square of the height (m).
Fat-free mass
Bioelectrical impedance spectroscopy (Hydra 4200, Xitron Technologies) was performed to assess FFM. Whole-body resistance was measured with four surface electrodes placed on the right wrist and ankle, as previously described(Reference Lukaski, Bolonchuk and Hall18). Briefly, the principle was based on the application of a variable electrical current between 50 and 700 μA produced by a generator and applied to the skin using adhesive electrodes (3M red Dot Ag/AgCl) with the subject lying supine(Reference Houtkooper, Lohman and Going19). FFM was calculated from resistance and reactance at the frequency of capacitance by using the Kyle Geneva equation(Reference Kyle, Genton and Karsegard20).
The phase angle of bioelectrical impedance at 50 kHz was calculated using the following equation: phase angle = (resistance/reactance) × (180/π). The cut-off point for patients with lung cancer, described by Gupta et al. (Reference Gupta, Lammersfeld and Vashi21), was used to classify patients with a low ( ≤ 5·3) and high (>5·3) phase angle.
Energy expenditure
Resting energy expenditure (REE) was measured by a ventilated hood system (Deltatrac, Datex); CO2 production (VCO2) and O2 consumption (VO2) were measured at complete rest for a period of 30 min. REE was calculated using a modified Weir equation(Reference Weir22, Reference Mansell and Macdonald23). To estimate total energy expenditure (TEE), 30 % was added to REE, assuming a physical activity level of 1·3 for sedentary patients with cancer(Reference Moses, Slater and Preston24).
Anorexia
Patients recorded their appetite on a visual analogue scale (VAS), 10 cm in length(Reference Wilson, Thomas and Rubenstein25). Patients' energy intake, assessed by a 24 h dietary recall, was expressed as percentage of TEE. Anorexia and/or reduced food intake were identified by the presence of appetite < 5 cm (VAS), energy intake < 84 kJ/kg body weight per d (84 kJ (20 kcal)/kg)(Reference Laviano and Meguid11) or energy intake < 70 % of TEE(Reference Muscaritoli, Anker and Argiles1).
Inflammation
Non-fasting blood samples were taken simultaneously with usual blood samples for chemotherapy. Plasma concentrations of C-reactive protein (CRP) were measured with an automated latex-enhanced immunoturbidimetric assay on a Modular P analyser (reference: 0–8 mg/l)(Reference Burtis, Ashwood and Tietz26). Serum IL-6 was measured by commercially available ELISA (Pelikine compact human ELISA kits, Sanquin) (reference: 0–4 pg/ml). Whole-blood Hb was determined by spectrophotometry on a Cell-Dyn Sapphire analyser (Abbott Diagnostics) (reference: ≥ 7·3 mmol/l or ≥ 117 g/l)(Reference Purich and Allison27). Serum albumin concentrations were chemically determined on a Modular P analyser (ACN 760, 11815148 216, Roche Diagnostics) (reference: ≥ 320 g/l)(Reference Dati, Johnson and Whicher28).
Muscle strength
Muscle strength was measured by handgrip strength in the non-dominant hand using a hydraulic hand dynamometer (Baseline, Fabrication Enterprises). The patient performed two maximal isometric contractions while sitting, with the shoulder adducted and neutrally rotated, elbow flexed at 90° and the forearm and wrist in neutral position. The average of two measurements was recorded, and compared with age- and sex-dependent reference values for handgrip strength(Reference Bohannon, Peolsson and Massy-Westropp29).
Additional parameters
We assessed additional parameters that could be related to cancer cachexia, such as quality of life, physical activity level and survival.
The investigator recorded the Karnofsky Performance Score, a valid and widely used instrument to quantify the functional status of cancer patients. The Karnofsky Performance Score ranges from 0 to 100, with a higher score indicating a better ability to carry out normal daily activities and work(Reference Yates, Chalmer and McKegney30, Reference Karnofsky, Burchenal and MacLeod31).
Patients filled out the European Organisation for Research and Treatment of Cancer – Quality of Life Questionnaire C30 (EORTC-QLQC30) questionnaire, a multidimensional validated cancer-specific measure that includes global health status, physical status, functional and symptom scales (i.e. fatigue)(Reference Simons, Schols and Buurman12, Reference Meij van der, Langius and Smit17, Reference Lukaski, Bolonchuk and Hall18).
