INTRODUCTION
Community-based prevention strategies for seasonal and pandemic influenza are essential to minimize their potential threat to public health [Reference Molinari1, Reference Carcione2]. Vaccination is the cornerstone of prevention of seasonal and pandemic influenza virus infections [Reference Fiore3]. Although existing evidence demonstrates that vaccination can be an effective approach to protect the population against influenza [Reference Jefferson4–Reference Ohmit6], uptake in some populations remains low [Reference Bohmer7–Reference Sammon9]. In the event of a novel influenza pandemic, vaccines that provide good protection against the new strain might not be available for 4–6 months, and other control measures would be required in the interim, including non-pharmaceutical interventions such as hand hygiene [Reference Bell10]. Hand hygiene interventions are appealing because they can be applied in both developed and lesser developed regions at low cost [Reference Bell10, Reference Smith11].
Influenza virus spreads among humans either by inhalation of virus-loaded droplets into the respiratory tract, by direct contact, e.g. hand shaking, or by indirect contact with infected individuals via contaminated objects (fomites) [Reference Brankston12–Reference Weber and Stilianakis14]. The relative importance of alternative modes of transmission is controversial, while the potential for efficacy of hand hygiene implicitly requires that direct or indirect contact is an important mode of transmission [Reference Boyce15]. Recent research has suggested that the importance of contact transmission may vary in different regions [Reference Lowen and Palese16]. For instance, ambient temperature and relative humidity may modify the mode of influenza transmission. Because small droplet transmission is enhanced by low or very high humidity [Reference Yang and Marr17], it has been hypothesized that in temperate zones with a cool and dry winter, influenza transmission is predominantly by aerosol while in tropical zones with a warm and humid environment, the virus is more often transmitted by the contact route [Reference Lowen and Palese16]. If this hypothesis is correct, the effectiveness of hand hygiene interventions would be expected to vary by latitude, ambient temperature and humidity. If virus transmission in temperate zones primarily occurs by aerosol, then hand hygiene interventions would be expected to be less effective.
Since the World Health Organization highlighted the need for controlled trials in formulating the use of non-pharmaceutical interventions in preventing influenza transmission in 2006 [Reference Bell10], various randomized controlled trials (RCTs) and systematic reviews [Reference Warren-Gash, Fragaszy and Hayward8, Reference Jefferson18] on the effectiveness of hand hygiene interventions in reducing influenza and other respiratory virus infections have been published. By contrast, there are three existing meta-analyses assessing the effectiveness of hand hygiene interventions in preventing respiratory diseases, none of which focused on influenza viruses specifically [Reference Aiello and Larson19–Reference Rabie and Curtis21]. This systematic review and meta-analysis aims to evaluate the impact of hand hygiene interventions in preventing influenza virus transmission in the community setting and to investigate the possible modifying effects of latitude, temperature and humidity on hand hygiene efficacy for influenza virus infection.
METHODS
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analyses recommendations (PRISMA) statement [Reference Moher22].
Search strategy
We searched the Medline (January 1946 to November 2013), PubMed (January 1960 to November 2013), EMBASE (1974 to November 2013), and Cochrane Library databases and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2013, Issue 11) databases using the following search terms in all fields regardless of publication date and language:
-
#1: ‘hand hygiene’ OR ‘hand washing’ OR ‘handwashing’ OR ‘hand-wash’ OR ‘hand sanitizers’ OR ‘hand sanitizer’ OR ‘hand rub’
-
#2: ‘influenza’ OR ‘flu’ OR ‘respiratory infection’ OR ‘respiratory virus’ OR ‘respiratory tract infection’ OR ‘respiratory illness’ OR ‘fever’ OR ‘cough’ OR ‘sore throat’ OR ‘runny nose’ OR ‘nasal congestion’ OR ‘sneezing’ OR ‘malaise’ OR ‘muscle aches’ OR ‘headache’
-
#3: #1 AND #2
To identify further studies of interest, manual search was performed with the reference lists of retrieved review articles.
Eligibility criteria
We included any RCT comparing the effect of hand hygiene interventions with no intervention in reducing influenza virus transmission in community settings, in which study subjects or cluster units in a population were assigned prospectively into intervention and control groups using random allocation [23]. A community setting was defined as an open setting without confinement and special care for the participants. Articles describing any hand hygiene-related interventions alone were included.
Study selection
The primary outcome was the relative reduction of influenza virus infections confirmed by reverse-transcriptase–polymerase chain reaction (RT–PCR), virus culture or rapid antigen test in the hand hygiene intervention group compared to the control group. The secondary outcome measure was the relative reduction of influenza-like illnesses (ILI) confirmed by either professional clinical diagnosis or reported symptoms. We adopted a febrile acute respiratory illness (FARI) definition which defines cases as the presence of fever with cough or sore throat [Reference Babcock, Merz and Fraser24].
