Obsessive–compulsive disorder (OCD) is a serious and disabling disorder that often begins in childhood.Reference Heyman, Fombonne, Simmons, Ford, Meltzer and Goodman1, Reference Freeman, Garcia, Coyne, Ale, Prezeworski and Himle2 OCD causes significant disruption to the child's academic, family and social life and impairs the child's cognitive and psychosocial development.Reference Freeman, Garcia, Coyne, Ale, Prezeworski and Himle2–Reference O'Kearney, Anstey, von Sanden and Hunt4 Because OCD is often a chronic condition, it imposes substantial long-term economic and social burdens at both the individual and national levels.Reference Dupont, Rice, Shiraki and Rowland5, Reference McCrone, Dhanasiri, Patel, Knapp and Lawton-Smith6 The direct ($2.1 billion) and indirect costs ($6.2 billion) of OCD was estimated to be $8.4 billion a year in 1990 USD prices, accounting for 5.7% of the costs of all mental illnesses.Reference Dupont, Rice, Shiraki and Rowland5 In the UK, the total costs of anxiety disorders (service costs and lost earnings), including OCD, was projected to be £14.2 billion (at 2007 prices) in 2026.Reference McCrone, Dhanasiri, Patel, Knapp and Lawton-Smith6
Despite the well-documented effectiveness of cognitive–behaviour therapy (CBT) in treating this patient group,Reference Watson and Rees7 underdiagnosis and undertreatment are common, partly because of inequalities in access to treatment.Reference Clark, Layard, Smithies, Richards, Suckling and Wright8–Reference Lovell and Richards11 Following the call from the National Service Framework for Mental Health to improve accessibility of effective treatments for common mental health problems,12 alternative treatment modalities using current technologies such as telephones and computers are increasingly being researched and developed.Reference Mataix-Cols and Marks10, Reference Kaltenthaler, Brazier, De Nigris, Tumur, Ferriter and Beverley13 Evidence in adults with OCD suggests that telephone CBT (TCBT) shows promising advantages over face-to-face CBT in terms of reduced service and patient costs, and improved accessibility and convenience.Reference Taylor, Thordarson, Spring, Yeh, Corcoran and Eugster14–Reference Lovell, Cox, Haddock, Jones, Raines and Garvey16 This study reports the results of an economic evaluation of TCBT in a group of young people with OCD carried out alongside a randomised controlled trial.Reference Turner, Mataix-Cols, Lovell, Krebs, Lang and Byford17
Method
Hypothesis
The economic aim of the trial was to compare the cost-effectiveness of TCBT with face-to-face CBT in treating young people with OCD. We hypothesised that TCBT would be cost-effective at a service level compared with face-to-face CBT.
Trial design
Participants were recruited by referral from primary care general practitioners, and from mental health professionals within secondary and tertiary care settings within the National Health Service (NHS) to a specialist OCD clinic between 2008 and 2011. Information about the study was conveyed by word of mouth, letter to referring agencies, advertisements published on webpages of national OCD charities within the UK and by a research support organisation within the NHS (the Mental Health Research Network). The trial was registered on the International Standard Randomized Controlled Trial Number Register (ISRCTN27070832).
Inclusion criteria were: (a) primary OCD according to DSM-IV criteria,18 (b) a Children's Yale-Brown Obsessive-Compulsive Scale (CY-BOCS)Reference Scahill, Riddle, McSwiggin-Hardin, Ort, King and Goodman19 score of 16 or greater, indicating moderate to severe impairment, (c) aged 11 to 18 years; (d) medication free or on a stable dose of medication for a period of 12 weeks or longer, (e) no suicidal intent, drug or alcohol misuse, or psychotic symptoms, (f) no intellectual disability (also known in UK health services as learning disability) or pervasive developmental disability, (g) need and want CBT, and agreeable to randomisation, and (h) agreeable to parental involvement in treatment.
Exclusion criteria were: (a) current diagnosis of psychosis, current alcohol or substance misuse/dependence, (b) English too poor to engage in treatment, (c) severe disabling neurological disorder, (d) diagnosed global intellectual disability or pervasive developmental delay, and (e) characteristics interfering with completion of treatment within trial (for example a life-threatening or unstable medical illness).
After initial clinical assessments, eligible participants attended a second clinic appointment approximately 8 weeks later. Participants who remained symptomatic were randomised to CBT or TCBT in a 1:1 ratio using a computer-generated randomisation sequence prepared before the study commenced. There were no restrictions or matching. A repeated measures design was used and assessments were conducted immediately before treatment (i.e. baseline), immediately after treatment (i.e. post-treatment) and at follow-up points scheduled at 3 months, 6 months and 12 months post-treatment.
Ethics and consent statements
The study protocol was approved by the Joint South London and Maudsley/Institute of Psychiatry Research Ethics Committee (08/H0807/12). Written informed consent was obtained from all parents and participants over 16 years, and informed assent from participants under 16 years after a detailed description of the study had been given.
Interventions
Treatment consisted of 14 sessions of CBT, lasting approximately 60 min, delivered by six experienced clinical psychologists following a detailed treatment manual. Treatment was identical within conditions except that participants randomised to TCBT received all treatment sessions via telephone.
