INTRODUCTION
Despite the availability of virally suppressive antiretroviral therapy (ART), human immunodeficiency virus (HIV)-associated neurocognitive impairment (NCI) continues to be common and impactful (Heaton et al., Reference Heaton, Marcotte, Mindt, Sadek, Moore and Bentley2011). NCI is observed in up to half of HIV-infected (HIV+) persons (Heaton et al., Reference Heaton, Franklin, Ellis, McCutchan, Letendre and LeBlanc2011), and is associated with worse every day functioning outcomes (e.g., medication nonadherence, driving problems, unemployment; Gorman, Foley, Ettenhofer, Hinkin, & van Gorp, Reference Gorman, Foley, Ettenhofer, Hinkin and van Gorp2009; Heaton et al., Reference Heaton, Marcotte, Mindt, Sadek, Moore and Bentley2004; Thames, Arentoft, Rivera-Mindt, & Hinkin, Reference Thames, Arentoft, Rivera-Mindt and Hinkin2013).
HIV is an especially significant public health concern for Hispanics/Latinos/as, who represent the largest ethnic/racial minority group in the United States (U.S. Census Bureau, 2015). Hispanics/Latinos/as are three-times more likely to have HIV infection compared with non-Hispanic Whites (Centers for Disease Control and Prevention, 2015), hereafter referred to as “Latinos” and “Whites,” respectively. Latinos tend to be disadvantaged at nearly every component of the HIV disease treatment cascade (Gardner, McLees, Steiner, del Rio, & Burman, Reference Gardner, McLees, Steiner, del Rio and Burman2011), including delayed HIV testing and delayed care engagement (Chen, Gallant, & Page, Reference Chen, Gallant and Page2012; Dennis, Napravnik, Sena, & Eron, Reference Dennis, Napravnik, Sena and Eron2011; Shapiro et al., Reference Shapiro, Morton, McCaffrey, Senterfitt, Fleishman, Perlman and Consortium1999; Sheehan, Trepka, & Dillon, Reference Sheehan, Trepka and Dillon2015; Turner et al., Reference Turner, Cunningham, Duan, Andersen, Shapiro, Bozzette and Zierler2000). Thus, not surprisingly, Latinos tend to present with worse HIV disease characteristics, including higher rates of acquired immunodeficiency syndrome (AIDS) and opportunistic infections, and lower CD4 cell counts than Whites (Swindells et al., Reference Swindells, Cobos, Lee, Lien, Fitzgerald, Pauls and Anderson2002).
Latinos also might be at increased risk for HIV-associated NCI in the era of combination ART (Durvasula, Miller, Myers, & Wyatt, Reference Durvasula, Miller, Myers and Wyatt2001; Heaton et al., Reference Heaton, Franklin, Deutsch, Letendre, Ellis and Casaletto2015; Rivera-Mindt et al., Reference Rivera-Mindt, Byrd, Ryan, Robbins, Monzones, Arentoft and Henniger2008, Reference Rivera-Mindt, Miranda, Arentoft, Byrd, Monzones, Fuentes and Morgello2014; Wojna et al., Reference Wojna, Skolasky, Hechavarria, Mayo, Selnes, McArthur and Nath2006). In a recent longitudinal study of HIV+ persons in the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study, Latino ethnicity was a significant, independent predictor of neurocognitive decline over an average of three years (Heaton et al., Reference Heaton, Franklin, Deutsch, Letendre, Ellis and Casaletto2015). While the latter study was not focused on examining ethnic differences, and thus statistical models were not specifically constructed to address this question, a small number of studies have focused on investigating HIV-associated NCI among Latinos (Rivera-Mindt et al., Reference Rivera-Mindt, Byrd, Ryan, Robbins, Monzones, Arentoft and Henniger2008, Reference Rivera-Mindt, Miranda, Arentoft, Byrd, Monzones, Fuentes and Morgello2014; Wojna et al., Reference Wojna, Skolasky, Hechavarria, Mayo, Selnes, McArthur and Nath2006). A study of 49 HIV+ women with nadir CD4 cell counts of <500 cells/mm3 living in Puerto Rico found elevated rates of NCI in this group compared with other HIV+ cohorts (global NCI=78%; Wojna et al., Reference Wojna, Skolasky, Hechavarria, Mayo, Selnes, McArthur and Nath2006).
Two additional studies investigated NCI among HIV+ Latinos primarily of Caribbean (i.e., Puerto Rican/Dominican) origin/descent and living in the northeastern United States (i.e., New York). One of these studies included participants in the Manhattan HIV Brain Bank, with advanced HIV disease (51 Latino and 49 White; Rivera-Mindt et al., Reference Rivera-Mindt, Byrd, Ryan, Robbins, Monzones, Arentoft and Henniger2008). Findings indicated Latinos performed worse than Whites on tests of abstraction/executive function in a model adjusting for socioeconomic status, English reading level (i.e., Wide Range Achievement Test , WRAT-3), and CD4 cell counts. However, there were no significant ethnic group differences in similar adjusted models on global neurocognitive function, learning, and attention/working memory.
Another study in the northeastern United States (Rivera-Mindt et al., Reference Rivera-Mindt, Miranda, Arentoft, Byrd, Monzones, Fuentes and Morgello2014) found worse global and domain (processing speed, learning, and memory) neurocognitive performance among older (50+ years) HIV+ Latinos (n=22) compared with older HIV+ Whites (n=22), but no significant differences in younger HIV+ Latinos (n=62) and Whites (n=20). Taken together, results from these studies indicate that at least some subgroups of Latinos might be at increased risk for HIV-associated NCI. However, none of the studies to date has examined NCI among large groups of Latinos, which would allow for comparisons among subgroups of Latinos.