Two independent reviewers (V.W.Y.C., B.J.C.) screened all titles of studies identified by the search strategy individually, then subsequently reviewed the abstracts of the potential relevant studies. If the studies described hand hygiene interventions and influenza transmission, the reviewers read the full-length text. Further discussion was held if a consensus was not reached.
Evidence quality assessment
We evaluated the methodological quality of each outcome with GRADEprofiler (GRADEpro) [Reference Brozek, Oxman and Schünemann25], as recommended by the Cochrane Collaboration. We ranked the quality of evidence of each outcome as high, moderate, low, and very low based on its risk of bias, consistency, directness, precision of the results and publication bias.
Statistical analysis
The effect estimates were summarized as risk ratios (RRs) and their corresponding 95% confidence intervals (CIs). Due to substantial variation in RRs, the summary statistic was estimated with the more conservative Mantel–Haenszel (MH) random-effects model since it accounts for both the potential variability in effects and also the random variability across studies associated with different study designs and settings. We assessed publication bias graphically with Begg's funnel plot [Reference Begg and Berlin26] and also implemented Egger's test [Reference Egger27] and the Begg & Mazumdar rank correlation [Reference Begg and Berlin26] to quantify the evidence of publication bias statistically. For Egger's test, we considered evidence of publication bias if the two-tailed P value was <0·05. For rank correlation, we considered evidence of publication bias if the two-tailed P value was <0·10 since this test statistic has been shown to be less sensitive than Egger's test [Reference Sterne, Gavaghan and Egger28]. We calculated the I 2 statistic to assess the extent of inconsistency for each pooled estimate. The I 2 statistic quantified the proportion of total variations across effect estimates due to heterogeneity but not sampling error, and ranges from 0% to 100% such that 0% indicates homogeneity and 100% reflects substantial heterogeneity [Reference Higgins29].
We performed separate analyses of studies in developed and developing countries due to their systematic differences such as cultural background, educational level, etc., and performed a subgroup analysis of hand hygiene interventions with or without facemask use for both outcomes. Meta-regression was conducted to further assess if any covariates could explain the variation across studies in the effect of hand hygiene on laboratory-confirmed influenza, i.e. the primary outcome. To test for a modifying impact of temperature and humidity on efficacy of hand hygiene, we constructed univariate random-effects regression models with a number of covariates including latitude, average temperature and humidity during studies. We calculated the mean of the average temperature and relative humidity during the recorded study months by using the data provided by WeatherSpark [Reference Diebel and Norda30], which is a weather website summarizing historical data for the world from the National Oceanic and Atmospheric Administration. We carried out the meta-analysis using RevMan version 5.1 software [31] and the Comprehensive Meta-analysis version 2 software [Reference Borenstein32].
RESULTS
Search results
We identified 979 articles in the initial database search, of which 41 were retrieved based on their title and abstract content. Of the 41 retrieved articles, ten were eligible for meta-analysis based on our inclusion criteria (see Fig. 1). We excluded 31 studies after the full-length assessment [Reference Apisarnthanarak33–Reference Miller63] for the following reasons: studies were not RCTs, ineligible definition on ILI, no definition on respiratory diseases outcomes, hand hygiene interventions as a part of infection control programme, or no control group (see online Appendix). The characteristics of the ten eligible RCTs are summarized in Table 1, which comprised nine studies assessing laboratory-confirmed influenza [Reference Aiello64–Reference Stebbins72] and ten studies assessing ILI [Reference Aiello64–Reference Hubner73].

Fig. 1. Flow diagram of the process and results of study selection.
Table 1. Characteristics of included studies
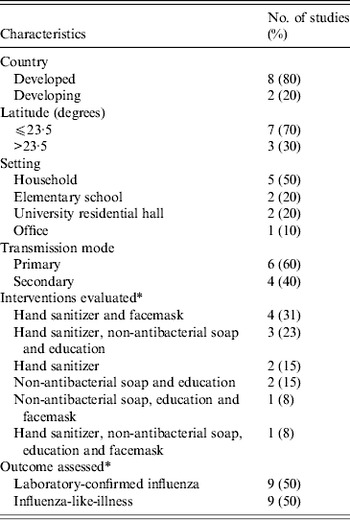
* Some studies assess more than one intervention and outcome.