Physical activity was assessed by the Physical Activity Monitor accelerometer. Patients were instructed to wear the Physical Activity Monitor for seven consecutive days on the hip (model AM101, 28 g, 59 × 43 × 10 mm, PAM B.V.)(Reference Slootmaker, Chinapaw and Schuit32). The Physical Activity Monitor produces a single index score, which is a proxy measure of total daily physical activity. Every three points of the physical activity score reflects about 10 min walking. The Physical Activity Monitor also produces minutes of low- and moderate-intensity activities; low-intensity physical activity corresponds with small in-house movements and moderate-intensity activity corresponds with walking(Reference Slootmaker, Chinapaw and Schuit33).
Definition of pre-cachexia and cachexia
We used two consensus-based frameworks to define cachexia: a cancer-specific and a non-disease-specific general framework. With the cancer-specific framework, we defined pre-cachexia, cancer cachexia and refractory cancer cachexia, as proposed by, respectively, the European Society for Parenteral and Enteral Nutrition (ESPEN) Special Interest Group ‘cachexia–anorexia in chronic wasting diseases’(Reference Muscaritoli, Anker and Argiles1) and an international panel of experts in clinical cancer cachexia research(Reference Fearon, Strasser and Anker4). Second, we used the general framework for cachexia in chronic illness, as described by Evans et al. (Reference Evans, Morley and Argiles5). Some of the parameters and cut-off points not specifically given a reference citation in the sections below were not described in frameworks, and therefore retrieved from available unspecified literature and, where necessary, from experts.
Cancer-specific framework for cachexia
Cancer pre-cachexia(Reference Muscaritoli, Anker and Argiles1):
(1) Unintentional weight loss of 0 to ≤ 5 % during the previous 6 months.
(2) Anorexia (the presence of either: appetite < 5 cm (VAS), energy intake < 84 kJ/kg body weight per d (84 kJ (20 kcal)/kg)(Reference Laviano and Meguid11) or energy intake < 70 % of TEE(Reference Muscaritoli, Anker and Argiles1)).
(3) Systemic inflammation (CRP ≥ 8 mg/l, the upper limit of normality).
Cancer cachexia(Reference Fearon, Strasser and Anker4):
(1) Weight loss >5 % during the previous 6 months or BMI < 20 kg/m2 and weight loss >2 % or sarcopenia (FFM index < 5th percentile of age- and sex-specific reference values(Reference Schutz, Kyle and Pichard34) and weight loss >2 %).
(2) Reduced food intake (the presence of either: appetite < 5 cm (VAS), energy intake < 84 kJ/kg body weight per d (84 kJ (20 kcal)/kg)(Reference Laviano and Meguid11) or energy intake < 70 % of TEE(Reference Muscaritoli, Anker and Argiles1)).
(3) Systemic inflammation (CRP ≥ 8 mg/l, the upper limit of normality)
Refractory cancer cachexia(Reference Fearon, Strasser and Anker4):
(1) Variable degree of ‘cancer cachexia’.
(2) Cancer disease both pro-catabolic and not responsive to anticancer treatment.
(3) Low performance score (Karnofsky Performance Score < 50, indicating that a patient is unable to care for self).
(4) < 3 months expected survival.
General framework
The non-disease-specific general framework for cachexia(Reference Evans, Morley and Argiles5) includes the combination of weight loss of ≥ 5 % in 6 months or BMI < 20 kg/m2, combined with at least three of the following five criteria:
(1) Decreased muscle strength. Handgrip strength below the lowest tertile extracted from age- and sex-specific reference values(Reference Bohannon, Peolsson and Massy-Westropp29).
(2) Fatigue (score of 3 or 4 according to the EORTC-QLQC30 symptom scale(Reference Simons, Schols and Buurman12)).
(3) Anorexia (the presence of: appetite < 5 cm (VAS), energy intake < 84 kJ/kg body weight per d (84 kJ (20 kcal)/kg)(Reference Laviano and Meguid11) or energy intake < 70 % of TEE(Reference Muscaritoli, Anker and Argiles1)).
(4) FFM index below the 10th percentile by age- and sex-specific reference values(Reference Staal-van den Brekel, Dentener and Schols13).
