Se is an essential micronutrient that is required by dogs and cats to sustain the basic functions of life, such as antioxidant, immune and thyroid functions( 1 ). There is a large variability in Se content within and between raw materials used in pet foods( Reference Souci, Fachmann and Kraut 2 ). For example beef muscle contains on average 11·9 μg Se/MJ with a range of 6·6–22·0 μg Se/MJ, and whole grain wheat contains on average 1·7 μg Se/MJ, ranging between 0·8 and 4·6 μg Se/MJ( Reference Souci, Fachmann and Kraut 2 ). Moreover, the bioavailable Se fraction, i.e. the Se fraction that reaches the systemic circulation( Reference Stahl, van den Berg and Arthur 3 ), can also vary considerably between raw ingredients and processed pet foods, although information available is limited. Wedekind et al. ( Reference Wedekind, Cowell and Combs 4 ) used a chicken bioassay and found that Se bioavailability (BA) of pet food ingredients, relative to Na2SeO3, ranged from 9 % in mackerel to 38 % in beef spleen; these authors found the Se BA in canned dog and cat foods to be 25 and 17 %, respectively. Using the same methodology, subsequent studies reported( Reference Wedekind, Bever and Combs 5 ) greater relative Se BA of canned (30 %) and dry pet foods (53 %) respectively. Todd et al. ( Reference Todd, Thomas and Bosch 6 , Reference Todd, Thomas and Hendriks 7 ) reported the Se BA of two canned cat foods, as measured in Se balance studies in adult cats, to be 25·3 and 21·2 %, respectively. The reason for these variable and often low values is unknown.
There are several factors that might underlie variations in Se BA. An important factor is the chemical form of Se: organic Se gets absorbed in the intestine through active transport, whereas sodium selenite appears to get absorbed through diffusion( Reference Reasbeck, Barbezat and Weber 8 ). Due to competition for absorption sites, S and methionine (Met) can also influence Se absorption( Reference Stahl, van den Berg and Arthur 3 , Reference Wolffram, Berger and Grenacher 9 ). Moreover, Se BA might also be affected by factors such as fibre content( Reference Reeves, Leary and Gregoire 10 – Reference Smits, Veldman and Verstegen 14 ) and food processing( Reference Hendriks, Emmens and Trass 15 , Reference Todd 16 ), due to their effect on overall nutrient digestibility. Dietary fat is another potential factor, since it correlates negatively with the concentration of Se containing enzymes (glutathione peroxidase) in plasma of chickens( Reference Mutanen and Mykkänen 17 ), suggesting that dietary fat reduces the intestinal transport of Se. In vitro, dietary fat is also negatively associated with Se accessibility (Se Aiv), i.e. the dietary Se fraction in the filtrate after in vitro digestion, in milk( Reference Shen, Van Dael and Luten 18 ).
The aim of the present study was to identify dietary factors that affect the Se Aiv in commercial pet foods. Se Aiv was used as an estimate for Se BA. For this study, sixty-two pet foods were selected based on their variability in nutrient content and diet type.
Experimental methods
Diet selection
A set of fifty-four commercial (dog, n 44; cat, n 10) and eight unprocessed pet foods were sourced to meet a broad range of diet types (kibble (extruded), n 24; pellet (pressed), n 8; can (retorted), n 21; meat, n 9, (six raw/frozen and three steamed)) and nutrient composition (protein content, mean 34·4 % of DM, range 7·9–93·8 %; fat content, mean 20·7 % of DM, range 3·1–52·4 %; and fibre content, mean 4·0 % of DM, range 0·7–13·2 %). A broad range in nutrient composition was achieved based on listing label information of the parameters mentioned above. A small number of cat foods were included to broaden the range of dietary protein content. The number of diets per diet type was chosen to reflect the market availability of these diet types. Diet types differed by virtue of both their processing (heat and pressure treatment) and raw ingredients (cereals, fresh meat products, meat and/or bone meal). As the majority of pet food producers did not include Se content or speciation on the package or website, this information was not available for diet selection. It is possible that diet types differ in their typical amount and speciation of Se, but this was considered as an inherent trait of the diet types.