Sessions 1–2 consisted of psychoeducation, sessions 3–12 consisted of graduated exposure with response prevention (E/RP) and incorporated various cognitive strategies as appropriate, sessions 13–14 consisted of relapse prevention and ongoing symptom management (if required). The treatment protocol incorporated 10 min of parental discussion at the end of each treatment session. Homework E/RP tasks were assigned between sessions and participants were encouraged to complete daily E/RP.
The treatment protocol has been validated in previous trials.Reference Turner, Heyman, Futh and Lovell20, Reference Mataix-Cols, Turner, Monzani, Isomura, Murphy and Krebbs21 All 14 sessions were required to be completed within 17 weeks, allowing illness, missed appointments or holidays to be accommodated. Treating therapists received supervision by senior clinical psychologists who were specialists in CBT for OCD and all sessions (wherever possible) were audio-recorded. A random sample of n = 225 (25%) recorded sessions were audited and independently rated for integrity to protocol. The rate of adherence to the manual was 93% and there were no differences in adherence ratings between conditions.Reference Turner, Mataix-Cols, Lovell, Krebs, Lang and Byford17
Outcomes
Research assessments were completed in face-to-face interviews at baseline, post-treatment, 3 months, 6 months and 12 months post-treatment. The primary outcome measure for the economic evaluation was the CY-BOCS,Reference Scahill, Riddle, McSwiggin-Hardin, Ort, King and Goodman19 which was administered by an independent clinician masked to treatment condition. The CY-BOCS is a detailed semi-structured clinician-administered interview, incorporating a ten-item inventory of paediatric OCD symptoms severity, with an obsession severity score and a compulsion severity score. Using a five-point scale for each item (score 0 to 4), the total scores range from 0 to 40, where higher scores indicate worse outcomes. The CY-BOCS has demonstrated robust psychometric properties, with good internal consistency, convergent and divergent validity reportedReference Scahill, Riddle, McSwiggin-Hardin, Ort, King and Goodman19 and has been shown to respond to change.
Secondary analysis explored cost-effectiveness in terms of quality-adjusted life-years (QALYs), using the self-report EQ-5D-3L (5 dimensions, three levels) measure of health-related quality of life.Reference Brooks22 The EQ-5D is a generic questionnaire that assesses health-related quality of life on five dimensions including mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension has three levels, leading to a total of 243 possible health states, each of which is associated with a score used to calculate QALYs. The questionnaire also contains a visual analogue scale that enables participants to rate their current health state between 0 (worst imaginable health state) and 100 (best imaginable health state).
Being a generic health state measure, the EQ-5D allows policymakers to make comparisons, and most importantly, resource allocation decisions, across competing interventions within the same patient group or more broadly across different disease areas and populations. The EQ-5D is used extensively in economic evaluations of mental health disorders, despite a lack of evidence to support the relevance and validity of the measure in all mental health populations, particularly young populations. Psychometric assessment of the EQ-5D in young people with persistent major depression provides evidence of weak to moderate validity and responsiveness.Reference Byford23 However, further research is needed to test the generalisability of these results to other child and adolescent mental health populations.Reference Byford23 For this reason, the EQ-5D is used to supplement results from the primary cost-effectiveness analysis (CEA) in this study.
Costs
Economic data were collected in interview at baseline, post-treatment and 3-, 6- and 12-month follow-ups. The economic evaluation took a health and social care perspective but additionally included carer costs, which were expected to be influenced by treatment delivery method (telephone or face-to-face). Service use information was recorded using the Child and Adolescent Service Use Schedule (CA-SUS), which included hospital and community health and social services, and concomitant psychotropic medications. Travel costs and productivity losses of the primary carer were recorded using the Carer Service Use Schedule (CARER-SUS). Both schedules have been designed based on previous economic evaluations in child and adolescent mental health populations.Reference Byford, Barrett, Roberts, Clark, Edwards and Smethurst24, Reference Byford, Barrett, Roberts, Wilkinson, Dubicka and Kelvin25 All unit costs are reported in pounds sterling and were for the financial year 2010–2011, which was the most recent year over which the trial data were collected. No discounting was necessary because of the short duration of the trial.
A nationally applicable unit cost for CBT for young people of £115 per hour of face-to-face contact was applied to all CBT sessions young people attended in the trial.Reference Curtis26 Sessions that young people did not attend were assumed to have a zero cost on the basis that the clinician would be able to make use of the time available to do something else. This unit cost was based on estimates from a randomised controlled trial of interventions for adolescents with major depressionReference Byford, Barrett, Roberts, Wilkinson, Dubicka and Kelvin25 and includes the cost of supervision and relevant overheads (management, administrative, capital, estates etc.). Expert opinion was sought that confirmed that this unit cost was reasonable, given similarities in the grade and seniority of the therapists involved and the length of the sessions. In addition, data collected by therapists at each session, which included session length, confirmed that the average length of time spent delivering TCBT sessions was equal to that of face-to-face sessions (mean 62 min in both groups), hence the same cost was applied to both treatment conditions.
