Human epidemiological and experimental studies have provided considerable evidence to suggest that nutritional imbalance and metabolic disturbances during critical developmental time windows have persistent effects on the health of the offspring and may be responsible for in utero programming of common disorders such as obesity, diabetes and hypertension in adult life(Reference Barker1–Reference Alexander3). Moderate and marginal Zn deficiency observed in pregnant women could be a nutritional insult to fetal and postnatal development(Reference Vallee and Falchuk4, Reference Sandstead5). Moreover, this micronutrient could program adult pathologies by an epigenetic mechanism since it controls methylation reactions and epigenetic modifications of DNA and histones(Reference Keen, Hanna and Lanoue6, Reference Maret and Sandstead7).
Zn is found in a wide variety of foods such as whole-grain cereals, legumes, meat, chicken and fish. However, moderate Zn deficiency is mostly due to nutritional imbalances in those stages of life when Zn requirements are increased, such as postnatal growth and pregnancy(Reference Sandstead5, Reference Sandström8).
We had previously reported that moderate Zn restriction during fetal life, lactation and/or post-weaning growth of rats induces an increase in arterial blood pressure (BP) and impairs renal function in adult life. These alterations were associated with an increase in renal oxidative stress, activation of renal apoptosis and fibrosis, and a reduction in the renal filtration surface area(Reference Tomat, Inserra and Veiras9). Moreover, we also reported that animals exposed to low Zn intake only since weaning and up to adult life showed an impairment in the vascular and renal NO system(Reference Tomat, Weisstaub and Jáuregui10, Reference Tomat, Costa and Girgulsky11). Up to now there has been no evidence about the influence of Zn deficiency during fetal life and lactation on this system.
It has been demonstrated that NO is an important factor in the regulation of blood flow and BP in mammals since it exerts a basal tonic relaxing action on systemic vasculature(Reference Ribeiro, Antunes and Nucci12, Reference Navarro, Sanchez and Saiz13). Moreover, NO plays a prominent role in the homeostatic regulation of glomerular, vascular and tubular functions in the kidney(Reference Kone14, Reference Majid and Navar15). Therefore, it is likely that NO system impairment could be involved in the increase in BP levels and in the reduction in glomerular filtration rate (GFR) previously observed in rats exposed to Zn restriction during fetal and postnatal development(Reference Tomat, Inserra and Veiras9).
It is known that NO is a small gaseous molecule produced in vivo by NO synthase (NOS). The NOS family consists of three isoforms: neuronal (nNOS), endothelial (eNOS) and inducible (iNOS), which are expressed in many tissues, including endothelium and vascular smooth muscle and kidney. All NOS isoforms contain a zinc thiolate (ZnS4) cluster that plays an essential role in the catalytic activity of this enzyme by maintaining stability of the dimer interface and integrity of the tetrahydrobiopterin binding site(Reference Kone14, Reference Zou, Shi and Cohen16).
Moreover, Zn deficiency could be a cardiovascular risk factor associated with alterations in lipoprotein, cholesterol and TAG metabolism. It has been reported that severe Zn deficiency in adult rats induces increased fatty acid de novo synthesis, decreased fatty acid oxidation and reduced activity of lipoprotein lipase, which may contribute to an increase in TAG concentration(Reference Kettler, Eder and Kettler17, Reference Daniel and Dieck18). Other authors have shown that severe Zn deficiency leads to a decrease in total and HDL-cholesterol, an increase in VLDL- and intermediate-density lipoprotein-cholesterol, or no changes in cholesterol lipoproteins(Reference Fischer, Giroux and Belonje19–Reference Faure, Roussel and Richard22).
Therefore, the aim of the present study was to investigate whether moderate Zn restriction during early growth periods, fetal life and lactation would induce impairment in the vascular and renal total NOS activity and on the expression of the different NOS isoforms associated with high arterial BP levels and functional renal alterations in adult life. Moreover, we evaluated whether Zn deficiency induces alterations in plasma lipid profile, which is considered a cardiovascular risk factor. We also investigated if the effects of Zn restriction in utero and during lactation persisted into adult life, even when a Zn-replete diet was provided after weaning.