(5) One or more abnormal serum biochemistry parameters: CRP >5 mg/l, Hb < 120 g/l or 117 g/l, serum albumin < 320 g/l or IL-6 >4 pg/ml(Reference Bozzetti and Mariani6).
Statistical analysis
Statistical analysis was performed using SPSS for Windows (version 17.0, SPSS, Inc.). Groups with no cachexia, pre-cachexia and cachexia were compared for serum biochemistry, REE, quality of life and physical role, emotional, cognitive and social functioning. Independent samples t tests were performed to compare groups with no cachexia and cachexia. For variables that were not normally distributed, non-parametric tests were performed to compare group differences. Frequencies within groups for nominal characteristics were compared by Pearson's χ2 tests. Differences between three groups (no cachexia, pre-cachexia and cachexia) were tested by one-way ANOVA. Correlations between variables were investigated by Pearson's correlation tests.
Group survival, from the date of the start of concurrent chemoradiotherapy (from 15 March 2005 until 30 October 2007) until death or follow-up visit (17 November 2011), was generated by the method of Kaplan and Meier and compared by means of the log-rank test. Second, the multivariate Cox's regression proportional hazards model was used to analyse hazard ratios (HR) for survival. Cachexia was the independent variable, and the model was adjusted for confounding factor(s) (based on a >10 % change of OR, after adding a single factor: sex, age and/or tumour stage: IIIa v. IIIb). Median survival was displayed with the standard error; P values < 0·05 were considered to be statistically significant.
Results
Patients
A total of forty patients with histologically or cytologically proven stage IIIa (n 16) or stage IIIb (n 24) NSCLC were studied, nineteen females and twenty-one males, with a median age of 57 (range 39–80) years. The average amount of weight loss during the previous 6 months was 1·9 (sd 6·5) % of pre-illness weight. The overall median survival was 25·0 (sd 8·7) months. Baseline patient characteristics are displayed in Table 1.
Table 1 Baseline characteristics for patients with stage III non-small-cell lung cancer, specified for groups with no cachexia, pre-cachexia and cachexia, as defined by different consensus-based frameworks (Mean values and standard deviations; number of participants and percentages)
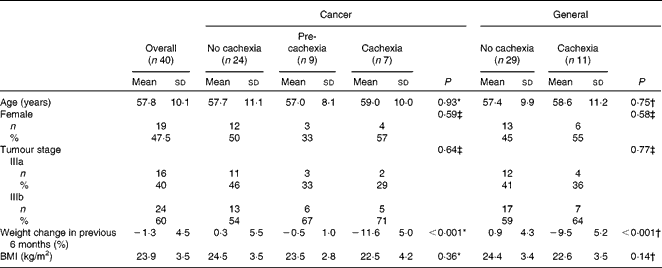
* ANOVA (comparing no-cachexia, pre-cachexia and cachexia groups).
† Independent samples t test for equality of means (comparing no-cachexia and cachexia groups).
‡ Pearson's χ2 test (comparing no-cachexia and (pre)cachexia groups).
Cancer-specific framework
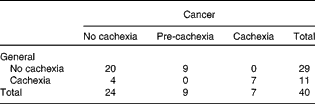
Using the two consensus-based frameworks of the ESPEN Special Interest Group(1) and Fearon et al.(Reference Fearon, Strasser and Anker4), we classified pre-cachexia in nine patients (23 %) and cachexia in seven patients (18 %). The remaining twenty-four patients were classified as no-cachexia patients (Table 2). None of the patients met the criteria of refractory cancer cachexia: measurements were carried out at diagnosis, just before starting anticancer treatment, and the Karnofsky performance score was relatively high (70–100) and the expected survival was at least 3 months in all patients.
Table 2 Number of patients with stage III non-small-cell lung cancer classified as having no cachexia, pre-cachexia and cachexia
Quality of life was significantly different among no-cachexia, pre-cachexia and cachexia groups (P= 0·03), but other function scales (such as physical function) did not significantly differ between groups. Survival was non-significantly different between no-cachexia, pre-cachexia and cachexia groups in univariate analysis (24 (sd 11·6) v. 32 (sd 1·5) v. 9 (sd 9·2) months, respectively; P= 0·21) (Fig. 1). Multivariate analysis with no cachexia as the reference category, corrected for sex and tumour stage, showed a significantly shorter survival in patients with cancer cachexia (HR 2·93; 95 % CI 1·03, 8·34; P= 0·04), but not in patients with pre-cachexia (HR 0·78; 95 % CI 0·30, 2·03; P= 0·62).