Latinos are highly heterogeneous, comprising multiple national origins, racial groups, patterns of immigration, and linguistic and educational backgrounds (Guarnaccia et al., Reference Guarnaccia, Martinez Pincay, Alegria, Shrout, Lewis-Fernandez and Canino2007; Llorente, Reference Llorente2008). Latinos are also known to possess genetic backgrounds shaped by admixture from several source continental populations, with often the precise contribution from each source population unknown (Bryc, Durand, Macpherson, Reich, & Mountain, Reference Bryc, Durand, Macpherson, Reich and Mountain2015; Conomos et al., Reference Conomos, Laurie, Stilp, Gogarten, McHugh, Nelson and Laurie2016; Libiger & Schork, Reference Libiger and Schork2013). Recent findings from a large population-based study of Latino health, indicate differential neurocognitive performances among subgroups of middle-aged and older Latinos (González et al., Reference González, Tarraf, Gouskova, Gallo, Penedo, Davis and Mosley2015).
Furthermore, there are documented differences in the HIV care continuum among subgroups of Latinos based on their country of origin (Chen et al., Reference Chen, Gallant and Page2012; Lee & Held, Reference Lee and Held2015; Sheehan et al., Reference Sheehan, Trepka and Dillon2015), which might have important implications for both general health and mental health care use and outcomes, including NCI. Latinos born in Mexico have an increased risk for late HIV diagnosis (Sheehan et al., Reference Sheehan, Trepka and Dillon2015), and HIV+ Latinos of Puerto Rican origin/descent are more likely to use services compared with other HIV+ Latino subgroups (Lee & Held, Reference Lee and Held2015; Sheehan et al., Reference Sheehan, Trepka and Dillon2015). Yet, HIV+ Puerto Ricans are at increased risk of early mortality (Sheehan et al., Reference Sheehan, Trepka and Dillon2015). They also have the highest rates of HIV transmission through injection drug use of all Latino subgroups (Chen et al., Reference Chen, Gallant and Page2012), which is linked to worse retention in care, ART adherence, and viral suppression (Gray, Valverde, Tang, Siddiqi, & Hall, Reference Gray, Valverde, Tang, Siddiqi and Hall2015). While these findings suggest that there are important differences among HIV+ Latino subgroups, we do not know whether these differences translate to differential rates of NCI.
There were two main purposes to the present study. First, we aimed to investigate differences in rates and correlates of NCI in HIV+ Latinos and Whites, and expand on prior studies by: (a) including a large cohort of participants, who were broadly representative of HIV+ persons receiving care at academic medical centers in the United States, and (b) by examining the role of HIV disease characteristics and comorbidities on ethnic differences in HIV-associated NCI. We hypothesized that HIV+ Latinos would show increased rates of NCI compared with HIV+ Whites.
The second aim was to investigate differences in rates and correlates of NCI among U.S. Latino subgroups based on self-reported country of origin/descent, that is, Puerto Rico and Mexico. These were the two Latino subgroups for whom we had considerable data available and they also represent the two largest subgroups of Latinos in the United States (Ennis, Rios-Vargas, & Albert, Reference Ennis, Rios-Vargas and Albert2011). We refer to these subgroups as Puerto Rican and Mexican for simplicity, but Latinos were living in the continental United States at the time of study participation, and we did not have consistent data on country of birth or duration of residence in the continental United States. In addition to these two main aims, we were also interested in investigating the role of genetically defined ancestry on ethnic group differences in NCI among a subgroup of participants with available genetic data. Better characterization of NCI among Latino subgroups is key for the development of targeted, culturally relevant interventions aimed at preventing and/or ameliorating NCI disparities in this large, vulnerable, heterogeneous, and often understudied segment of the U.S. population.
METHODS
Participants
The sample included 794 HIV+ adults (194 Latinos and 600 Whites) who were participants in the CHARTER study (Heaton et al., Reference Heaton, Clifford, Franklin, Woods, Ake and Vaida2010, Reference Heaton, Franklin, Deutsch, Letendre, Ellis and Casaletto2015), a longitudinal multisite cohort study of HIV+ persons. All participants were fluent in English, as ascertained by participant self-report and determined by study staff. Only baseline data were used for present analyses. Study visits took place between 2002 and 2010, with 98% of study visits occurring by 2007. Inclusion criteria were self-identifying as Latino or White, and having neurocognitive data.
Materials and Procedures
Assessments were performed by trained staff, who were certified by the coordinating center (UC San Diego). All data were obtained in compliance with the Helsinki Declaration and Human Research Protections Programs at the study sites.
Demographic characteristics
Demographic information (age, years of education, gender, ethnicity/race) was obtained via self-report. Self-reported ethnicity was ascertained following NIH guidelines, which define “Hispanic/Latino” as a person of Mexican, Puerto Rican, Cuban, South or Central American, or other Spanish-speaking culture of origin regardless of race (Office of Management and Budget, 1997). Per this methodology, participants were asked whether they were of Spanish/Hispanic/Latino origin, and provided with the following response options: (1) No, not Spanish/Hispanic/Latino; (2) Yes, Mexican, Mexican American, Chicano; (3) Yes, Puerto Rican; (4) Yes, Cuban; and (5) Yes, Other.
Neuromedical evaluation
Participants underwent a comprehensive neuromedical evaluation that included assessment of medical history, structured medical and neurological examinations, and the collection of blood, cerebrospinal fluid (CSF), and urine samples, as described in Heaton and colleagues (2010). Central nervous system (CNS) penetration effectiveness (CPE) was included as an estimate of the degree of CNS penetration of current ART regimen (Letendre, Reference Letendre2011; Letendre et al., Reference Letendre, Marquie-Beck, Capparelli, Best, Clifford, Collier and Grp2008). Neurocognitive comorbidity status was ascertained by pre-established international criteria outlined in Antinori and colleagues (Reference Antinori, Arendt, Becker, Brew, Byrd, Cherner and Wojna2007). In short, comorbidity status was based on self-reported history of HIV-related opportunistic CNS conditions and unrelated developmental, psychiatric, and neuromedical confounds, and categorized as incidental, contributing, or confounding.
Details about the procedure and its inter-rater reliability in the CHARTER study are available in Heaton and colleagues (2010). The Veterans Aging Cohort Study (VACS) Index score was computed as described previously (Justice et al., Reference Justice, Freiberg, Tracy, Kuller, Tate and Goetz2012, Reference Justice, Modur, Tate, Althoff, Jacobson, Gebo and Gange2013). It is a composite marker of disease severity in HIV, which includes “traditional” HIV biomarkers (current CD4 count and HIV-1 plasma RNA), and non-HIV biomarkers [indicators of renal and liver function, anemia, and hepatitis C virus (HCV) co-infection]. HCV co-infection was ascertained based on HCV serostatus (in 83% of the sample) or self-report.