Costs of psychotropic medication were taken from the British National Formulary,27 and costs of hospital contacts, including in-patient and out-patient appointments, and accident and emergency attendance, were obtained from the National Schedule of Reference Costs.28 Contacts with community health and social services were taken from national publications.Reference Curtis26 Unit costs were multiplied by the corresponding service use data to generate total service costs per patient.
Productivity losses of the primary carers were valued using the human capital approach.Reference Drummond, Sculpher, O'Brien, Stoddart and Torrance29 This involves multiplying the individual's salary by hours of absence from work because of their child's illness. Travel costs of public transport, such as train and bus, were self-reported in the CARER-SUS. To estimate travel cost by private car, mileage between the clinic and home address was multiplied by the national average standing (basic costs of keeping the car for use on the road, including annual car tax, insurance, cost of capital used for the car and depreciation) and running (costs that depend directly on using the car, including fuel costs, parking and tolls, tyres, servicing and repair costs) cost per mile.30
Statistical method
The trial was designed to test non-inferiority in effects of the two competing interventions, so one may consider it legitimate to conduct a cost-minimisation analysis (CMA), which is an analysis method involving comparison of costs alone, given equal outcomes. However, CMA has been criticised for leading to biased results, causing overestimation or underestimation of the probability that treatment is cost-effective.Reference Dakin and Wordsworth31 For this reason, CEA is recommended, regardless of non-inferiority, for exploration of uncertainty surrounding the cost and effectiveness data and to help interpret the economic results.Reference Dakin and Wordsworth31, Reference Briggs and O'Brien32
Analyses were carried out on an intention-to-treat basis, with the primary objective of comparing the costs and cost-effectiveness of TCBT and face-to-face CBT at the final 12-month follow-up point. In order to best utilise all available data, multiple regression was used to impute missing total cost, QALY and CY-BOCS data in the main CEAs using the impute command in Stata. Factors included in the multiple regression were treatment arm and the following baseline characteristics: gender, age, CY-BOCS scores and EQ-5D scores. All analyses were adjusted for baseline characteristics including gender, age, CY-BOCS scores and EQ-5D scores using multiple regression techniques. Results from the smaller sample with full economic data were reported in sensitivity analyses to explore the robustness and validity of the imputed data.
Results from CEAs were expressed in terms of incremental cost-effectiveness ratios (ICERs), defined as the difference in mean costs divided by the difference in mean effects, calculated using the net benefit approach.Reference Stinnett and Mullahy33 Non-parametric bootstrapping (random and repeat re-sampling from the costs and outcome data) was used to generate a large number of sets of expected incremental costs and effects for both treatment groups (1000 replications).Reference Drummond, Sculpher, O'Brien, Stoddart and Torrance29 The proportion of these that were greater than zero gives the probability that TCBT is the optimal choice, i.e. cost-effective compared with face-to-face CBT, subject to a range of thresholds that represent decision-makers’ willingness-to-pay for a unit improvement in outcome.
These probabilities were used to generate cost-effectiveness acceptability curves (CEACs), which are the recommended alternative to confidence intervals around ICERs to overcome problems associated with ratio estimators in standard statistical methods.Reference Briggs and Fenn34, Reference Fenwick and Byford35 CEACs account for the uncertainty surrounding the estimates of expected costs and outcomes, and act as a useful tool to inform decision-makers on the probability that an intervention will be cost-effective at different thresholds.Reference Fenwick and Byford35 Cost-effectiveness planes were used to illustrate the distribution of bootstrapped mean differences in costs and outcomes.
Sensitivity analyses were carried out to investigate the robustness of the economic evaluation, and to account for uncertainty that exists around some of the input parameters and assumptions. First, as noted above, a complete case sensitivity analysis was undertaken to explore the validity of the imputation method used for dealing with missing data. Second, we considered the ongoing debate about the inclusion of various non-healthcare related costsReference Stant, Ten Vergert and den Boer PCAM36 and repeated the economic analyses by employing the NHS and personal social services perspective preferred by the National Institute for Health and Care Excellence (NICE) in guideline development, which involved the removal of all costs borne by the carers. Finally, we considered the hypothesis that face-to-face CBT overhead costs may be higher than TCBT overhead costs as a result of the need for potentially more expensive clinical space, compared with office space, administrative costs related to the booking of clinical space, and time spent preparing the clinic space. Whereas the main analysis was conservative, assuming equal overheads for TCBT and face-to-face CBT, the sensitivity analysis reduced the cost of TCBT by 10%.
Results
Participants
In total, 72 participants were recruited into the trial with 36 randomised to TCBT and 36 to face-to-face CBT. Baseline demographic and clinical characteristics of the two treatment groups are shown in supplementary Table 1 available at https://doi.org/10.1192/bjo.2018.73. The current paper focuses on the economic results; further detail on participant characteristics and clinical results are reported elsewhere.Reference Turner, Mataix-Cols, Lovell, Krebs, Lang and Byford17
At final 12-month follow-up, full clinical data was available for 27 (75%) participants in the CBT group and 25 (69%) participants in the TCBT group and full economic data was available for 21 (58%) in the CBT group and 22 (61%) in the TCBT group. Comparison of baseline characteristics between those with available and those with missing data revealed a significant difference in baseline CY-BOCS scores (P = 0.033), with those missing having poorer baseline scores, but no differences in any other variables.