In addition to the commercially available diets, eight standard recipes of unprocessed diets (kibble, n 4; can, n 4) were included in the diet selection to assess the effect of processing. The unprocessed diets came from the same batch as the corresponding commercially available diet that was selected. Each of the sixty-two diets was analysed for DM, crude ash, crude protein (CP, 6·25 × N), crude fat, S, amino acid profile, total dietary fibre (TDF), gross energy and total Se (methodologies as described in ‘Chemical analyses’).
Sample preparation
Dry diets were ground over a 1 mm sieve in a centrifugal mill (Retsch ZM200; Retsch GmbH). The wet diets (canned and meat) were homogenised using a hand mixer (Philips HR1561), and then frozen at − 20°C until in vitro digestion. A portion of each wet diet was also freeze-dried and ground over a 1 mm sieve for chemical analyses.
In vitro digestion
Stomach and small intestinal digestion were simulated using a modified procedure described by Hervera et al. ( Reference Hervera, Baucells and Blanch 19 ). The method consisted of a 2 h pepsin (2000 International Pharmaceutical Federation (FIP) U/g, expressed as μmol of tyrosine equivalents liberated per min at 25°C, Merck article. no. 7190) incubation step at a pH of 2·0, and a second pancreatin (porcine pancreas grade VI, Sigma no. P-1750) incubation step for 4 h at a pH of 6·8. The method was scaled up tenfold to increase the amount of residue required for chemical analyses. The amount of fresh matter required that equated to 10 g of DM ( ± 0·75) was calculated, to account for the different moisture contents of each diet. De-mineralised water was added to all diets to achieve a moisture content of 85 %. A hypoxic environment was used by addition of CO2 for 30 s before every incubation step, to prevent Se oxidation, which might have an influence on the Se Aiv ( Reference Swanson, Patterson and Levander 20 – Reference Young, Nahapetian and Janghorbani 22 ). Glass covers were placed over the beakers during incubation, and pH was measured after every incubation step. As dietary fat content may influence Se Aiv ( Reference Shen, Van Dael and Luten 18 ), samples were not defatted before incubation, as was done in the original method, but 1·5 g bile extract (Sigma Porcine Bile Extract B8631; Sigma Aldrich) was added to the small intestine incubation step to mimic fat digestion. Type and amount of bile extract is based on publications of Hedrén et al. ( Reference Hedrén, Diaz and Svanberg 23 ), Clegg et al. ( Reference Clegg, Thondre and Henry 24 ) and Intawongse & Dean( Reference Intawongse and Dean 25 ). Due to the larger amount of the sample, the filtration step as described by Hervera et al. ( Reference Hervera, Baucells and Blanch 19 ) was not feasible. Filtration was performed with a Büchner funnel and a nylon cloth, based on a method of Jha et al. ( Reference Jha, Bindelle and Van Kessel 26 ), resulting in a digested (filtrate) and undigested (residue) fraction.
The filtrates were stored at − 20°C for total Se analysis. Residues were scraped from the cloth and dried overnight in an oven at 70°C and stored at room temperature. Residues were pooled per diet and ground to a powder before analyses for DM, crude ash and CP. In order to obtain at least 3 g of residue for analyses, in vitro digestion was repeated two to nine times according to the digestibility of the diets. Diets with an in vitro DM digestibility higher than 97 % were eliminated from the study, because more than ten repeats would have been necessary. With every new batch of buffers, quality controls (blanks and one of the pelleted study diets) were incubated for assessment of repeatability between runs. The CV for DM digestibility over the incubation runs was 0·5 %. Diet filtrates were corrected for total Se in the blank filtrates (2·31 μg/l, only containing de-mineralised water, buffers, pepsin, pancreatin and bile solutions).