The present study used the same design as our previous study, but it was an independent experiment. Offspring, derived from a second set of dams, were fed similar diets and followed an identical experimental protocol as those used previously(Reference Tomat, Inserra and Veiras9).
Materials and methods
Animals and study design
Female Wistar rats from the breeding laboratories of the Facultad de Farmacia y Bioquímica (Universidad de Buenos Aires, Argentina) were mated by exposure to Wistar males during 1 week. Immediately afterwards, female rats were randomly fed either a moderately Zn-deficient diet (L; 8 parts per million (ppm)) or a control-Zn diet (C; 30 ppm) during the pregnancy and lactation periods. After birth, offspring were weighed and no more than nine rat pups remained with each mother. After weaning, male offspring from each L mother were randomly assigned to low- (8 ppm; Ll; n 10) or control- (30 ppm; Lc; n 10) Zn diets during 60 d. Meanwhile, male offspring from C mothers were fed a control diet (Cc).
Diet composition is outlined in Table 1. Both diets had the necessary nutrients, except Zn content, to meet rat requirements for the periods of pregnancy, lactation and growth according to American Institute of Nutrition (AIN)-93 recommendations(Reference Reeves, Nielsen and Fahey23).
Table 1 Composition of the experimental diets (g/kg diet)

* Nestlé Argentina S.A., contaning 85·1 g protein/100 g.
† Mineral mix composition according to American Institute of Nutrition (AIN)-93 diet(Reference Reeves, Nielsen and Fahey23).
‡ Composition identical to mineral mix but without zinc chloride.
§ Vitamin mix composition according to AIN-93 diet(Reference Reeves, Nielsen and Fahey23).
Animals were allowed food and deionised water ad libitum. All laboratory material was previously washed with nitric acid (20 %) and deionised water. Male offspring were housed separately in plastic cages in a humidity- and temperature-controlled environment, with a 12 h light–dark cycle.
At day 56 after weaning, rats were fasted overnight, tail blood was collected and centrifuged and serum samples were stored at − 20°C until the moment of lipid analysis. TAG, total cholesterol and HDL-cholesterol were measured by standardised enzymic methods, under good quality-control conditions (Selectra 2 Vitalab analyser, multiple calibrator CEFAS; Roche Diagnostics GmbH, Mannheim, Germany). VLDL was determined in the supernatant fraction after LDL selective precipitation(Reference Assman, Jabs and Kohnert24). Non-HDL-cholesterol, as an indicator of apoB-containing lipoproteins, was calculated as the difference between total cholesterol and HDL-cholesterol. Values for plasma lipids are fasting measurements in order to standardise results, given the great variability in these parameters.
Systolic BP (SBP) was measured indirectly in awake animals by the tail-cuff method using a Grass polygraph (model 79H; Grass Instrument Co., Quincy, MA, USA) at 60 d after weaning, as described previously(Reference Tomat, Weisstaub and Jáuregui10).
At day 60 after weaning, blood samples were collected from rats' tails and animals were placed in plastic metabolism cages in order to collect 24 h urine and faeces samples. Urine volume was determined gravimetrically. Plasma and urinary creatinine levels were measured by a colorimetric method (Wiener Laboratory, Rosario, Argentina). Creatinine clearance was calculated to estimate GFR. Nitrites and nitrates (NOx) were measured in urine samples by a colorimetric method, according to the procedure described by Verdon et al. (Reference Verdon, Burton and Prior25). Zn concentration in plasma, faeces, kidneys and diet was determined using an atomic absorption spectrophotometer (Varian Spectrophotometer Spectr AA-20, air acetylene flame, 0·5 nm slit, wavelength of 213·9 nm; Perkin Elmer Corp., Norwalk, CT, USA), as described previously(Reference Tomat, Weisstaub and Jáuregui10, Reference Tomat, Costa and Girgulsky11).
At the end of the dietary treatment, rats were killed by cervical decapitation and the kidneys, the thoracic aorta artery and a segment of small intestine were immediately removed.
Nitric oxide synthase activity
NOS activity was measured in the thoracic aorta artery and in the renal cortex of Cc, Lc and Ll animals with l-[U-14C]arginine as the substrate (specific activity: 13 319 MBq (360 mCi)/mmol; Perkin Elmer Life and Analytical Sciences, Boston, MA, USA) using a liquid scintillation counter (Wallac 1414 WinSpectral; EG&G Company, Turku, Finland), as described previously(Reference Costa, Loria and Elesgaray26).