Outcomes
For the primary clinical outcome, CY-BOCS, at all assessment points through to 6-month follow-up, the difference between conditions was non-significant and the 95% confidence interval lies below the five-point difference margin, indicating that TCBT was not inferior to face-to-face CBT. For the 12-month follow-up point, the difference remained non-significant but non-inferiority of TCBT could not conclusively be demonstrated as the 95% confidence interval included the margin of difference.Reference Turner, Mataix-Cols, Lovell, Krebs, Lang and Byford17 All secondary measures included in the clinical trial confirmed non-inferiority at all assessment points.Reference Turner, Mataix-Cols, Lovell, Krebs, Lang and Byford17
Table 1 reports the results for the EQ-5D. Both groups show improvements in health-related quality of life over time but there were no significant differences between the groups.
Table 1 Outcomes and costs by treatment groups
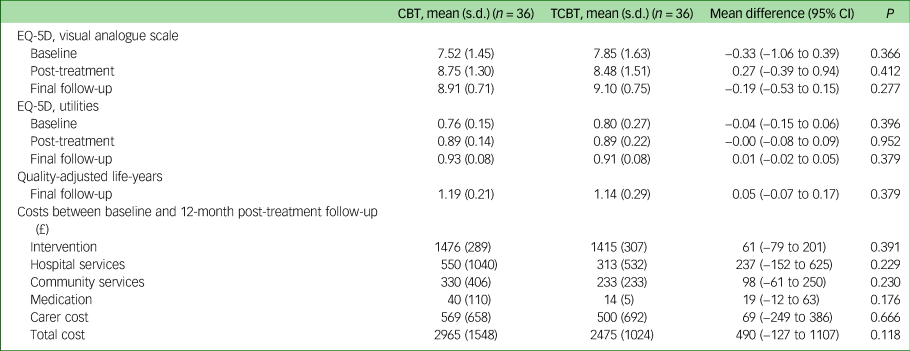
CBT, cognitive–behavioural therapy; TCBT, telephone CBT.
Resource use
Mean number of service contacts for participants with full economic data over the treatment and 12-month follow-up period are shown in supplementary Table 2. There were few differences in use of services between the two groups, although participants in the face-to-face CBT group had slightly more out-patient appointments and more contacts with community health and social services than those in the TCBT group, particularly general practitioner and clinical psychologist contacts. Despite the different modes of delivery, intervention attendance was similar in each group (12.3 sessions in the face-to-face CBT group versus 12.8 sessions in the TCBT group out of a possible 14 sessions).
Total costs
Total costs per participant over the treatment and 12-month follow-up period are reported in Table 1. Intervention costs were similar in the two groups, as a result of the similar number of sessions attended (mean costs in the CBT group £1476, s.d. = 289; mean cost in TCBT group £1415, s.d. = 307). On average, total cost per participant in the face-to-face CBT group was £2965 (s.d. = 1548), which was £490 more costly than the TCBT group (£2475, s.d. = 1024). This difference was not statistically significant (P = 0.118). For both groups, the CBT interventions accounted for the greatest proportion of the total costs (53%), followed by carer costs (20%) and hospital services (16%).
Carer costs were relatively low and differed little between groups. Only a small proportion of parents reported taking any time off work (n = 13 at the post-treatment follow-up point) and travel costs reported in the face-to-face CBT group were small.
CEA
Figure 1 shows a scatterplot of the bootstrapped replications for incremental cost and incremental CY-BOCS score for TCBT on the cost-effectiveness plane. Because lower CY-BOCS scores are associated with improved outcomes, the standard cost-effectiveness plane is reversed (outcomes deteriorate when moving from left to right on the x-axis). Compared with TCBT, face-to-face CBT has higher bootstrapped mean cost per participant (£697) and slightly better bootstrapped mean effects on the CY-BOCS (−0.07367), giving rise to an ICER of £9461 per unit reduction (improvement) in CY-BOCS. In other words, a one-point improvement in CY-BOCS can be realised if decision-makers are willing to pay an additional £9461 for face-to-face CBT.