Neurocognitive evaluation
Participants completed a comprehensive neurocognitive battery assessing seven cognitive domains (see Heaton et al., Reference Heaton, Clifford, Franklin, Woods, Ake and Vaida2010, for a list of tests by domain). Raw scores for each test were converted to T-scores adjusting for demographic characteristics (age, education, gender). T-scores were then converted to deficit scores ranging from 0 (no impairment) to 5 (severe impairment). Deficit scores for tests comprising each domain were averaged to compute domain deficit scores. Deficit scores from all tests were averaged to compute Global Deficit Scores that reflect the number and severity of deficits across the entire neurocognitive battery (Carey et al., Reference Carey, Woods, Gonzalez, Conover, Marcotte and Grant2004). Presence of domain and global NCIs were defined as domain deficit score >.5 and global deficit score≥.5, respectively. Two Processing Speed tests in the battery (i.e., Digit Symbol and Symbol Search from the Wechsler Adult Scale Intelligence Scale-Third Edition; WAIS-II; Wechsler, Reference Wechsler1997) have Latino ethnicity-corrected T-scores available (Heaton, Taylor, & Manly, Reference Heaton, Taylor and Manly2002), and T-scores on these tests were also examined separately. All other tests did not include adjustments for ethnicity.
Psychiatric and substance use characteristics
Current (past 30 days) and lifetime histories of major depressive and substance use disorders were evaluated using the computer-assisted Composite International Diagnostic Interview (Wittchen et al., Reference Wittchen, Robins, Cottler, Sartorius, Burke and Regier1991), which follows Diagnostic and Statistical Manual-Fourth Edition criteria (American Psychiatric Association, 1994). Substance use disorder was determined as meeting criteria for substance abuse or dependence for alcohol, marijuana, cocaine, hallucinogens, inhalants, methamphetamine, opioids, phencyclidine, sedatives, or other substances. History of injection drug use was ascertained by self-report. Current mood was assessed using the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, Reference Beck, Steer and Brown1996), and recent substance use was identified via urine toxicology for various substances (i.e., marijuana, cocaine, methamphetamine, amphetamine, barbiturates, benzodiazepines, and phencyclidine) on the day of testing.
Genotyping and genetic ancestry
Genome-wide genotype information was available on a subset of participants (127 Latinos and 419 Whites) from whom genomic DNA was isolated from whole blood and subjected to genotyping using the Affymetrix Genome-Wide Human SNP Array 6.0 platform (Affymetrix, Inc., Santa Clara, CA). These data were subjected to rigorous quality control analyses with exclusion of variants with less than 95% genotyping efficiency or minor allele frequencies less than 1% in the study population.
To assess genetic ancestry based on genotype information for this subset of participants, the method developed by Libiger and Schork (Reference Libiger and Schork2013) was used. Specifically, an ancestry-informative reference panel was constructed by bringing together genotype data from 2513 individuals of known ancestry from 63 populations around the world using several publically available sources (Cann et al., Reference Cann, de Toma, Cazes, Legrand, Morel, Piouffre and Cavalli-Sforza2002; International HapMap 3 Consortium et al., Reference Altshuler, Gibbs, Peltonen, Altshuler, Gibbs and McEwen2010; Nelson et al., Reference Nelson, Bryc, King, Indap, Boyko, Novembre and Lai2008; Xing et al., Reference Xing, Watkins, Witherspoon, Zhang, Guthery, Thara and Jorde2009). The reference panel was created in a stepwise manner to ensure that the included individuals were not admixed among six major continental populations (African, Central Asian, East Asian, European, Native American, and Oceanic) and that each continental population was represented by a reasonably large number of diverse individuals originating in the relevant continent. The assembled reference panel contained genotype information at 16,433 strand-unambiguous SNPs. These markers exhibited low linkage disequilibrium (r-squared less than 0.1 was observed between 99% of marker pairs), and allele frequency was higher than 1%.
To assess ancestry and admixture proportions a supervised clustering approach implemented in the ADMIXTURE software (Alexander, Novembre, & Lange, Reference Alexander, Novembre and Lange2009) probabilistically assigned each participant to six clusters corresponding to the six major continental populations listed above. The genotype profiles of the six populations were defined by the individuals who made up the reference panel. Based on this approach, the degree of ancestry of each participant was determined, effectively quantifying the proportion of their genome that is likely to be derived from each of the six populations (African, Central Asian, East Asian, European, Native American, and Oceanic). Major genetic ancestry was defined as having a genetic ancestry proportion of ≥.5 for a given region. Participants with a hybrid pattern of ancestries (all with frequencies <.5) were assigned to “no single major ancestry.”
Statistical Analyses
To investigate differences between Latinos and Whites, we conducted independent sample t tests and Chi-square tests on demographic, HIV disease, medical and psychiatric comorbidity, genetic ancestry, and global and domain NCI by group. To examine whether differences in rates of NCI might be explained by HIV disease characteristics and comorbidities we ran a logistic regression model on global and domain NCI, including as predictors ethnic group and significant covariates. Covariates examined included HIV disease characteristics, and medical and psychiatric comorbidity (Table 1). To build the models, we entered covariates that were significantly different by ethnic group at p<.10 into a stepwise backward regression models (minimum Akaike information criterion) on global NCI. Variables selected via this method were entered in final multivariable models on global and domain NCI. We also examined the impact of these covariates on ethnic differences in NCI via path analyses (for continuous mediators, see Preacher & Hayes, Reference Preacher and Hayes2004; for categorical mediators, see Imai, Keele, & Tingley, Reference Imai, Keele and Tingley2010).