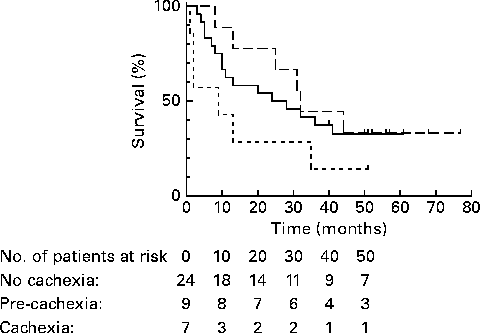
Fig. 1 Kaplan–Meier survival functions for no cachexia (![]() ; n 24), pre-cachexia (
; n 24), pre-cachexia (![]() ; n 9) and cachexia (
; n 9) and cachexia (![]() ; n 7) in patients with stage III non-small-cell lung cancer, defined by the European Society for Parenteral and Enteral Nutrition Special Interest Group and cancer-specific framework of Fearon et al. (Reference Fearon, Strasser and Anker4, Reference Fearon, Voss and Hustead35). P= 0·21.
; n 7) in patients with stage III non-small-cell lung cancer, defined by the European Society for Parenteral and Enteral Nutrition Special Interest Group and cancer-specific framework of Fearon et al. (Reference Fearon, Strasser and Anker4, Reference Fearon, Voss and Hustead35). P= 0·21.
General framework
Using the general framework to define cachexia, we identified eleven (28 %) out of forty patients with cachexia and twenty-nine patients (72 %) as having no cachexia (Table 2). The four patients who were classified as cachectic using the general definition, but not when using the cancer-specific framework, did not experience anorexia, but scored positive in at least three other features of the general definition (Table 3). Cachexia tended to be associated with a trend for a lower quality of life (P= 0·08). Between the general no-cachexia and the cachexia groups, median survival was significantly different (respectively, 32·0 (sd 4·5) v. 10·0 (sd 3·7) months; P< 0·01) (Fig. 2). In multivariate analysis, corrected for confounding by sex and tumour stage, cachexia remained significantly associated with a shorter survival (HR 4·2; 95 % CI 1·7, 10·0; P= 0·001).
Table 3 Number of patients with stage III non-small-cell lung cancer scoring on cachexia features according to the applied criteria*,†,‡ (Number of participants and percentages)

FFM, fat-free mass; BIS, bioimpedance spectroscopy; EORTC-QLQC30, European Organisation for Research and Treatment of Cancer – Quality of Life Questionnaire C30; CRP, C-reactive protein.
* Cancer-specific framework for pre-cachexia.
† Cancer-specific framework for cachexia.
‡ General framework for cachexia.
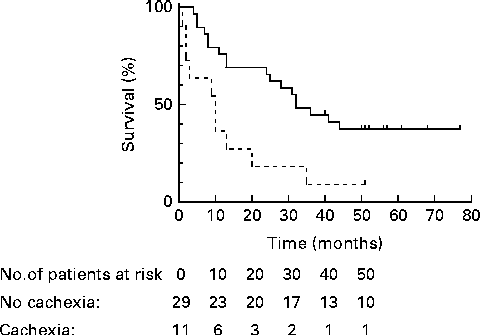
Fig. 2 Kaplan–Meier survival functions for no cachexia (![]() ; n 29) and cachexia (
; n 29) and cachexia (![]() ; n 11) in patients with stage III non-small-cell lung cancer, defined by the non-disease-specific, general framework of Evans et al. (Reference Evans, Morley and Argiles5). P< 0·01.
; n 11) in patients with stage III non-small-cell lung cancer, defined by the non-disease-specific, general framework of Evans et al. (Reference Evans, Morley and Argiles5). P< 0·01.
Cachexia features
Approximately 50 % of non-cachectic patients scored positively on cachexia features, such as fatigue, anorexia, reduced handgrip strength and upper arm circumference, or increased CRP. In general, low percentages of patients scored positively on a reduced FFM index, albumin or Hb (Table 3).