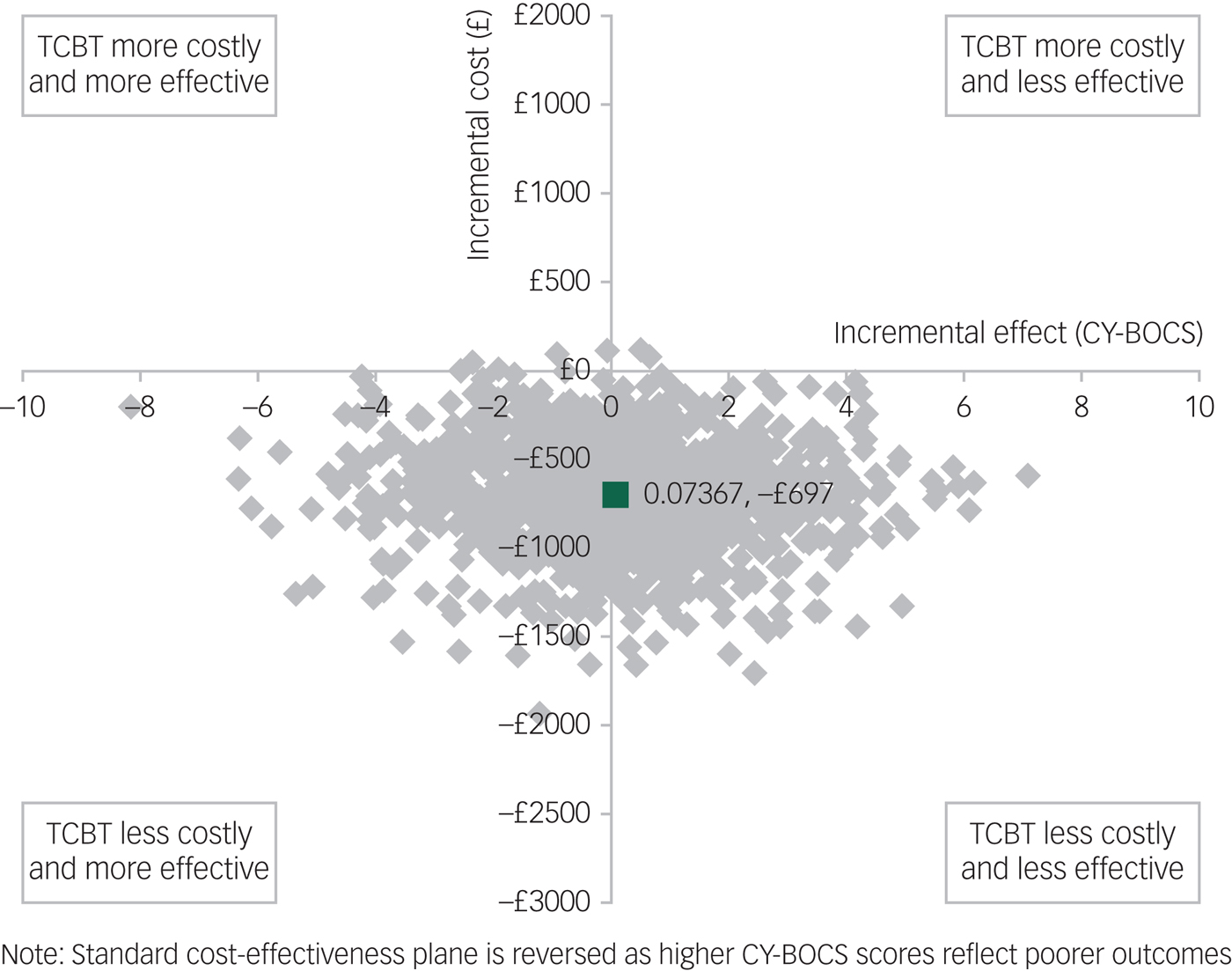
Fig. 1 Bootstrapped mean differences in costs and effects in terms of the Children's Yale-Brown Obsessive-Compulsive Scale (CY-BOCS) for telephone cognitive–behavioural therapy (TCBT) compared with face-to-face CBT.
It should be noted that, although the cost-effectiveness results presented are based on a unit improvement in CY-BOCS, a clinically meaningful reduction in symptoms has been suggested to be at least a 35% reduction in CY-BOCS score.Reference Mataix-Cols, Fernandez de la Cruz, Nordsletten, Lenhard, Isomura and Blair Simpson37 Taking the minimum for inclusion in this study of a CY-BOCS score of 16, a 35% reduction would be six points. Thus, while the incremental cost per unit improvement in CY-BOCS is £9461, willingness to pay for a clinically meaningful improvement would need to be a minimum of £56 766 for face-to-face CBT to be considered cost-effective compared with TCBT using the CY-BOCS. This minimum would increase with increasing severity of impairment at baseline. For example, taking the average baseline score for trial participants of approximately 25, a 35% reduction would be equivalent to approximately nine points on the CY-BOCS and thus willingness to pay for a clinically meaningful improvement would need to be at least £85 149 per participant for face-to-face CBT to be considered cost-effective compared with TCBT.
The results for QALYs are shown in Fig. 2, where, in this case, lower scores are associated with poorer outcomes so the standard cost-effectiveness plane applies (outcomes improve when moving from left to right on the x-axis). Face-to-face CBT was again associated with higher bootstrapped mean cost per participant (£697) and improved bootstrapped mean effects in QALY (0.0794) compared with TCBT, giving rise to an ICER of £8778 per unit increase in QALY. Thus, for both measures of outcome, TCBT is associated with lower costs but also slightly poorer outcomes.