NADPH diaphorase activity
The thoracic aorta artery, the kidney and a segment of the small intestine from Cc, Lc and Ll rats were processed by the NADPH diaphorase (NADPH-d) histochemical method, as described previously(Reference Tomat, Weisstaub and Jáuregui10, Reference Tomat, Costa and Girgulsky11, Reference Rothe, Canzler and Wolf27). This technique is used as a marker of isozyme-independent NOS.
The NADPH-d-stained cells from the different groups were measured using a Nikon E400 light microscope (Nikon Instrument Group, Melville, NY, USA) equipped with a digital camera connected to Image-Pro Plus 4.5.1.29 software (Media Cybernetics, LP, Silver Spring, MD, USA) and computerised acquisition and analysis software (Scion Image Beta 4.02; Scion Corporation, Walkersville, MD, USA). The mean of each optical density (OD) value was calculated by the measurement of OD in different tissue areas of the same section and in different sections of the same organ. Each set of OD measurements (control and experimental groups) was performed blindly and under similar light, gain, offset and magnification conditions.
Western blot
Samples of renal cortex and thoracic aorta containing equal amounts of protein (10 μg protein/lane) were separated by electrophoresis in 7·5 % SDS-polyacrylamide gels (Bio-Rad, Munich, Germany), transferred to a nitrocellulose membrane (Bio-Rad), and then incubated with rabbit polyclonal anti-NOS antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA, dilution 1:500, anti-iNOS: epitope at the carboxy terminus, anti-eNOS: epitope at the amino terminus, and anti-nNOS: epitope at the amino terminus) and a secondary immunoreaction with a goat anti-rabbit antibody conjugated with horseradish peroxidase was performed (dilution 1:5000; Amersham Pharmacia Biotech, Uppsala, Sweden). Samples were revealed by chemiluminescence using ECL reagent for 2–4 min (Amersham Pharmacia Biotech). Quantification of the bands was performed by digital image analysis using a Hewlett-Packard scanner and TotalLab analyser software (Nonlinear Dynamics Ltd, Newcastle upon Tyne, UK). All experiments were performed in triplicate.
Statistical analysis
Values are means with their standard errors. Prism Graph Pad Software (San Diego, CA, USA) was used for statistical analysis. Data were analysed using one-way ANOVA followed by a Bonferroni multiple-comparison post hoc test. TAG were analysed by the non-parametric Dunn test for multiple comparisons. Linear regression analysis was used to determine the relationship between birth weight and SBP and NOS activity in the renal cortex and GFR at 60 d. P < 0·05 was considered a significant difference.
Ethical approval for animal experimentation
Animals were cared for according to regulation 6344/96 of Argentina's National Drug, Food and Medical Technology Administration and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996). Experimental procedures were approved by the ethics committee of the School of Biochemistry and Pharmacy (CEFFB), Buenos Aires University, Argentina.
Results
The mothers fed either a moderately Zn-deficient diet or a control diet exhibited similar daily food intake during the experimental period (L, 30·5 (sem 3·7); C, 29·2 (sem 2·3) g/d; NS).
At birth, male offspring of L mothers exhibited lower body weights compared with male offspring of C mothers (C, 7·7 (sem 0·1); L, 6·8 (sem 0·2) g; P < 0·01). However, there were no significant differences in body weight among the various dietary groups at 60 d (Cc, 406 (sem 9); Lc, 386 (sem 10); Ll, 384 (sem 11) g, NS). Further, because daily food intake in all groups was similar (Cc, 22·6 (sem 0·8); Lc, 22·4 (sem 0·6); Ll, 22·0 (sem 0·8) g/d; NS), it was not necessary to pair feed control rats.