For all instruments, groups with cachexia showed higher levels of CRP and IL-6 and a lower Hb and serum albumin than patients with no cachexia (P< 0·01) (Table 4). CRP was positively correlated with IL-6 (r 0·55, P< 0·01), and negatively correlated with Hb (r − 0·47, P< 0·01) and serum albumin (r − 0·71, P< 0·01). The remaining inflammatory parameters were also significantly correlated with one another. Using different cut-off points for CRP (>5 or 10 mg/l instead of >8 mg/l) did not change the presence of pre-cachexia and cachexia in individual patients (data not shown).
Table 4 Differences in biochemistry, phase angle, resting energy expenditure (REE), physical activity and quality of life between cachexia groups with stage III non-small-cell lung cancer (Mean values and standard deviations)

CRP, C-reactive protein; FFM, fat-free mass; PAM, Physical Activity Monitor; EORTC-QLQC30, European Organisation for Research and Treatment of Cancer – Quality of Life Questionnaire C30.
* ANOVA (comparing no-cachexia, pre-cachexia and cachexia groups).
† Independent samples t test for equality of means (comparing no-cachexia and cachexia groups).
‡ 1 kJ = 0·239 kcal.
Of all patients, twelve (30 %) had a weight loss of at least 5 % in the previous 12 months or less, three (8 %) had a FFM index below the 5th percentile of reference values, twenty-seven (68 %) had decreased handgrip strength (below the lowest tertile of reference values), nineteen (48 %) experienced fatigue and twenty-three (58 %) experienced anorexia or reduced food intake. When comparing individual levels of inflammatory parameters with their reference values, CRP and serum IL-6 were elevated in, respectively, twenty-eight (70 %) and twenty (50 %) patients, and Hb and serum albumin were decreased in eight (20 %) and seven (18 %) patients, respectively (Table 3).
Additional parameters
REE per kg FFM, physical activity and phase angle were non-significantly different between groups. However, physical activity appeared to be lower in cachexia patients (Table 4).
Discussion
The purpose of the present explorative study was to study the presence of pre-cachexia and cachexia in patients with stage III NSCLC, by using consensus-based conceptual frameworks, which have not yet been applied or validated in populations of patients with cancer. Second, we explored the association of (pre)cachexia with survival and quality of life.
Although we are gaining knowledge on the pathophysiology and treatment of cancer cachexia, little is known about the typical profile and staging of cachexia. We chose to apply the only two available consensus-based frameworks to define cachexia. These frameworks were both comprehensive, but differed in the kind of parameters to define cachexia. The cut-off point of essential parameters, e.g. weight loss, is still a subject of debate. Therefore, we were interested in the outcomes of these two instruments when applied in a small, heterogeneous population of patients with locally advanced cancer.
These frameworks defined cachexia and described the clinical features associated with cachexia. More recently published proposals that aimed to grade the severity of cachexia led to the definition of pre-cachexia(Reference Muscaritoli, Anker and Argiles1, Reference Fearon, Strasser and Anker4). Using these proposals, it is possible to identify cancer patients with pre-cachexia: early-stage cachexia, characterised by moderate systemic inflammation and metabolic alterations, and minimal weight loss. Patients with pre-cachexia are not always recognised by clinicians or nutritional screening instruments, while nutritional support is expected to prevent progressive loss of body weight and FFM. On the contrary, treatment options for cachexia are limited.
In the present population of patients at diagnosis of stage III NSCLC, pre-cachexia was prevalent in 23 %, but only the framework proposed by the ESPEN Special Interest Group(Reference Muscaritoli, Anker and Argiles1) defines pre-cachexia, while the general framework of Evans et al. (Reference Evans, Morley and Argiles5) only defines cachexia. Cachexia was also prevalent in the present population, but the cancer-specific framework and the general framework for cachexia found a different number of patients with cachexia (respectively, 18 and 28 % by the cancer-specific and non-disease-specific general framework).
A number of studies showed the association between survival and weight loss in general cancer populations(Reference Dewys, Begg and Lavin10, Reference Ovesen, Hannibal and Mortensen14, Reference Fearon, Voss and Hustead35) and in patients with gastrointestinal(Reference Andreyev, Norman and Oates36) and lung cancer(Reference Ross, Ashley and Norton9). One of the first papers on this topic found the combination of weight loss, food intake and systemic inflammation to be related to poor outcome in pancreatic cancer patients(Reference Fearon, Voss and Hustead35). Because the definition of cachexia includes the presence of severe weight loss, the association with survival in the present study is consistent with these findings. The difficulty is that, in the literature, weight loss and cachexia are used disorderly, and that it is not possible to isolate starvation from cancer cachexia. Another component of the cachexia definition is inflammation. Systemic inflammation, amongst others reflected by elevated CRP and hypoalbuminaemia, is also negatively associated with survival(Reference McMillan, Crozier and Canna37, Reference Jiang, Hiki and Nunobe38).