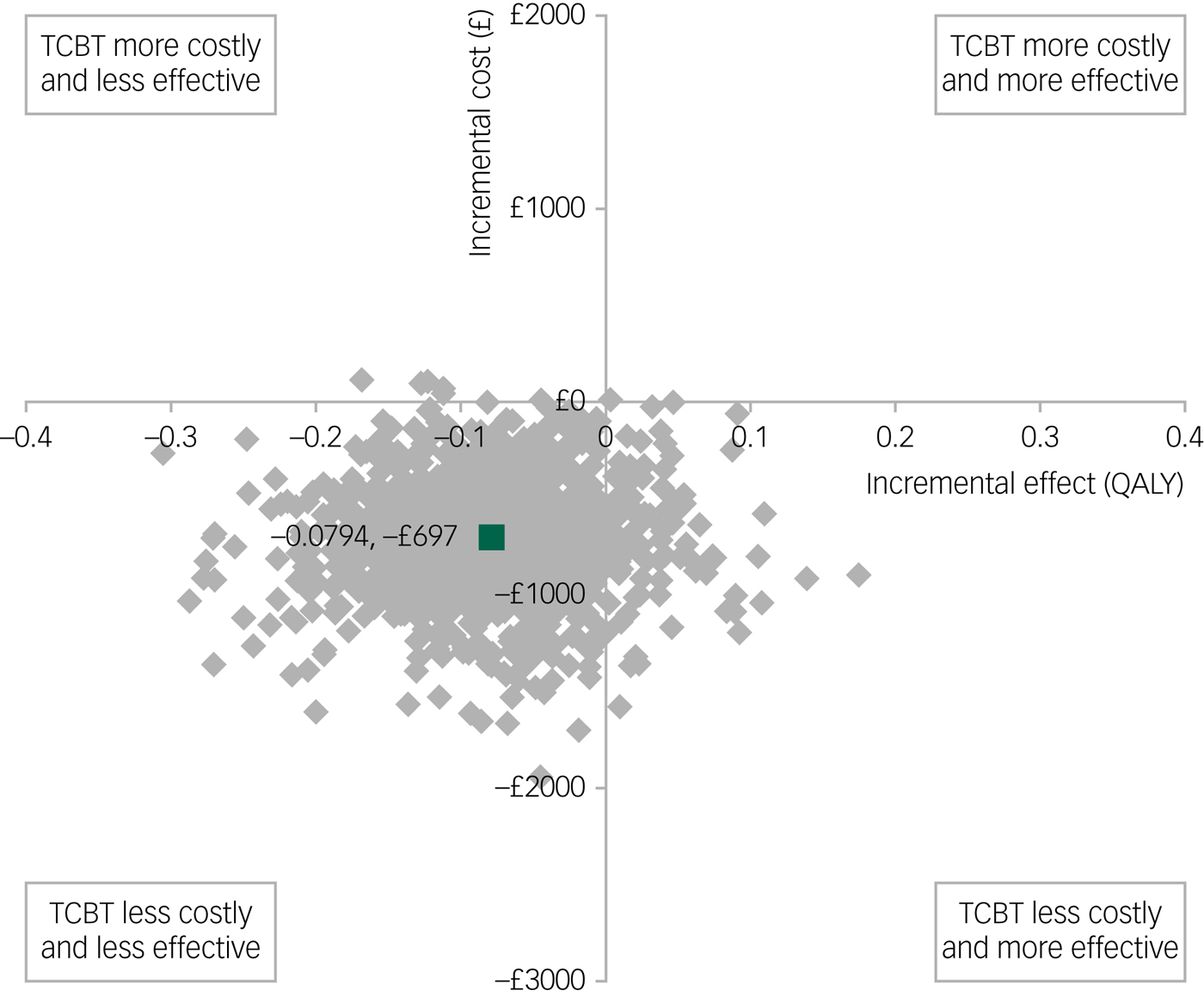
Fig. 2 Bootstrapped mean differences in costs and effects in terms of quality-adjusted life-years (QALYs) for telephone cognitive–behavioural therapy (TCBT) compared with face-to-face CBT.
The CEAC shown in Fig. 3 illustrates that at the standard NICE (2008) willingness-to-pay threshold of £20 000 per QALY, the probability of TCBT being the dominant option is 26% and thus the probability of face-to-face CBT being cost-effective compared with TCBT is 74%. There is no clear consensus threshold for a unit improvement in CY-BOCS. Figure 3 suggests that at low levels of willingness to pay (£4000 and below), there is a higher probability of TCBT being the cost-effective option. However, as willingness to pay rises above this amount, the probability of either intervention being cost-effective is around 50%.