Table 1 Characteristics of the study cohort by ethnic group

Note. CSF=cerebrospinal fluid; CNS=central nervous system; IQR=interquartile range; HCV=hepatitis C virus; VACS=Veterans Aging Cohort Study.
a Based on independent sample t-tests and Chi-Square tests.
b Major genetic ancestry refers to having a genetic ancestry proportion of ≥.5 for a given region (Africa, Europe, America, East Asia, Central Asia, and Oceania), with participants that have all frequencies <.5 being assigned to “no single major ancestry”; Whites: n=419; Latinos: n=127.
c Among those on ART (antiretroviral therapy).
To investigate the impact of other HIV-disease characteristics relevant to persons on ART (detectable plasma and CSF RNA, and CPE), we ran a logistic regression model on global NCI within our subset of participants on ART, including significant covariates yielded by the overall model in addition to ART-relevant variables that differed significantly between groups. To examine predictors of global NCI by ethnic group, we first ran univariable analyses investigating the association of each of the HIV disease characteristics and psychiatric and medical comorbidities on global NCI separately by ethnic group. We then ran separate multivariable logistic regression models by ethnic group with predictors being all the variables that were associated with global NCI in univariable analyses at p<.10. To examine differences and associations among subgroups of Latinos, we conducted similar analyses comparing Puerto Ricans and Mexicans.
To examine whether factors possibly linked to genetic ancestry might explain differences in global NCI by ethnic group (and country of origin/descent) among HIV+ persons, we ran a logistic regression model on global NCI among our subset of participants with available genetic ancestry data, including self-reported ethnic (or country of origin) group and major genetic ancestry as predictors. We also ran separate univariable analyses examining the association of proportion of genetic ancestry from each region with global NCI by ethnic and country of origin group.
RESULTS
Cohort Characteristics by Ethnic Group
Table 1 shows characteristics of the study cohort by ethnic group. Compared with Whites, Latinos were slightly younger, had less formal education, and were more likely to be female. All participants who self-identified as White had a European major genetic ancestry. As expected, there was considerable variability in major genetic ancestry within the self-identified Latino group. Latinos were recruited primarily from sites in New York (NY), San Diego (CA), and Galveston (TX). Latinos were more likely to have AIDS, had lower nadir and current CD4 cell counts, and among those on ART, were more likely to have detectable HIV RNA in plasma and CSF (failure of viral suppression on ART). There were no significant differences on other HIV disease characteristics or overall comorbidity ratings, except for a trend for higher VACS Index scores (indicative of worse health) among Latinos. Regarding psychiatric characteristics, Latinos were somewhat less likely to have a lifetime history of major depressive and alcohol use disorders.
Neurocognitive Impairment by Ethnic Group
Figure 1 shows results from ethnic group comparisons on global and domain NCI. In unadjusted analyses, Latinos had higher rates of global NCI than Whites [odds ratio (OR)=1.61; confidence interval (CI)=1.16–2.23; p<.01). Within neurocognitive domains, Latinos had higher rates of NCI on executive function, working memory, learning and memory/delayed recall, and speed of information processing.
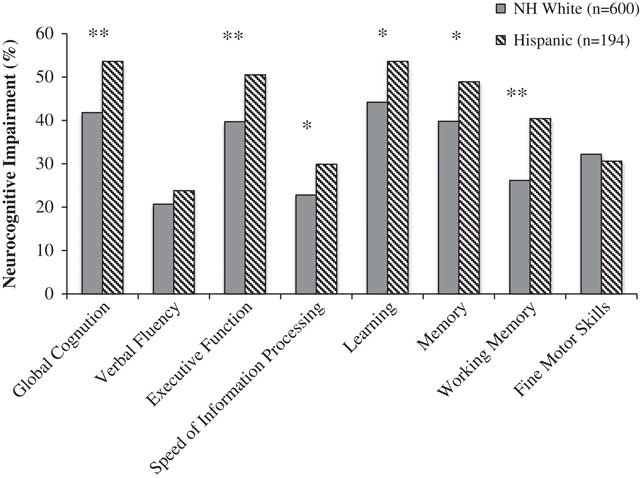
Fig. 1 Rates of global and domain neurocognitive impairment among HIV+ Latinos and Whites. *p<.01; **p<.001.
HIV disease characteristics and medical and psychiatric comorbidities, which were significantly different by ethnic group at p<.10 (see Table 1), were entered into a backward stepwise regression model on global NCI along with ethnicity. This method selected current and nadir CD4, and the VACS Index as significant covariates. After including these variables, ethnic differences in global NCI remained significant (OR=1.59; 95% CI=1.13–2.23; p<.01). Path analyses showed nadir CD4 (indirect effect=0.049; 95% CI=0.006–0.121) was the only significant partial mediator.
Multivariable models adjusting for significant covariates on neurocognitive domains, revealed that ethnic group differences were significant for executive function (OR=1.54; 95% CI=1.10–2.15; p=.01), working memory (OR=1.98; 95% CI=1.39–2.81; p<.001), learning (OR=1.49; 95% CI=1.07–2.09; p=.02), and delayed recall (OR=1.44; 95% CI=1.03–2.03; p=.03), but not for speed of information processing (OR=1.40; 95% CI=0.96–2.02; p=.08). Within the subset of participants on ART, unadjusted models showed increased rates of NCI among Latinos than Whites (OR=1.92; 95% CI=1.30–2.85; p<.001). A model including significant covariates identified by the overall model, in addition to detectable plasma and CSF RNA, again revealed increased rates of global NCI among Latinos than Whites (OR=1.80; 95% CI=1.14–2.85; p=.01).
Correlates of NCI by Ethnic Group
Among Latinos, results from univariable models showed that lower nadir CD4, being on ART, and increased comorbidity status were all significant predictors of global NCI (p<.001). Results from a multivariable model showed that being on ART (ON–OFF: OR=2.30; 95% CI=1.02–5.35; p=.04) and comorbidity status (confounding-incidental: OR=7.77; 95% CI=2.86–25.29; p<.001; contributing-incidental; OR=3.21; 95% CI=1.61–6.61; p<.001) were both significant independent predictors of increased risk for global NCI among Latinos.