After carrying out baseline measurements at diagnosis, patients received different anticancer treatments and participated in a placebo-controlled randomised controlled trail comparing oral nutritional supplements containing n-3 PUFA with an isoenergetic placebo. Yet, the percentages of patients with (pre)cachexia and survival did not significantly differ among groups with different cancer treatments (data not shown). Preclinical studies suggest that an increased intake of n-3 PUFA decreases the risk of cancer development and progression. A few clinical studies support the potential benefit of n-3 PUFA on chemotherapy efficacy(Reference Murphy, Mourtzakis and Chu39) or cancer cell proliferation(Reference Aronson, Kobayashi and Barnard40). In the present population, patients who received oral nutritional supplements containing n-3 PUFA did not show a significantly different presence of cachexia or survival than control patients (data not shown).
On average, the present population of patients with stage III NSCLC showed a moderate amount of weight loss (on average 1·9 % of pre-illness weight) during the previous 6 months and, consequently, a low prevalence of malnutrition (20 %). Other studies in patients with lung cancer (all types and stages) reported high percentages of malnutrition, i.e. 15·6(Reference Fox, Brooks and Gandra7), 30(Reference Staal-van den Brekel, Schols and ten Velde41), 36(Reference Dewys, Begg and Lavin10) and 50–61 %(Reference Laviano and Meguid11). A study by Bozzetti & Mariani(Reference Bozzetti and Mariani6) showed an average weight loss of 9·5 % in outpatients with lung cancer. Consequently, the percentage of patients with cachexia in the present patient population was relatively low (18 % by the cancer-specific framework and 28 % by the general definition). This could be explained by the selection of stage III NSCLC. As this is one of the first studies to assess pre-cachexia in stage III lung cancer, it is hard to compare these findings with other data. Op den Kamp et al. (Reference Op den Kamp, Langen and Minnaard42) found a comparable amount of weight loss (average 3·1 %) in a group of sixteen newly diagnosed patients with stage I to III NSCLC. Compared with healthy controls, these patients also showed systemic inflammation, but no apparent loss of FFM. However, the present exploratory study did not describe pre-cachexia features (such as inflammation and anorexia) in individual patients(Reference Op den Kamp, Langen and Minnaard42).
Weight loss was associated with an elevated REE(Reference Simons, Schols and Buurman12, Reference Staal-van den Brekel, Schols and ten Velde41), systemic inflammatory response(Reference Simons, Schols and Buurman12, Reference Staal-van den Brekel, Schols and ten Velde41) and a reduced dietary intake(Reference Staal-van den Brekel, Schols and ten Velde41) in patients with SCLC and NSCLC. Metabolic and inflammatory derangements seemed to be mainly related to the tumour; after resection(Reference Fredrix, Soeters and Wouters43) or chemotherapeutic treatment(Reference Staal-van den Brekel, Schols and Dentener44), REE in patients with lung cancer decreased. We did not find differences for REE per kg FFM between cachexia groups, probably due to high CRP and inflammation in the majority of patients. When uncorrected for FFM, REE in patients with cachexia (defined by the general framework for cachexia) was significantly lower, but this could be explained by the lower body weight in patients with cachexia. Because we used bioelectrical impedance spectroscopy to assess FFM (and not the ‘gold standard’ dual-energy X-ray absorptiometry), this may have resulted in over-estimation or under-estimation of FFM.
We also showed that approximately 50 % of non-cachectic patients scored positively on cachexia features, such as moderate weight loss, systemic inflammation, fatigue, anorexia, reduced handgrip strength and upper arm circumference. The frameworks that we used define patients as pre-cachectic or cachectic when they experience a combination of cachexia features, inflammation and weight loss, which is consistent with the existing knowledge on the pathophysiology of cachexia. Other cachexia frameworks, e.g. the proposal of the SCReening the Nutritional Status in Oncology (SCRINIO) working group(Reference Bozzetti and Mariani6) and the cachexia score(Reference Argiles, Lopez-Soriano and Toledo8), were not consensus-based and therefore not selected to address the present research question.