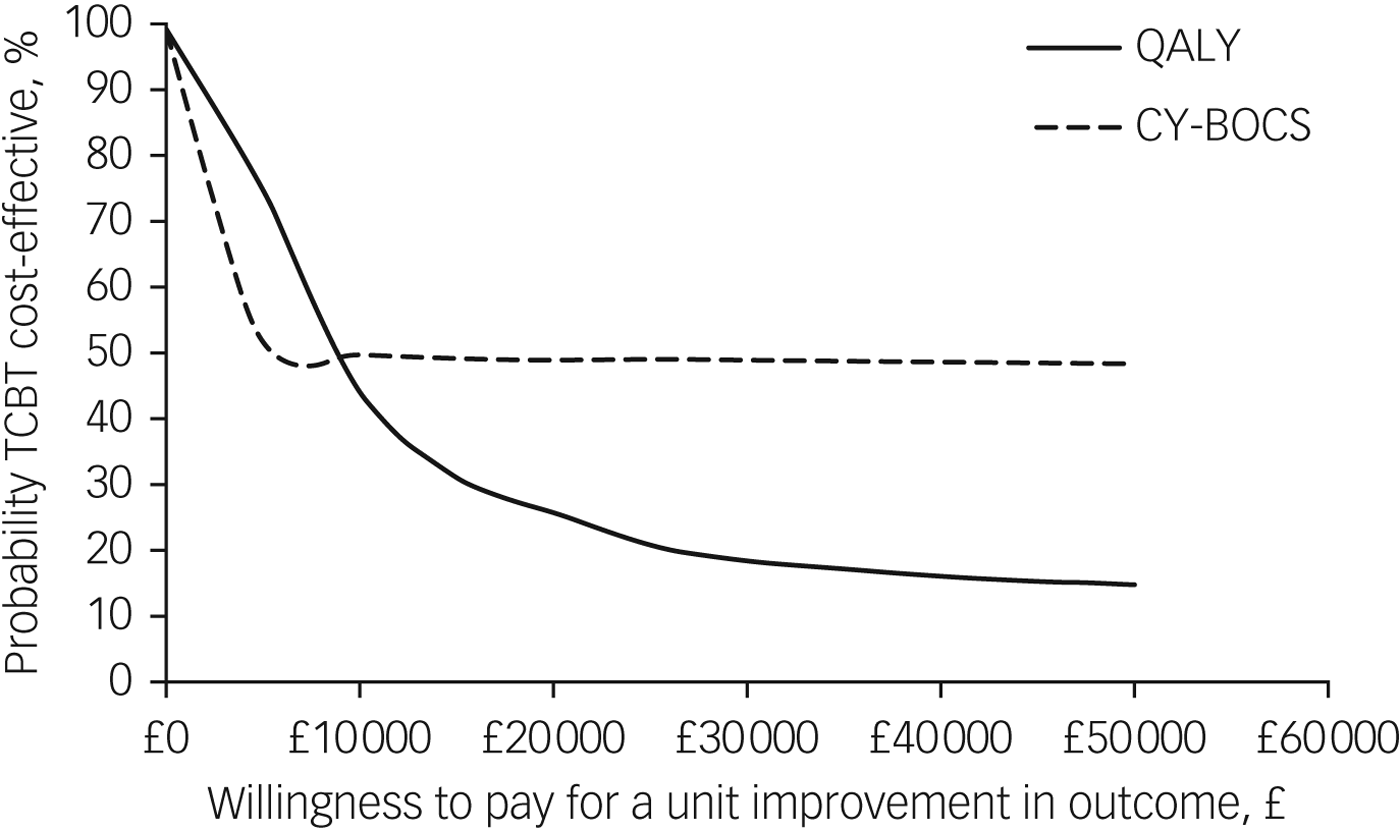
Fig. 3 Cost-effectiveness acceptability curves showing the probability that telephone cognitive–behavioural therapy (TCBT) is cost-effective compared with face-to-face CBT.
Sensitivity analyses
Sensitivity analyses, reported in supplementary Table 3, did not alter the overall findings of the CEAs. The complete case and the narrower NHS/social services perspective reduced the mean cost per participant in each group, but the difference between groups remained very similar (£490 primary analysis; £542 complete case analysis; £421 narrow perspective) and these differences remained non-significant. Differences in costs became statistically significant between the two groups when the cost of TCBT was reduced by 10% to £104 per session (mean difference £631, P = 0.044). However, this did not alter the cost-effectiveness results.
Discussion
Main findings
The results of this economic evaluation, and the associated clinical trial,Reference Turner, Mataix-Cols, Lovell, Krebs, Lang and Byford17 suggest there is strong evidence to support the clinical non-inferiority of TCBT compared with face-to-face CBT for young people with OCD, and no evidence to suggest any statistically significant differences in total cost per participant between the two groups, albeit with lower observed costs in the TCBT group.
In terms of cost-effectiveness, although our secondary CEA based on QALYs favoured face-to-face CBT, our primary CEA based on the CY-BOCS was less clear. This analysis suggests that TCBT may be the preferred option at low levels of willingness to pay for additional improvements in CY-BOCS scores, whereas at higher levels of willingness to pay, the probability of either intervention being cost-effective is around 50%.
Taking into consideration evidence to suggest that TCBT is clinically non-inferior to CBT, evidence from our primary CEA to suggest TCBT has a 50% or higher probability of being cost-effective compared with face-to-face CBT, and potential cost-savings for TCBT (which were statistically significant in sensitivity analysis hypothesising a 10% reduction in the cost of TCBT given the potential for lower overhead costs), TCBT presents an effective alternative for young people with OCD who are unable or unwilling to access face-to-face CBT.
Limitations
There are a number of limitations of the work presented. First, there is currently no evidence of the validity or responsiveness of the EQ-5D in young people with OCD, and some evidence to suggest that the youth version of the EQ-5D (EQ-5D-Y) is not correlated with clinical outcomes in such populations,Reference Lenhard, Ssegonja, Andersson, Feldman, Ruck and Mataix-Cols38 so the sensitivity of the EQ-5D to clinically important changes is in doubt. Lack of sensitivity of broadly focused outcome measures compared with disease-specific measures has been demonstrated in a previous paediatric OCD population,Reference Lee, Jones, Goodman and Heyman39 so this is a real possibility in the current sample. However, both measures of effect showed consistent improvements over the post-treatment and follow-up periods and there were no significant between-group differences. This suggests that the EQ-5D may be a relatively robust and sensitive measure of effect in this patient group, although more research is required to substantiate this.