Chemical analyses
Diet sample preparation for total Se analysis was adapted from Lavu et al. ( Reference Lavu, Willekens and Vandecasteele 27 ). Diets were prepared using closed-vessel microwave acid digestion. 1 g of each sample with an accuracy of 0·03 was weighed into a vessel, and 10 ml nitric acid 65 % was added. Vessels were closed and placed into a microwave (MARS 5 CEM) for 25 min in total (1200 W, 600 psi, 195°C for 13 min). Vessels were cooled and the contents were filtered over a no. 42 Whatman filter paper into a 50 ml flask, and diluted with ultra-pure water up to 50 ml. Filtrates resulting from the in vitro digestion were pooled per sample and transferred into a 20 ml centrifuge tube. Samples were centrifuged for 10 min at 10 000 g (Sorvall RC-5B Refrigerated Superspeed Centrifuge, SA-600 rotor; DuPont Instruments). Supernatants were pipetted into 1·5 ml Eppendorf tubes and stored at − 20°C until analysis. Samples were analysed for total Se using inductively coupled plasma-MS (Elan DRC-e; PerkinElmer), as described by Lavu et al. ( Reference Lavu, Willekens and Vandecasteele 27 ). Spikes with Certipur® selenium standard solution (SeO2 in HNO3, 1000 mg Se/l, Merck Art no. 119796) at a concentration of 200 or 400 μg/l were added as a quality control to four samples before and three samples after microwave destruction. Se recoveries of 87·7 % (sd 1·1 %) and 82·6 % (sd 4·5 %) were obtained when spikes were added after or before microwave destruction, respectively.
Diets and residues were analysed in duplicate for DM and crude ash, by drying to a constant weight at 103°C and combusting at 550°C, respectively. The Kjeldahl method (ISO 5983-1, 2005)( 28 ) was used to determine CP (6·25 × N). Crude fat in the diets was assayed according to the Berntrop-method (ISO 6492, 1999)( 29 ), and gross energy was analysed by bomb calorimetry. The microwave digests prepared for Se analyses in diets, were also analysed for S using inductively coupled plasma-optical emission spectrometry (ICP-OES, Iris intrepid II XSP, Thermo Fisher Scientific, Inc.) according to ISO 11 885 (2007)( 30 ). Diets were defatted by fat extraction with petroleum diethyl ether and extracted in line with the procedure of the Commission Directive (98/64/EC)( 31 ) for amino acid analyses; an HPLC method was used (Agilent 1100; Fluorescence Detector; ZORBAX eclipse AAA Rapid Resolution 4·6 × 150 mm, 3·5 micron column, PN 963400-902, Agilent Technologies) according to the method of Henderson et al. ( Reference Henderson, Ricker and Bidlingmeyer 32 ). TDF analyses were performed using the enzymatic-gravimetric method described by Prosky et al. ( Reference Prosky, Asp and Furda 33 )(AOAC 985.29).