Among Whites, univariable analyses showed that AIDS, lower nadir CD4, more months of ART exposure, increased comorbidity status, and depressed mood (BDI scores) were significantly associated with global NCI (p<.01). In a multivariable model, comorbidity status was the only significant predictor of increased risk of global NCI among Whites (confounding-incidental: OR=10.45; 95% CI=5.69–20.41; p<.001; contributing-incidental: OR=2.81; 95% CI=1.89–4.19; p<.001; confounding-contributing: OR=3.72; 95% CI=1.94–7.48; p<.001).
Differences Among Subgroups of Latinos by Country of Origin/Descent
There was considerable heterogeneity within our Latino group in regards to self-reported country of origin/descent (79 Mexican, 60 Puerto Rican, 6 Cuban, 18 South or Central American, 29 from another Spanish culture or origin, and 2 did not respond). Analyses comparing Puerto Ricans and Mexicans (Table 2) showed that Puerto Ricans were significantly older and more likely to be female. A majority of individuals in both groups had a European major genetic ancestry, but Puerto Ricans were somewhat more likely to have African as their major genetic ancestry, and Mexicans were more likely to have no single major genetic ancestry.
Table 2 Characteristics of Latinos by country of origin/descent (Mexican and Puerto Rican)

Note. HIV=human immunodeficiency virus; AIDS=acquired immunodeficiency syndrome; ART=antiretroviral therapy; IQR=interquartile range; CSF=cerebrospinal fluid; CNS=central nervous system; HCV=hepatitis C virus; VACS=Veterans Aging Cohort Study.
a Based on independent sample t-tests and Chi-Square or Fisher’s exact tests.
b Major genetic ancestry refers to having a genetic ancestry proportion of ≥.5 for a given region (Africa, Europe, America, East Asia, Central Asia, and Oceania), with participants that have all frequencies <.5 being assigned to “no single major ancestry”; Mexican: n=62; Puerto Rican: n=33.
c Among those on ART (antiretroviral therapy).
Most Puerto Ricans were tested in New York, and most Mexicans were tested in San Diego and Galveston (Table 2). Puerto Ricans had longer duration of infection and more months of exposure to ARTs. Among those on ARTs, Puerto Ricans also were more likely to have detectable HIV RNA in CSF (i.e., failure to suppress HIV replication in the CNS). Furthermore, Puerto Ricans had higher VACS Index scores and were more likely to have contributing and confounding comorbidity ratings (as compared with incidental) than Mexicans. Puerto Ricans were also more likely to test positive for illicit substances on the date of testing based on urine toxicology.
As Figure 2 shows, Puerto Ricans had significantly higher rates of global NCI than Mexicans, and this difference was primarily driven by the domains of learning and memory/delayed recall (with significant differences for both verbal and visual tests of learning/recall, ps<.05).
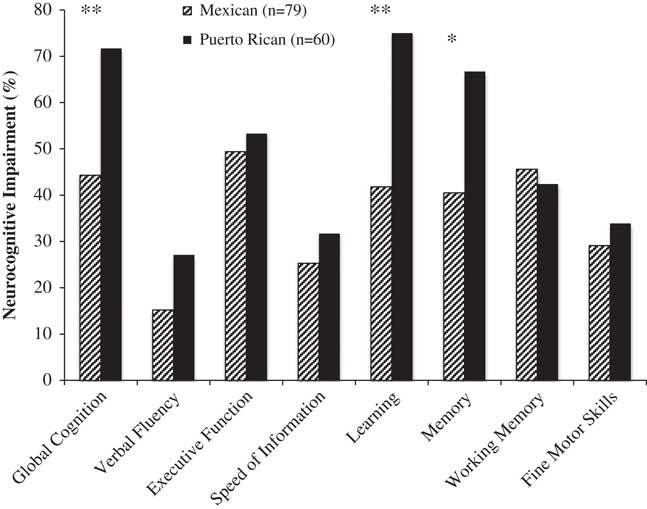
Fig. 2 Rates of global and domain neurocognitive impairment by Latino group (Mexican and Puerto Rican origin/descent). *p<.01 **p<.001.
HIV disease characteristics, and medical and psychiatric comorbidities which were significantly different by country of origin group at p<.10 (see Table 2) were entered into a backward stepwise regression model on global NCI as predictors along with country of origin. This method selected ART and comorbidity status as covariates. Multivariable models adjusting for these variables, continued to show a significant effect of country of origin on global NCI (Puerto Ricans-Mexicans: OR=2.40; 95% CI=1.11–5.29; p=.03), as well as learning and memory/delayed recall (Puerto Ricans-Mexicans: Learning, OR=3.09; 95% CI=1.44–6.81; p<.01; Memory, OR=2.22; 95% CI=1.06–4.71; p=.03).
Path analyses on global NCI, showed that comorbidity status was the only significant partial mediator of differences associated with country of origin (indirect effect=0.07; 95% CI=0.02–0.15; p=.02). Multivariable regression analyses on global NCI within the subset of participants on ART, adjusting for comorbidity status and detectable CSF RNA, also showed a significant effect of group (Puerto Ricans-Mexicans: OR=3.10; 95% CI=1.08–9.92; p=.04).
Regarding predictors of NCI among Puerto Ricans, being on ART and increased comorbidity status were significantly associated with NCI in univariable analyses. In a multivariable model, being on ART (OR=9.71; 95% CI=1.78–71.09; p<.001) and comorbidity status (confounding-incidental: OR=13.39; 95% CI=1.86–290.95; p=.02; contributing-incidental: OR=5.89; 95% CI=1.35–35.34; p=.02) were both significant independent predictors of NCI.
Among Mexicans, univariable analyses showed that lower nadir CD4 and increased comorbidity status were significant predictors of global NCI. In multivariable analyses, both lower nadir CD4 (OR/100 units=0.75; 95% CI=0.53–1.00; p=.05), and comorbidity status were marginally associated with global NCI (confounding-incidental: OR=7.01; 95% CI=1.00–141.7; p=.05), but with a high ORs.