No differences were observed in kidney, plasma and faeces Zn content between Cc and Lc groups. Ll animals showed lower Zn concentrations in kidneys (16·2 (sem 1·0) μg/g tissue) than both the Cc animals (26·6 (sem 0·9) μg/g tissue; P < 0·05) and the Lc animals (24·9 (sem 0·8) μg/g tissue; P < 0·05) at 60 d after weaning. They also had lower plasma Zn concentrations (0·95 (sem 0·09) mg/l) than the Cc animals (1·62 (sem 0·10) mg/l; P < 0·05) and the Lc animals (1·45 (sem 0·10) mg/l; P < 0·05). Similarly, faeces concentrations of Zn were lower in the Ll group (139 (sem 16) μg/d) than in the Cc group (656 (sem 105) μg/d; P < 0·05) and the Lc group (586 (sem 98) μg/d; P < 0·05).
Animals exposed to a moderately Zn-deficient diet during pre-weaning and/or post-weaning growth (Ll, Lc groups) exhibited higher values (P < 0·01) of SBP at the end of the dietary treatment compared with the Cc group (Cc, 129 (sem 2); Lc, 148 (sem 5); Ll, 149 (sem 6) mmHg). However, there were no significant differences in SBP levels among the Ll and Lc dietary groups. Moreover, an inverse correlation was observed between birth weight and SBP (r 0·8305; P < 0·001) at 60 d.
Lipid profile is shown in Table 2. Serum TAG concentration was higher in Ll animals compared with the Cc and Lc groups. However, total cholesterol, HDL-cholesterol, non-HDL-cholesterol and VLDL-cholesterol concentrations were not altered by the dietary treatments.
Table 2 Effects of low-zinc diet during fetal life, lactation and post-weaning growth on lipid profile at 56 d‡
(Mean values with their standard errors)

Cc, control Zn during pregnancy, lactation and post-weaning; Lc, low Zn during pregnancy and lactation, control Zn post-weaning; Ll, low Zn during pregnancy, lactation and post-weaning.
* Mean value was significantly different from that of the Cc group (P < 0·05).
† Mean value was significantly different from that of the Lc group (P < 0·05).
‡ TAG were analysed by the non-parametric Dunn test for multiple comparisons and the other parameters were analysed using one-way ANOVA followed by a Bonferroni post hoc test.
Animals exposed to Zn deficiency showed lower levels of urinary NOx than Cc rats at 60 d. However, Lc rats showed higher urinary NOx compared with the Ll group. Ll rats had lower urinary NOx (0·69 (sem 0·11) nmol/ml × min × 100 g) than both the Cc (1·45 (sem 0·14) nmol/ml × min × 100 g; P < 0·01) and the Lc (1·14 (sem 0·06) nmol/ml × min × 100 g; P < 0·01) rats. Lc animals exhibited lower levels of urinary NOx than Cc animals (P < 0·01).
Fig. 1 shows NOS activity, determined with the l-[U-14C]arginine in vitro method, in the thoracic aorta and the renal cortex. Renal and vascular tissues from the Lc and Ll groups showed decreased NOS activity compared with Cc rats. The Ll and Lc groups also exhibited lower levels (P < 0·01) of GFR at the end of the dietary treatment compared with the Cc group (Cc, 0·72 (sem 0·09); Lc, 0·39 (sem 0·01); Ll, 0·28 (sem 0·03) ml/min × 100 g). Moreover, a positive correlation was observed between NOS activity in the renal cortex and GFR (r 0·6131; P < 0·001).