Calculations
Se Aiv was calculated with the following formula:
 $$\begin{eqnarray} Se\,A_{iv}\,(\%) = (Se\,\,in\,the\,\,filtrate\,(\mu g/l)\times (dilution\,during\, in \, vitro \,digestion\,(ml)/1000))/sample\,weighed\,in\,for\, in \, vitro \,digestion\,(g\,DM)\times 100/Se\,in\,the\,diet\,(\mu g/g\,DM). \end{eqnarray}$$
$$\begin{eqnarray} Se\,A_{iv}\,(\%) = (Se\,\,in\,the\,\,filtrate\,(\mu g/l)\times (dilution\,during\, in \, vitro \,digestion\,(ml)/1000))/sample\,weighed\,in\,for\, in \, vitro \,digestion\,(g\,DM)\times 100/Se\,in\,the\,diet\,(\mu g/g\,DM). \end{eqnarray}$$
Digestibility coefficients were calculated with the formulae:
 $$\begin{eqnarray} Organic\,matter\,digestibility\,(\%) = 100 - (residue\,(g\,DM)\times (100 - ash\,in\,residue\,(\%\,DM))/100)\times 100/(sample\,weighed\,in\,for\, in \, vitro \,digestion\,(g\,DM)\times (100 - ash\,in\,the\,diet\,(\%DM))/100). \end{eqnarray}$$
$$\begin{eqnarray} Organic\,matter\,digestibility\,(\%) = 100 - (residue\,(g\,DM)\times (100 - ash\,in\,residue\,(\%\,DM))/100)\times 100/(sample\,weighed\,in\,for\, in \, vitro \,digestion\,(g\,DM)\times (100 - ash\,in\,the\,diet\,(\%DM))/100). \end{eqnarray}$$
 $$\begin{eqnarray} CP\,digestibility\,(\%) = 100 - (CP\,in\,the\,residue\,(g\,DM)\times 100/CP\,in\,the\,sample\,weighed\,in\,for\, in \, vitro \,digestion\,(g\,DM)). \end{eqnarray}$$
$$\begin{eqnarray} CP\,digestibility\,(\%) = 100 - (CP\,in\,the\,residue\,(g\,DM)\times 100/CP\,in\,the\,sample\,weighed\,in\,for\, in \, vitro \,digestion\,(g\,DM)). \end{eqnarray}$$
Statistical analyses
Data were analysed using the Statistical Analysis System (SAS) version 9.3 for Windows (SAS Institute, Inc.). Data were initially screened for linearity, normality, outliers and homogeneity of variance. The effect of diet type on Se Aiv was analysed using ANOVA (PROC GLM). Pairwise comparisons between diet types were tested at a total significance level of 0·05 using the Tukey–Kramer adjustment for multiple comparisons. The effect of the variables gross energy, CP, fat, TDF, S, Se, lysine (Lys), cysteine (Cys) and Met of the diets, and calculated variables CP (g/MJ), Met/CP, Met (g/MJ), Cys/CP, Cys (g/MJ), Lys/CP, Lys (g/MJ), Se (μg/MJ), DM digestibility, organic matter digestibility and CP digestibility on Se Aiv was analysed, per diet type, using regression (PROC REG). The effect of processing was analysed with a paired Student's t test. In all cases statistical significance was evaluated at P≤ 0·05.
Results
One commercial canned diet and one commercial extruded diet were eliminated for further analyses based on an in vitro DM digestibility >97 %. The inter-assay CV for DM digestibility over the incubation runs was 0·5 % and pH remained constant within each incubation step. Total Se in the blank samples was on average 2·31 μg/l, which was used to correct Se in filtrates. Se analyses had a recovery of 87·7 % as measured using spiked samples after microwave destruction, and 82·6 % when samples were spiked before microwave destruction. Table 1 provides an overview of the chemical composition and digestibility results of the diets per diet type. The high variation in CP, crude fat and TDF reflect the diet selection criteria. The large range in Se content of the diets was mainly due to two high values from raw meat diets (1173·2 and 1195·5 μg/100 g DM), which also increased the mean value over all diets (98·4 μg/100 g DM, results not shown). The median for dietary Se overall diets was 44·8 μg/100 g DM (results not shown).
Table 1 Chemical composition (g/100 g DM, except where specified), gross energy content (MJ/kg DM) and in vitro digestibility (%, w/w) of pet foods (n 60) per diet type (Mean values and standard deviations)

* Including four unprocessed diets.
Se Aiv differed among diet types (P <0·001; Table 2). Canned and steamed meat diets had lower Se Aiv than pelleted and raw meat diets. In all diet types, a large range of Se Aiv was found, but the range was the largest for kibble and canned diets. There was no significant correlation between Se Aiv and CP digestibility when all diet types were pooled (Fig. 1; r − 0·095, P =0·504). However, when diet type was considered, there was a positive correlation between Se Aiv and in vitro CP digestibility in the kibble diets (r 0·540, P =0·017) v. a negative correlation in the canned diets (r − 0·611, P =0·012).