Association of Genetic Ancestry and Self-Reported Ethnicity or Country of Origin/Descent to NCI
Stratified analyses by ethnic group showed there were no significant differences between the samples with and without genetic data on demographic variables (age, gender, education). Among participants with genetic ancestry data, a multivariable model in the overall sample on global NCI showed a significant effect of ethnicity (Latino-White: OR=1.65; 95% CI=1.02–2.66; p=.04) and no significant effect of genetic ancestry (p=.45). Separate univariable analyses by ethnic group showed proportions of European, African, or American Indian genetic ancestry were not significantly associated with NCI in Latinos (ps=.59, .68, .46, respectively) or in Whites (ps=.47, .12, .85, respectively).
A multivariable analysis on global NCI among our subset of Mexicans and Puerto Ricans with available genetic ancestry data yielded a significant effect of country of origin/descent (Puerto Ricans-Mexicans: OR=3.08; 95% CI=1.23–8.27; p=.02) and a significant overall effect of major genetic ancestry (p=.02). Specifically, the group with No single major ancestry had marginally higher rates of NCI than the European major ancestry group (OR=2.65; 95% CI=0.89–8.70; p=.08), who, in turn, had marginally higher rates of NCI than those with another major ancestry (African, American, or East Asian combined; OR=3.83; 95% CI=0.97–19.72; p=.06); with significant differences between this latter group and the No single major ancestry group (p<.01). Separate univariable analyses by country of origin group showed proportions of European, African, or American Indian genetic ancestry were not significantly associated with NCI in Puerto Ricans (ps=.64, .67, .24, respectively) or Mexicans (ps=.70, .71, .12, respectively).
Secondary Analyses
Subgroup of Whites matched to Latinos on demographics
To further control for differences in age, education and gender between ethnic groups, in exploratory analyses, we selected a subset of Whites (n=194) who were one-to-one matched with Latinos on age (±5 years), education (±2 years), and gender (all but one participant were matched on gender), and re-ran all core analyses. Latinos had higher rates of global NCI than the matched White group, as well as higher rates of NCI in the domains of learning, delayed recall and working memory. Results of analyses adjusting for significant covariates in this matched subset of participants (nadir CD4 and alcohol use disorder) on global and domain NCI yielded comparable findings to those of the overall cohort (i.e., while p values only approached significance in some cases, OR were similar or higher).
We also compared Mexicans and Puerto Ricans to their respective matched group of Whites on global and domain T-scores. Figure 3 shows Cohen’s d effect sizes for these comparisons. For Puerto Ricans, there were medium-to-large effect sizes for global neurocognition, which appeared to be driven primarily by large effect sizes in learning and memory, and small-to-medium effects on verbal fluency, speed of information processing, executive function and working memory. For Mexicans, there were small-to-medium effect sizes on executive function and working memory.
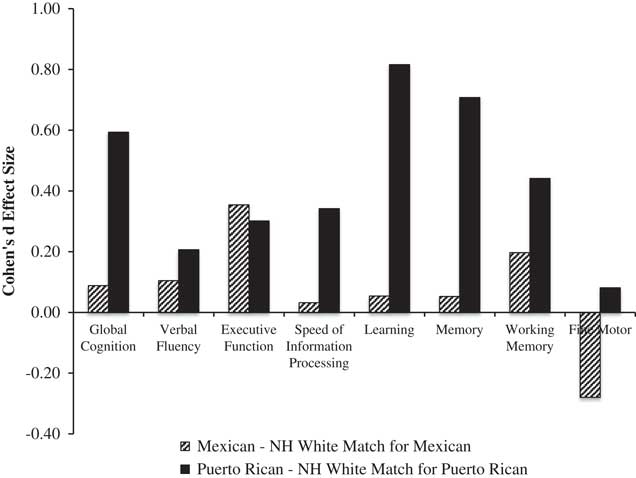
Fig. 3 Effect sizes on global and domain neurocognitive function (T-scores) by subgroup of Latinos (i.e., Mexicans, n=79; and Puerto Ricans, n=60) compared with their respective matched subgroups of non-Latino Whites (matched on age, gender, and education). Positive effect sizes indicate worse performance among Latino subgroups. Effect sizes: 0.20=small; 0.50=medium; 0.80=large (Cohen, Reference Cohen1988).
Norms with ethnicity corrections
For both components of the Processing Speed Index of the WAIS-III (Digit Symbol Coding and Symbol Search subtests; Heaton et al., Reference Heaton, Taylor and Manly2002) we had normative data for English-speaking Latinos living in the United States. To explore whether group differences might be attributed to a lack of appropriate norms, we ran a linear regression model on the average adjusted T-scores of the subtests comprising the Processing Speed Index (with Whites as the reference group). Results again showed a significant effect of group, F(2,793)=2.77, p=.04, and indicated that these group differences were primarily driven by Puerto Ricans performing significantly lower than Whites (Estimate=−2.96; SE=1.39; p=.03).
Impact of study site
Because of the distribution of Latinos in the United States and location of the study sites, ethnicity and country or origin/descent were highly confounded with study site (Tables 1 and 2). We explored the potential effect of study site by running a multivariable model on NCI with predictors being ethnicity and study site among participants tested at sites with reasonable numbers of Whites and Latinos (i.e., San Diego, New York, Galveston, and Seattle; n=658). The model showed significant independent effects of ethnicity (p=.015) and site (p<.001). Given that none of the sites had adequate numbers of both Mexicans and Puerto Ricans, we were unable to run comparable analyses among Latino subgroups. Instead, we ran separate chi-square tests on NCI, comparing Mexicans and Whites in San Diego (Mexicans: n=36; NCI=47%; Whites: n=165; NCI=32%; p=.09) and Puerto Ricans and Whites in New York (Puerto Ricans: n=47; NCI=79%; Whites: n=61; NCI=54%; p=.007).
DISCUSSION
Present findings support that HIV+ Latinos receiving care at academic medical centers in the United States are at increased risk for concurrent NCI compared with their White counterparts. This study also highlights that there are important differences among Latino subgroups, and one relevant way in which Latinos differ is their country of origin/descent. In the present study of HIV+ persons, Latinos of Puerto Rican origin/descent showed significantly increased rates of NCI compared with those of Mexican origin/descent.