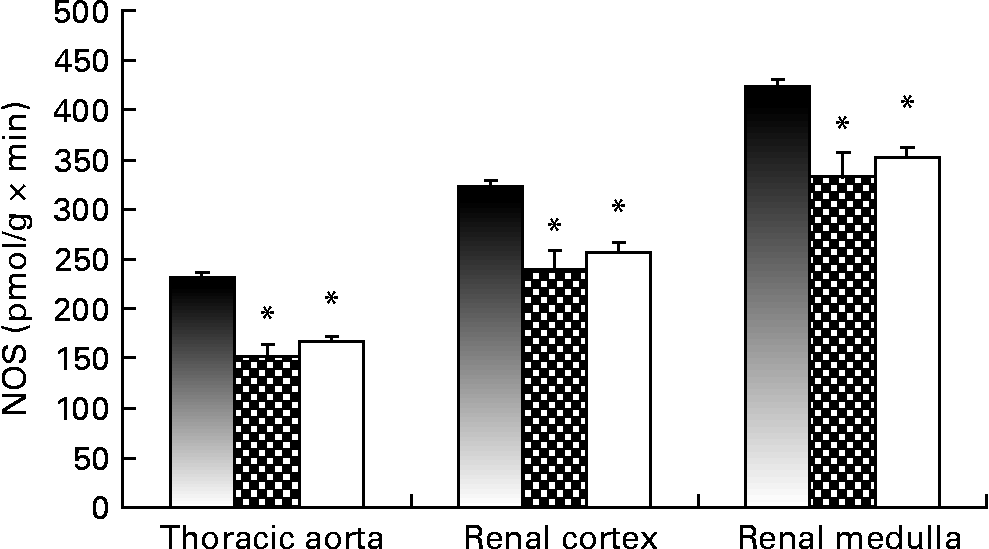
Fig. 1 NO synthase (NOS) activity measured, using l-[U-14C]arginine as the substrate, in thoracic aorta and renal cortex of Control control (Cc, ■), Low control (Lc, ![]() ) and Low low (Ll, □) Zn diet groups. Values are means (n 10 per group), with their standard errors represented by vertical bars. Data were analysed by one-way ANOVA followed by a Bonferroni post hoc test. * Mean value was significantly different from that of the Cc group (P < 0·001).
) and Low low (Ll, □) Zn diet groups. Values are means (n 10 per group), with their standard errors represented by vertical bars. Data were analysed by one-way ANOVA followed by a Bonferroni post hoc test. * Mean value was significantly different from that of the Cc group (P < 0·001).
Table 3 and Fig. 2 show NOS activity in the thoracic aorta, intestinal arterioles and renal cortex, evaluated by the NADPH-d activity technique. NADPH-d staining in the endothelium and smooth muscle sections of the aorta and arterioles was less intense in the Lc and Ll rats than in the Cc group. Animals exposed to Zn deficiency also showed lower NADPH-d activity in glomeruli, proximal tubules, distal tubules and collecting ducts compared with the Cc group. The data are in accordance with the results of NOS activity obtained with the l-[U-14C]arginine in vitro method.
Table 3 NADPH diaphorase activity (measured as optical density) in thoracic aorta, intestinal arterioles and nephron segments at 60 d after weaning†
(Mean values with their standard errors)
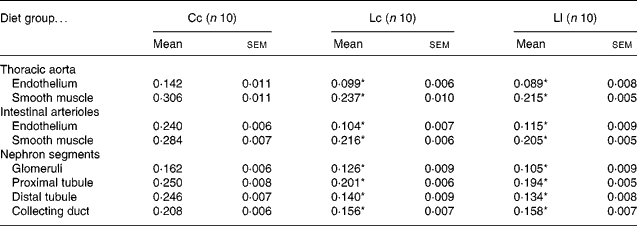
Cc, control Zn during pregnancy, lactation and post-weaning; Lc, low Zn during pregnancy and lactation, control Zn post-weaning; Ll, low Zn during pregnancy, lactation and post-weaning.
* Mean value was significantly different from that of the Cc group (P < 0·001).
† Data were analysed using one-way ANOVA followed by a Bonferroni post hoc test.

Fig. 2 NADPH-diaphorase staining in (a) thoracic aorta, (b) intestinal arterioles and (c) renal cortex of Control control (Cc), Low control (Lc) and Low low (Ll) Zn diet groups at the end of the experimental protocol (n 10 for each group). Arrows indicate staining in endothelium (E), vascular smooth muscle (VSM), intestinal arterioles (A), glomeruli (G), proximal tubule (PT) and collecting tubule (CT). All images are at the same magnification of × 400. Scale bar = 30 μm.
NOS expression was measured by Western blot analysis in the thoracic aorta and renal cortex. There was no difference in aortic nNOS protein abundance among the groups (Fig. 3(a)), and the aortic iNOS isoform was undetectable. Fig. 3(b) shows that eNOS expression was decreased in the thoracic aorta of Lc and Ll adult rats compared with the Cc group. All NOS isoforms were expressed in the renal cortex of Cc, Lc and Ll adult rats. Nevertheless, Zn dietary restriction during fetal life, lactation and post-weaning growth did not modify abundance of any of the NOS isoforms (Fig. 4(a)–(c)).