Table 2 In vitro selenium accessibility of pet foods (%, w/w) per diet type (Mean values and standard deviations; number of animals, minimum and maximum values)
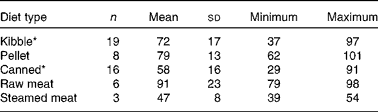
* Unprocessed diets are not included in the data.

Fig. 1 Correlations (r) between in vitro selenium accessibility (%, w/w) and crude protein digestibility (%, w/w) of pet foods: ![]() , all diets (n 52, r − 0·095);
, all diets (n 52, r − 0·095); ![]() and
and ![]() , kibble (n 19, r 0·540*);
, kibble (n 19, r 0·540*); ![]() , pellet (n 8);
, pellet (n 8); ![]() and
and ![]() , canned (n 16, r − 0·611*);
, canned (n 16, r − 0·611*); ![]() , raw meat (n 6); × , steamed meat (n 3). * Values show a significant correlation (P <0·05). Unprocessed diets are not included in the data.
, raw meat (n 6); × , steamed meat (n 3). * Values show a significant correlation (P <0·05). Unprocessed diets are not included in the data.
Se Aiv was not significantly correlated with any of the measured parameters in the pelleted diets. Raw meat diets showed a negative correlation between the amount of Se in the diet and the Se Aiv (in μg/g DM: r − 0·823, P =0·044; in μg/MJ: r − 0·843, P =0·035). For the steamed meat diets a positive correlation was found between Se Aiv and the amount of Cys per MJ (r 0·999, P =0·012). The correlations for the canned and kibble diets are displayed in Table 3. Among the kibble diets a negative correlation was found between Se Aiv and dietary CP, Lys, Cys, Met, S, ash, CP/MJ, Cys/MJ, and Lys/MJ. In canned diets Se Aiv was negatively correlated with dietary TDF.
Table 3 Correlations (r) between in vitro selenium accessibility (%, w/w) and parameters of kibble (n 19) and canned (n 16) diets in g/100 g DM, except where specified
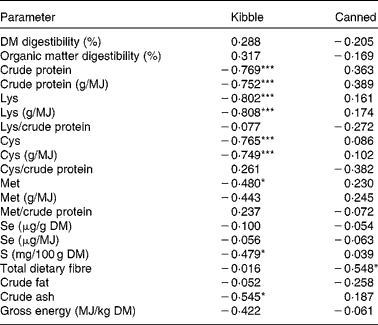
Values were significantly different: * P< 0·05, *** P< 0·001.
† Unprocessed diets are not included in the data.
The extrusion process did not affect Se Aiv (P =0·297, Table 4). In contrast, retorting almost halved Se Aiv (P =0·001). Dietary Se concentrations did not differ before and after processing (canned P =0·863, kibble P =0·335; results not shown). In the canned diets, the S content was higher before processing (P =0·028), and the TDF content tended to be higher after processing (P =0·085).
Table 4 In vitro selenium accessibility (%, w/w) in processed v. unprocessed canned and kibble diets (Mean values and standard deviations; number of animals, minimum and maximum values)
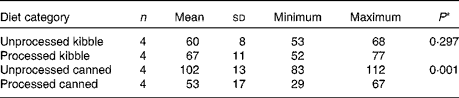
* P-value from paired Student's t test.