Our results are consistent with findings of two prior studies (Rivera-Mindt et al., Reference Rivera-Mindt, Byrd, Ryan, Robbins, Monzones, Arentoft and Henniger2008; Wojna et al., Reference Wojna, Skolasky, Hechavarria, Mayo, Selnes, McArthur and Nath2006), which indicated increased rates of global or domain NCI in HIV+ Latinos of primarily Caribbean descent and with advanced HIV disease. Somewhat inconsistent with present findings, another study (Rivera-Mindt et al., Reference Rivera-Mindt, Miranda, Arentoft, Byrd, Monzones, Fuentes and Morgello2014) found rates of NCI were not significantly different among HIV+ Latinos and Whites younger than 50 years of age. Non-significant findings in this prior study might have been partly due to the relatively small sample sizes (Whites: n=20; Latinos: n=68), particularly given that there were small to medium effect sizes (as measured by Cohen’s d) on the domains of verbal fluency, attention/working memory, learning, and fine motor skills, and a medium effect size on executive function. In addition, 20% of the present sample was 50 years or older, and this prior study (Rivera-Mindt et al., Reference Rivera-Mindt, Miranda, Arentoft, Byrd, Monzones, Fuentes and Morgello2014) found significantly increased rates of NCI among older (50+ years old) HIV+ Latinos than older HIV+ Whites. This suggests that the differences observed in the present study, might be exacerbated in older cohorts.
Ethnic differences on NCI were evident across the domains of executive function, working memory, speed of information processing, learning, and recall, with no significant differences on verbal fluency and fine motor skills. Most of the neurocognitive domains in which there were significant ethnic differences (with the exception of speed of information processing) are among the most commonly impacted by HIV in the combination ART era (Heaton et al., Reference Heaton, Franklin, Ellis, McCutchan, Letendre and LeBlanc2011), and are linked with problems in everyday functioning in HIV+ persons (Heaton et al., Reference Heaton, Marcotte, Mindt, Sadek, Moore and Bentley2004). This underscores the potential significance of our findings for the daily lives of HIV+ Latinos.
In addition to having different rates of NCI, ethnic groups differed in several other factors, including HIV-disease and non HIV-disease characteristics. Consistent with prior findings (Swindells et al., Reference Swindells, Cobos, Lee, Lien, Fitzgerald, Pauls and Anderson2002), Latinos in our study showed worse HIV disease burden than Whites, both in terms of historical and current HIV disease characteristics. While lower nadir CD4 among Latinos significantly accounted for some of the ethnic differences in NCI, differences in NCI were still significant in adjusted models. This suggests that other psychosocial (e.g., acculturation, socioeconomic status, healthcare access, health behaviors) and biomedical factors of particular relevance among Latinos living with HIV need to be considered.
As HIV+ persons are living longer, HIV has become a complex disease with multiple causes of morbidity. While there were no significant ethnic group differences on comorbidity status, there was a trend for Latinos to have higher VACS Index scores. Additionally, comorbidity status was among the most important predictors of NCI, both in Whites and Latinos, indicating that there might be certain specific comorbidities worth investigating in more detail. One such factor might be metabolic syndrome (Grundy et al., Reference Grundy, Brewer, Cleeman, Smith and Lenfant2004), which is highest among Latinos (Aguilar, Bhuket, Torres, Liu, & Wong, Reference Aguilar, Bhuket, Torres, Liu and Wong2015; Ford, Giles, & Dietz, Reference Ford, Giles and Dietz2002; Heiss et al., Reference Heiss, Snyder, Teng, Schneiderman, Llabre, Cowie and Aviles-Santa2014) and has been linked to HIV-associated NCI (Fabbiani et al., Reference Fabbiani, Ciccarelli, Tana, Farina, Baldonero, Di Cristo and Di Giambenedetto2013; McCutchan et al., Reference McCutchan, Marquie-Beck, FitzSimons, Letendre, Ellis and Heaton2012).
We did not have an HIV-uninfected comparison group, and most of the tests did not have norms available that adjusted for Latino ethnicity. Thus, we cannot rule out that some of the ethnic differences observed in this study of HIV+ persons might be observed in the non HIV-infected population. In the present study, however, group comparisons on neurocognitive tests that do have normative adjustments for Latino ethnicity yielded comparable findings to those in the overall battery of tests. This, together with the findings that significant ethnic differences were observed on neurocognitive domains that are most commonly impacted by HIV, that nadir CD4 partially mediated ethnic differenced in NCI, and that NCI within our Latino group(s) was associated with multiple indicators of HIV disease severity, supports the notion that these group differences are, at least partly, HIV-related. Consistently, a large longitudinal study using data on the CHARTER cohort (Heaton et al., Reference Heaton, Franklin, Deutsch, Letendre, Ellis and Casaletto2015), found that Latino ethnicity was an independent predictor of HIV-associated neurocognitive decline.
In line with prior studies among HIV+ persons (Sheehan et al., Reference Sheehan, Trepka and Dillon2015), we found Puerto Ricans fared worse than Mexicans on HIV-disease burden, comorbidity status and injection drug use. They were also significantly more likely to show global NCI than Mexicans, with these differences being primarily driven by differential rates of NCI in the domains of learning and delayed recall. Findings showing worse NCI among Puerto Ricans than Mexicans in this study are also consistent with other reports indicating worse health outcomes (Velasco-Mondragon, Jimenez, Palladino-Davis, Davis, & Escamilla-Cejudo, Reference Velasco-Mondragon, Jimenez, Palladino-Davis, Davis and Escamilla-Cejudo2016), and worse neurocognitive performance (González et al., Reference González, Tarraf, Gouskova, Gallo, Penedo, Davis and Mosley2015) among Puerto Ricans than other Latino subgroups living in the United States
While there might be behavioral factors related to healthcare use and adherence that might impact several health outcomes, there might also be some disease-specific genetic/biomedical vulnerabilities that help explain these disparities. In the present study, there is some indication that both biomedical and behavioral factors might be playing a role. Comorbidity status partially mediated differences in NCI among Latino subgroups, and was a key predictor of NCI in all groups, again highlighting the importance of investigating comorbidities in future studies. Of interest, Puerto Ricans are the subgroup of Latinos with the highest rates of metabolic syndrome (Heiss et al., Reference Heiss, Snyder, Teng, Schneiderman, Llabre, Cowie and Aviles-Santa2014), and, as mentioned above, metabolic syndrome has been linked to HIV-associated NCI.