Fig. 3 Western immunoblots showing the protein expression of (a) neuronal NO synthase (nNOS) and (b) endothelial NOS (eNOS) in the thoracic aorta of Control control (Cc, ■), Low control (Lc, ![]() ) and Low low (Ll, □) Zn diet groups. All experiments were performed in triplicate. Each blot was normalised to expression of the β-actin marker from the same gel. Values are means (n 10 per group), with their standard errors represented by vertical bars. Data were analysed by one-way ANOVA followed by a Bonferroni post hoc test. * Mean value was significantly different from that of the Cc group (P < 0·01).
) and Low low (Ll, □) Zn diet groups. All experiments were performed in triplicate. Each blot was normalised to expression of the β-actin marker from the same gel. Values are means (n 10 per group), with their standard errors represented by vertical bars. Data were analysed by one-way ANOVA followed by a Bonferroni post hoc test. * Mean value was significantly different from that of the Cc group (P < 0·01).

Fig. 4 Western immunoblots showing the protein expression of (a) neuronal NO synthase (nNOS), (b) inducible NOS(iNOS) and (c) endothelial NOS (eNOS) in the renal cortex of Control control (Cc, ■), Low control (Lc, ![]() ) and Low low (Ll, □) Zn diet groups. All experiments were performed in triplicate. Each blot was normalised to expression of the β-actin marker from the same gel. Values are means (n 10 per group), with their standard errors represented by vertical bars. Data were analysed by one-way ANOVA followed by a Bonferroni post hoc test.
) and Low low (Ll, □) Zn diet groups. All experiments were performed in triplicate. Each blot was normalised to expression of the β-actin marker from the same gel. Values are means (n 10 per group), with their standard errors represented by vertical bars. Data were analysed by one-way ANOVA followed by a Bonferroni post hoc test.
Discussion
In the present study we offer evidence that moderate Zn restriction during fetal life and postnatal growth induces alterations in lipid metabolism and in the vascular and renal NO system which could contribute to cardiovascular and renal alterations in adult life.
Animals exposed to moderate Zn deficiency during fetal life showed lower body weight at birth and higher BP levels in adult life. These results are in agreement with our previous study that used the same experimental design and showed that rats exposed to moderate Zn deficiency during fetal life and postnatal growth exhibited an increase in SBP and lower body weight at the time of weaning(Reference Tomat, Inserra and Veiras9). Therefore, the present and our previous results demonstrate the reproducibility of our model and support the hypothesis that Zn deficiency could be a risk factor for the development of CVD in adult life. Moreover, the close negative correlation between birth weight and SBP at 60 d, observed in the present study, is in accordance with many epidemiological studies proposing that factors present in the prenatal environment are responsible for in utero programming of CVD(Reference Eriksson, Forsén and Tuomilehto28–Reference Barker, Forsén and Eriksson30).
The ‘fetal origins hypothesis’ suggests that intra-uterine growth restriction results in low birth weight, programs the development of organs involved in BP regulation, such as the kidney and vessels, and may predispose to long-term health problems(Reference Barker31). In this regard, we demonstrated in the present study that Zn restriction during fetal and early postnatal life induced vascular and renal NO system impairment in adult life. Lower NOS activity was observed in different nephron segments and in the endothelium and smooth muscle of the aorta artery and resistance arterioles, even when renal and plasma Zn content was restored through an adequate-Zn diet during post-weaning growth. Additionally, animals exposed to Zn restriction showed lower urinary excretion of NO endproducts, whose concentration is an indicator of systemic NO production and/or bioavailability. Therefore, the above findings suggest that Zn deficiency in different periods of growth can impair NO production.
The reduction in vascular NOS activity, regardless of the period of growth, is associated with a lower protein expression of the eNOS isoform. Therefore, the lower eNOS-derived NO production in conduct arteries would impair vascular smooth muscle relaxation and would not allow the response to increases in flow and in shear stress, inducing a decrease in arterial compliance(Reference Moncada32–Reference Vapaatalo and Mervaala34).