A secondary aim of the present study was to explore quality of life and physical activity, and their association with cachexia. Overall, the present small sample size resulted in a low statistical power, which made it hard to demonstrate significant associations. Pre-cachexia and cachexia were associated with a reduced overall quality of life, but not with other quality of life parameters, such as physical function. In the literature, an association among nutritional status, inflammation and well-being in lung cancer has been described, but these studies did not assess cachexia in the way we did(Reference Giannousi, Gioulbasanis and Pallis45, Reference Gioulbasanis, Georgoulias and Vlachostergios46).
Physical activity is an important indicator of quality of life and performance status in cancer patients(Reference Coups, Park and Feinstein47), and found to be reduced in patients with SCLC(Reference Gibney, Elia and Jebb48) and pancreatic cancer(Reference Moses, Slater and Preston24). The present patients with stage III NSCLC also showed a lower physical activity than healthy subjects (approximately 6 v. 20)(Reference Slootmaker, Chinapaw and Schuit33), and patients with pre-cachexia and cachexia showed a non-significant lower physical activity than no-cachexia patients.
When using the selected frameworks, we encountered some issues. First, patients with weight loss as well as complaints and/or inflammation were incorrectly justified as having no cachexia by the general framework, which requires three positive scores on complaints and inflammation. Patients with ≥ 5 % weight loss, in combination with two positive scores on complaints and inflammation, were not classified as cachectic. Also, the ESPEN Special Interest Group did not classify these patients as pre-cachectic, as their weight loss was more than 5 %. Second, cut-off points for anorexia, CRP and FFM index were lacking for the pre-cachexia and cancer-specific frameworks. For pre-cachexia, weight loss ≤ 5 % was described, but it was unclear if this accounted for patients with a weight loss of 0 %. We solved these issues by consulting the authors. In line with current knowledge, we found a positive correlation between pro-inflammatory indexes (CRP and serum IL-6), and these were negatively correlated with Hb and serum albumin. Interestingly, when other cut-off points for inflammatory parameters were applied, we observed the same presence of (pre)cachexia.
Validation of cachexia instruments in large groups of patients with cancer is still required, but a ‘gold standard’ is lacking. The association of cachexia with survival is informative, but validation of instruments against one or more indicators of cachexia (e.g. standardised assessment of muscle mass) is preferable. Further studies in larger populations are warranted to validate these new instruments and to more extensively explore the prognostic value in patients with cancer. Ideally, worldwide cancer centres record a number of biomarkers and cachexia parameters, follow-up treatment adherence and survival, and merge these data in order to validate definitions and their prognostic value. A promising parameter might be proteolysis-inducing factor, which has been found in the urine of cachectic patients with cancer(Reference Tisdale49).
In conclusion, new consensus-based frameworks show that pre-cachexia and cachexia are prevalent in patients with stage III NSCLC. Cachexia appears to be associated with a shorter overall survival and a reduced quality of life.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114512004527
Acknowledgements
We would like to thank N. Kok (Department of Pulmonary Diseases, VU University Medical Center, Amsterdam, The Netherlands) and V. van Adrichem (Department of Nutrition and Dietetics, VU University Medical Center, Amsterdam, The Netherlands) for their assistance with patient inclusion. Conflict of interest statement: B. S. v. d. M., J. A. L. and P. A. v. L. were funded by Abbott Nutrition. Abbott Nutrition provided funding for data collection, but was not involved in protocol development, data analysis or manuscript writing. C. P. S., M. M. and E. F. S. do not declare any conflicts of interest. B. S. v. d. M. conceived the study, participated in the design and coordination of the study, performed statistical analysis and drafted the manuscript. J. A. L. conceived the study, participated in the design and coordination of the study and helped to draft the manuscript. C. P. S. performed statistical analysis and helped to draft the manuscript. M. M. helped to interpret the data and to draft the manuscript. E. F. S. conceived of the study and helped to draft the manuscript. P. A. v. L. conceived of the study, participated in the design and coordination of the study and helped to draft the manuscript. The present work was presented as a poster at ESPEN, Goteborg, 2011.