Additionally, among Puerto Ricans, being on ART conferred an increased risk for NCI. Being on ART is at least partly a marker of worse disease status, such that those who present to clinic with worse HIV-disease status are more likely to be prescribed ART. The fact that this factor was associated with NCI among Puerto Ricans, but not other groups in multivariable analyses, might be related to worse HIV-adherence among Puerto Ricans (Robbins et al., Reference Robbins, D’Aquila, Morgello, Byrd, Remien and Mindt2012). Consistent with this, among those on ART, Puerto Ricans were more likely to have detectable CSF HIV RNA. Regarding Mexicans, nadir CD4 also was an independent predictor of NCI, which is consistent with prior studies of HIV-associated NCI (Ellis et al., Reference Ellis, Badiee, Vaida, Letendre, Heaton and Clifford2011) and underscores the importance of early HIV treatment and testing in this group.
In light of prior evidence showing greater NCI risk among HIV+ females than males (Kabuba, Menon, Franklin, Heaton, & Hestad, Reference Kabuba, Menon, Frnaklin, Heaton and Hestad2016), it is worth noting that there was a higher percentage of females among Puerto Ricans than Mexicans. However, findings on the association between gender and HIV-associated NCI are inconsistent (Faílde-Garrido, Rodriguez Alvarez, & Simón-López, Reference Faílde-Garrido, Rodríguez Alvarez and Simón-López2008; Heaton et al., Reference Heaton, Franklin, Ellis, McCutchan, Letendre and LeBlanc2011, Reference Heaton, Franklin, Deutsch, Letendre, Ellis and Casaletto2015), and given our methodological approach, it is unlikely that NCI differences between Latino subgroups were accounted for by differential gender composition. Because of the small number of females (Mexican: n=10; Puerto Rican: n=23), we were unable to investigate gender differences in great detail, and it would be important for future studies to do so.
A unique aspect of the present study was the use of genomic data to characterize the genetic ancestry of self-reported ethnic groups. Present findings did not find a significant impact of genetic ancestry on NCI by ethnic or country of origin groups. Yet, genetic ancestry was a significant independent contributor to NCI among Latino subgroups in addition to country of origin/descent. Future larger studies investigating genetic markers of diseases that might contribute to HIV-associated NCI and be more common among subgroups of Latinos, might be key for elucidating the types of exposures that might contribute to ethnic differences in NCI.
Another important aspect of the present study concerns geographic differences in the distribution of the study groups, and the impact of geographic site on NCI. Latino/a ethnicity and country of origin both traveled with study site, which is consistent with U.S. Census Bureau data on the geographic distribution of Latinos in the United States (Ennis et al., Reference Ennis, Rios-Vargas and Albert2011). In the present study, study site did not appear to fully explain differences in rates of NCI among Latinos and Whites. Furthermore, we found a 15% increase in rates of NCI in Mexicans compared with Whites in San Diego and a 25% increase in rates of NCI in Puerto Ricans compared with Whites in New York.
While the difference in NCI between Mexicans and Whites was not statistically different, at least partly because of the smaller sample sizes, overall these findings indicate that geographic differences do not fully explain ethnic/cultural disparities in NCI. Of interest, however, there was a significant effect of study site. While this was not the focus of present analyses, understanding the factors driving these sites differences might shed light onto other social and environmental factors impacting NCI among HIV-infected persons in the United States.
There are several limitations to our study. We did not have data on several culturally relevant factors, such as language use history, bilingualism, socioeconomic status, country of birth, and acculturation. Future studies incorporating these types of assessments might shed light on factors underlying differences in NCI among subgroups of Latinos. The present study included English-speaking Latinos living in the United States, and thus caution is warranted in generalizing findings to Spanish-speaking and other subgroups of HIV+ Latinos.
In summary, HIV+ Latinos in the United States appear to be at higher risk for NCI compared with HIV+ Whites, with HIV+ Puerto Ricans being at particularly increased risk. In examining these ethnic differences, it is critical to recognize the heterogeneity within the Latino population, as well as the complex interplay of disease and non-disease factors, as well as genetic and environmental risks. Present findings underscore the need for the development of larger studies particularly focused on examining ethnic differences in NCI among HIV+ persons, that include different subgroups of Latinos and systematically consider and incorporate relevant sociocultural, biomedical, and genetic factors.
ACKNOWLEDGMENTS
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) is supported by awards N01 MH22005, HHSN271201000036C, and HHSN271201000030C from the National Institutes of Health. M.J. Marquine: K23MH105297. The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with the Johns Hopkins University, Mount Sinai School of Medicine, University of California, San Diego, University of Texas, Galveston, University of Washington, Seattle, Washington University, St. Louis and is headquartered at the University of California, San Diego and includes: Director: Igor Grant, M.D.; Co-Directors: Scott L. Letendre, M.D., Ronald J. Ellis, M.D., Ph.D., Thomas D. Marcotte, Ph.D.; Center Manager: Donald Franklin, Jr.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Terry Alexander, R.N.; Laboratory, Pharmacology and Immunology Component: Scott Letendre, M.D. (P.I.), Edmund Capparelli, Pharm.D.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Matthew Dawson; Virology Component: Davey M. Smith, M.D. (P.I.); Imaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Michael J. Taylor, Ph.D., Rebecca Theilmann, Ph.D.; Data Management Component: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman,; Statistics Component: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D.; Protocol Coordinating Component: Thomas D. Marcotte, Ph.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Johns Hopkins University Site: Justin McArthur (P.I.), Vincent Rogalski; Mount Sinai School of Medicine Site: Susan Morgello, M.D. (Co-P.I.) and David Simpson, M.D. (Co-P.I.), Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.), Will Toperoff, N.P..; University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Trudy Jones, M.N., A.R.N.P.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., Mengesha Teshome, M.D. The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government. There are no conflicts of interest to report.