Meanwhile, in the kidney, reduced NOS activity in the renal cortex could be one of the possible mechanisms involved in the decrease in GFR that we observed in Zn-deficient animals. This hypothesis is supported by the correlation observed between NOS activity in the renal cortex and GFR, as well as by evidence showing that NO plays a major role in the maintenance of renal perfusion, GFR and low renal vascular resistance in the kidney(Reference Kone14, Reference Majid and Navar15). Moreover, reduced renal NO production could also be associated with morphological renal alterations, proteinuria, renal fibrosis and apoptosis, leading to progressive hypertension and severe renal injury in adult Zn-deficient rats(Reference Tomat, Inserra and Veiras9, Reference Ribeiro, Antunes and Nucci12).
Restitution of renal Zn content by an adequate-Zn diet during post-weaning growth was not enough to normalise BP and GFR, probably due to irreversible alterations induced in utero, such as renal and vascular NO system impairment as well as the morphological renal alterations previously reported(Reference Tomat, Inserra and Veiras9).
Contrasting with the aorta artery, the decrease in renal NOS activity was not associated with lower expression of NOS isoform proteins. Contrasting information about NOS isoform expression in different tissues of animals exposed to Zn deficiency is found in the literature. In weanling rats exposed to severe Zn deficiency, iNOS expression has been induced in the lung, skin and intestine(Reference St Croix, Leelavaninchkul and Watkins35–Reference Cui and Okada37). On the other hand, Sato et al. found that severe Zn deficiency during adult life does not change NOS activity and aortic eNOS expression in normotensive rats(Reference Sato, Kurihara and Moridaira38) and enhances expression of eNOS mRNA and protein in the thoracic aorta of spontaneously hypertensive rats(Reference Sato, Yanagisawa and Nojima39).
Taking into account our evidence and that from other reports, we suggest that the enhanced oxidative stress condition would probably contribute to decreased NOS activity, since oxygen free radicals could trigger the uncoupling of the enzyme(Reference Tomat, Inserra and Veiras9, Reference Zou, Shi and Cohen16). In addition, we postulate that disturbances in Zn homeostasis during critical periods of growth would affect other regulation enzymic mechanisms, including alterations in the dimeric enzyme structure, in substrate and cofactor synthesis and transport, and/or in the activity of humoral and nervous factors(Reference Herrera and Garvin40, Reference Mount and Power41).
On the other hand, animals exposed to Zn restriction since fetal life and until adulthood showed augmented plasma TAG level, another cardiovascular risk factor. The increase in TAG level seems to be induced mainly by Zn dietary restriction during post-weaning growth. The present results are in agreement with previous work on severe Zn deficiency in adult rats(Reference Kettler, Eder and Kettler17, Reference Daniel and Dieck18). However, we did not observe changes in total cholesterol and in the other major serum lipoprotein fractions as observed in experimental models of severe Zn deficiency(Reference Fischer, Giroux and Belonje19–Reference Faure, Roussel and Richard22). These contrasting observations may indicate that the effects of Zn deficiency on lipid profile may be related to the duration of the low Zn dietary treatment, the degree of Zn restriction, the period of life involved and the environment.
In conclusion, the results of the present study give strong evidences that Zn deficiency during intra-uterine and postnatal growth can result in low birth weight and in the programming of renal and vascular NO system dysfunction. These alterations could be linked to the elevated BP observed in adult life.
Acknowledgements
The present study was supported by grants PIP11220080101121/09 CONICET and PICT 12126 FONCYT, Secretary of Science and Technology, Argentina and by grants B026 and B008 from the University of Buenos Aires and IQUIMEFA-CONICET, Argentina.
The authors thank Sebastián Finella (Fisiología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires) and Adriana Ruth Weisstaub (Nutrición, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires) for their technical assistance and Ana Borthwick for English language proofreading.
A. T. performed the experiments and participated in experimental design, data analysis and in writing the manuscript. R. E. participated in renal and vascular NOS activity determination.
V. Z. and L. S. carried out lipid analyses. A. F. carried out Western blot analysis. H. F. performed the determination of Zn content in plasma, faeces, kidneys and diets. A. M. B. assisted in scientific and technical supervision. M. A. C. participated in scientific and technical supervision, experimental design, data analysis and in writing the manuscript. C. A. participated in scientific and technical supervision, experimental design, data analysis and in writing the manuscript.
The authors declare that they have no conflicts of interest.