Discussion
To verify the factors that may contribute to the Se BA of pet foods, the present study examined a large range of commercially available pet foods differing in diet type and protein, fat and fibre content. There was a clear difference in Se Aiv between diet types (canned and steamed meat Se Aiv < pellet and raw meat Se Aiv) which may have been due to the way the diets were processed. A number of factors such as time, temperature, pressure and shear that influence the processing of pet foods may have contributed to the observed variation. Typically, raw meat diets and pelleted diets, which undergo no or relatively low heat treatment, showed a higher Se Aiv (91 and 79 %, respectively), compared to steamed meat and canned diets (47 and 58 %, respectively). These findings suggest a negative effect of heat processing on Se accessibility. This is in accordance with findings of Todd( Reference Todd 16 ), who found that non-processed inorganic Se in canned diets had a higher apparent Se absorption in cats (83·3 %) than processed inorganic Se (53·7 %)( Reference Todd 16 ). Canned diets had a higher Se Aiv before, compared to after processing, which also indicates an effect of processing on Se Aiv, which was not demonstrated in the kibble diets. The differences in the effect of processing on Se Aiv between canned and kibble diets may be due to variations in Maillard reactions caused by the different processing types. In the baking process, some Se may be lost because the Maillard reaction of selenomethionine and glucose yields volatile seleniferous compounds( Reference Tsai, Hiserodt and Ho 34 ). This did not appear to have a major influence on total Se in the present study, because total dietary Se did not differ before and after processing. Processing did decrease the S content in canned diets, which might be due to the conversion of dietary S to volatile compounds and volatilisation after opening the processed cans. Another possible effect in heat-processed diets is the occurrence of cross-linkages between amino acids, within and between proteins. Cross-linking reduces the rate of protein digestion by preventing enzyme penetration, or by masking the sites of enzyme attack( Reference Hurrel, Finot, Finley and Hopkins 35 ). Cys seems to be one of the most susceptible amino acids for cross-linking( Reference Singh 36 ), by which Se linked to Cys might be less available for digestion.
Hendriks et al. ( Reference Hendriks, Emmens and Trass 15 ) showed that heat processing a cat food at 121°C for 80–120 min did not destroy amino acids, but did decrease ileal apparent digestibility of the diet. In the present study, there was no impact on CP digestibility, when comparing the same diets pre- and post-processing. However, the effect of CP digestibility on Se Aiv differed between canned and kibble diets. In kibble diets, there was a positive relationship between CP digestibility and Se Aiv. This may indicate that when more protein is digested, more protein bound-Se becomes available. Similar findings were reported by Shen et al. ( Reference Shen, Van Dael and Luten 18 )in milk products. Interestingly, the opposite effect was found for canned diets. The lack of correlation between Se Aiv and Cys, Met and Lys suggests that they are not an explanation for the negative correlation between CP digestibility and Se Aiv in canned diets. Therefore, this correlation is likely due to a factor that was not accounted for in the present study.
The source of TDF in the canned diets could affect Se Aiv, because fibre is known to reduce nutrient digestibility( Reference Reeves, Leary and Gregoire 10 – Reference Smits, Veldman and Verstegen 14 ). TDF in canned diets in the present study was negatively correlated with Se Aiv, and tended to increas in canned diets after processing, compared to TDF in unprocessed diets (P =0·085). Azizah & Zainon( Reference Azizah and Zainon 37 ) also found an increase in TDF after roasting wheat, rice, mung beans and soyabeans at 80°C for 5 min. During heat treatment, fibre–protein complexes can be formed( Reference Dhingra, Michael and Rajput 38 ), which might be the cause for the negative impact on Se Aiv and the tendency of increase in TDF. A particular TDF component that is commonly used in canned diets is the soluble NSP guar gum. Guar gum increases viscosity and has been shown to decrease the digestibility of protein in diets fed to cats( Reference Harper and Siever-Kelly 39 ), which can be an additional explanation for the negative relationship between TDF and Se Aiv in canned diets, because protein bound-Se may then become unavailable. Choe & Kies( Reference Choe and Kies 40 ) reported an increase in faecal Se excretion by 14 % and a decreased Se balance (53 %) and whole blood glutathione peroxidase activity (9 %) in human subjects, when guar gum was supplemented to a standardised diet, although, in their study both Se and guar gum were not processed.