Sample sizes, estimated for the purpose of the primary clinical question,Reference Turner, Mataix-Cols, Lovell, Krebs, Lang and Byford17 were small, and thus the economic evaluation may have been underpowered. We attempted to minimise the further impact of data loss through imputation of missing data and, although the imputation method was robust in sensitivity analysis, results of the study still require careful interpretation because of the small sample sizes and large amount of missing economic data at the 12-month follow-up. Significant differences in the baseline CY-BOCS scores (P = 0.033) were found between those with missing data and those with full economic data, with those missing having marginally higher symptom scores at baseline, although this was less than two points on the CY-BOCS scale, which is unlikely to be clinically meaningful. No significant differences were detected in any other baseline characteristics.
Data collected at each therapy session confirmed that there were no differences in terms of length of sessions, grade of therapists and thus costs, between TCBT and face-to-face CBT, and that CBT sessions in young people with OCD are comparable with those with major depression, which is what the unit cost applied was based on. However, a more detailed micro-costing (bottom-up) in future research may still be valuable as it would provide more accurate estimates of treatment costs. In an attempt to compensate for the lack of a micro-costing approach, and the hypothesis that overhead costs associated with TCBT may be lower than those for face-to-face CBT, the cost of TCBT was reduced by 10% in sensitivity analysis, and the cost results, although not the cost-effectiveness results, were found to be sensitive to this parameter.
In terms of generalisability, all treatments within the trial were delivered by NHS therapists to NHS patients aged 11 to 18 with a clinical diagnosis of OCD. However, this was a single site study based in a specialist clinic in London, so generalisability across the UK or other countries is not proven.
Finally, the trial enabled comparisons to be made in terms of improving access to treatment by attempting to remove geographical, social or financial barriers between the two delivery modes for CBT in young people with OCD. It was not, however, designed to quantify the effect of TCBT on commonly long NHS waiting lists that result from therapist shortage.Reference Mataix-Cols and Marks10 As with greater access comes greater demands, improvement in access via waiting list reduction could only be achieved in this patient group if TCBT is proven to save therapists’ time, and if the treatment could be delivered by more therapists through increased training and effective dissemination of clinical and training materials.Reference Layard9 Thus, the implications for the NHS in terms of availability of resources to provide such a service and the impact of such provision on the NHS waiting list remain unclear. The full economic impact of TCBT in reducing waiting time or delayed access is unknown and further research is needed. Similarly, the analysis does not take into consideration resource implications in terms of therapist location, with face-to-face CBT requiring therapy rooms that are often in great demand, compared with TCBT, which can take place at a desk.
Policy implications
There is no evidence to suggest that TCBT is cost-effective compared with face-to-face, clinic-based CBT in this study, particularly in terms of QALYs, and therefore TCBT may not be the preferred strategy of policymakers by default. However, taking into consideration the non-inferiority of effects, the potential for cost-savings and the potential to overcome barriers to treatment, it should be recognised that TCBT has a place in supporting the UK government's initiative to increase accessibility of effective treatments for OCD12 and should be offered where access to specialist clinic-based CBT is limited or where patient or family preference for telephone therapy is high.
It is also important to consider the generalisability of the results and the context within which the study was undertaken. The study is not able to come to conclusions about the cost-effectiveness of TCBT for young people who were excluded from the study including those with mild impairment, with current alcohol or substance misuse or dependence, with psychosis or psychotic symptoms, or with chaotic medication use. In addition, the study is not able to come to any conclusion about the cost-effectiveness of TCBT in more rural settings, where specialist clinic-based services are likely to be particularly inaccessible.
Further research priorities in this field include (a) comparison of the cost-effectiveness of TCBT with other less resource-intensive modes of delivering evidence-based treatments, such as computerised or internet-based CBT for OCDReference Kaltenthaler, Brazier, De Nigris, Tumur, Ferriter and Beverley13 or therapist supported self-help programmes,Reference Mataix-Cols and Marks10 (b) investigation of the cost-effectiveness and feasibility of TCBT delivered by other health professionals within the community setting, such as CBT-trained nurses (mental health nurse or practice-based nurse), or generic Child and Adolescent Mental Health Service therapists, and (c) replication of the study with a larger sample of participants recruited from multiple sites, including both rural and urban sites.
Funding
This research was funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (grant reference number PB-PG-0107-12333). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Acknowledgements
The authors wish to thank the NHS Mental Health Research Network for assistance with recruitment of participants. The authors wish to thank Chloë Volz, CPsychol, Kristina Hilton, DPsy, Jacinda Cadman, MClinPsy, Holly Diamond, DPsy, Amy Shayle, DPsy, Amita Jassi, DPsy and Caroline Stokes, DPsy, of the South London and Maudsley NHS Foundation Trust, for their contributions to this project. All authors had full access to the data. Data available from the corresponding author upon request.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2018.73.







eLetters
No eLetters have been published for this article.