The difference in Se Aiv between the diet types might also be explained by a difference in the raw materials that are used in their manufacture, and consequently, that of the Se species in the diets. Supplemental Se in the form of sodium selenite or sodium selenate is commonly employed in dry pet foods (pellet and kibble), whereas in canned diets Se is mainly present in the form of selenomethionine from raw materials. Selenomethionine is absorbed through the same active transport mechanism as Met, whereas sodium selenite is absorbed through diffusion( Reference Reasbeck, Barbezat and Weber 8 ). Furthermore, reactions of sodium selenite with other components during storage may change its speciation, possibly to elemental Se( Reference Stadlober, Sager and Irgolic 41 ). Due to detection limit issues, Se speciation was not analysed in the present study; however, it may have an effect on the Se BA( Reference Todd, Thomas and Bosch 6 , Reference Swanson, Patterson and Levander 20 – Reference Young, Nahapetian and Janghorbani 22 , Reference Todd, Thomas and Hendriks 42 ).
Finally, the very high Se content in two of the raw meat diets could be due to the type of raw materials used. Tissues with a high rate of protein synthesis such as erythrocytes, skeletal muscle, pancreas, liver and kidney generally contain high amounts of Se( Reference Schrauzer 43 ).
The present study aimed to identify factors that influence Se Aiv. It is possible that the in vitro Se Aiv from the pet foods used in the present investigation may differ quantitatively from in vivo Se Aiv. Hervera et al. ( Reference Hervera, Baucells and González 44 ) confirmed that apparent in vivo CP digestibility is lower than that in vitro. The current European recommended allowance set by the European Pet Food Industry Federation( 45 ) and the adequate intake of Se set by the National Research Council( 1 ) for dogs and cats only take into account a fixed Se BA percentage, despite the large number of factors that influence the BA. The Association of American Feed Control Officials( 46 ) does not give any information on which BA factor they have used for the recommended allowance of Se in pet foods. The results of the present study can be used to help design in vivo studies to confirm and quantify the impact of diet composition and type on Se Aiv as found in the present study. This may enable the pet food industry to formulate diets that meet canine Se requirements by taking into account the Se BA for each specific diet type.
Conclusion
The present study found evidence for the hypothesis that diet type and processing do affect Se Aiv. Among other factors, CP digestibility is positively correlated with Se Aiv in kibble diets, but negatively in canned diets, and retorting strongly decreased Se Aiv. Further in vivo studies are warranted to confirm these in vitro findings and to verify if recommendations of Se inclusion levels in pet foods need to take such factors into account.
Acknowledgements
The authors express their special thanks to Joachim Neri of the Department of Applied Analytical and Physical Chemistry at the Faculty of Bioscience Engineering of Ghent University for total Se analyses and to Sofie Coelus of the Department of Food Safety and Food Quality at the Faculty of Bioscience Engineering of Ghent University for assistance during amino acid analyses.
The present study is part of a PhD project funded by the WALTHAM® Centre for Pet Nutrition (WCPN). Diets were provided for free by Royal Canin, Prins Petfoods, Happy Dog, InterPet Products, Arie Blok Diervoeding and FarmFood HE. The diets not provided by these firms were purchased from local stores. Apart from WCPN, none of these pet food companies had a role in the design, analysis or writing of this article.
The authors' responsibilities are as follows: M. v. Z, M. H., L. G. A., G. B., W. H. H. and G. P. J. J. conceived and designed the study; M. v. Z., L. G. A., G. P. J. J. and M. H. selected the study diets; M. v. Z. and G. B. conducted the in vitro experiment; M. v. Z., G. D. L. and B. D. M. were involved in the chemical analyses; M. v. Z. and Kl. Go. carried out the statistical analyses; M. v. Z., M. H., L. G. A., Ke. Gr. and G. P. J. J. interpreted the findings; M. v. Z. wrote the manuscript; M. H., L. G. A., Ke. Gr., G. B., W. H. H., G. D. L., B. D. M. and G. P. J. J. reviewed and edited the paper. All authors read and approved the final manuscript.
L. G. A. and Ke. Gr. were employed by the WALTHAM® Centre for Pet Nutrition at the time of the study. The other authors have no conflicts of interest to declare.