Quality of evidence
The methodological qualities of studies were assessed by GRADEpro. Studies that used a laboratory-confirmed influenza outcome were graded as high, while studies with an ILI-only outcome were graded as moderate. The evidence profile for each outcome is summarized in Table 2 (see also online Appendix). All included trials were RCTs with proper randomization and their allocation sequences were properly concealed. They were either single-blinded to the recruiting physician, principal investigator and statisticians or not blinded to any personnel. No significant publication bias was noted (see online Appendix). The imprecision was, however, significant in most of the trials due to small sample size, inadequate case ascertainment, poor compliance to interventions, and insufficient statistical power. Most (8/10) of the studies received funding from the United States Centers for Disease Control and Prevention, one study was supported by the German Federal Ministry of Health, and one from a pharmaceutical company.
Table 2. Grade evidence profile

CI, Confidence interval; FARI, febrile acure respiratory illness.
The superior numerical indicators refer to notes in Supplementary Table S2 (see online Appendix).
Efficacy of hand hygiene interventions
The forest plot for studies conducted in developed countries is shown in Figure 2. There was an insignificant relative risk reduction of 18% in the pooled analysis (RR 0·82, 95% CI 0·66–1·02, I 2 = 0%, P = 0·07) of laboratory-confirmed influenza outcome. While a significant reduction of 27% was reported for the hand hygiene and facemask group (RR 0·73, 95% CI 0·53–0·99, I 2 = 0%, P = 0·05), the hand hygiene only comparison was not statistically significant. A significant RR reduction of 22% (RR 0·78, 95% CI 0·68–0·90, I 2 = 0%, P = 0·0008) was found in the pooled analysis of ILI outcomes. In the subgroup analyses, similar to the result from the laboratory-confirmed influenza outcome, a significant reduction of 27% (RR 0·73, 95% CI 0·60–0·89, I 2 = 0%, P = 0·002) was noted for the combined comparison of hand hygiene and facemask use while the result from hand hygiene alone was not statistically significant.

Fig. 2. Risk ratios for the effect of hand hygiene interventions with or without facemask on laboratory-confirmed influenza in studies conducted in developed countries.
There were only two studies in less developed countries. The efficacy of hand hygiene was not significant in the pooled analysis for the laboratory-confirmed influenza outcome. For the ILI outcome, a non-significant relative increase was observed for the efficacy of combined comparison of hand hygiene and facemask use (see online Appendix).
Meta-regression
We used meta-regression to explore if any particular covariate could explain the observed heterogeneity across studies (Table 3). A systematic review suggests that facemasks can reduce aerosol transmission of influenza virus [Reference Cowling74]; therefore, we conducted meta-regression on hand hygiene interventions without facemask to assess the independent effects of hand hygiene even after adjusting for potential factors that could impact heterogeneity. For the studies conducted in developed countries, we found that a 10° rise in latitude [relative risk ratio (RRR) 1·28, 95% CI 0·91–1·79, P = 0·15], average temperature (RRR 0·82, 95% CI 0·59–1·13, P = 0·22) and average relative humidity (RRR 0·63, 95% CI 0·32–1·22, P = 0·17) were not statistically significantly associated with a change in the efficacy of hand hygiene in developed countries but the direction of the estimate for relative humidity was consistent with the hypothesis that influenza transmission is predominately by aerosol in temperate zones while the virus is commonly transmitted by contact route in tropical areas (see online Appendix).
Table 3. Univariate regression analyses on different covariates in relation to the risk of laboratory-confirmed influenza in combined countries' data and developed countries' data (hand hygiene intervention only)
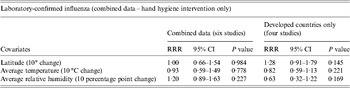
RRR, Relative risk ratio; CI, confidence interval.
DISCUSSION
We examined the efficacy of hand hygiene interventions in preventing influenza virus transmission in the community. The subgroup analysis from developed countries suggested that a combined intervention consisting of hand hygiene with facemasks is an effective strategy to prevent influenza, but we did not confirm the efficacy of hand hygiene alone for reducing influenza illness. This is consistent with evidence on the important role of aerosol transmission of influenza, such that interventions against contact transmission alone like hand hygiene may not be sufficient to control influenza transmission in the community [Reference Cowling75]. However, shortcomings related to statistical power to detect the impact of hand hygiene suggest that future studies should continue to study the impact of hand hygiene independently on laboratory-confirmed influenza outcomes.
Seasonal and pandemic influenza viruses cause a major burden of illness, hospitalization and death. Our review captured studies with the outcomes of laboratory-confirmed influenza or FARI (ILI) which is a fairly specific outcome to influenza. We did not include studies with broader definitions of respiratory illness, which could encompass many other outcomes such as other non-influenza viral infections, asthma exacerbation, allergic rhinitis or non-viral respiratory infections, because the efficacy of hand hygiene intervention on each respiratory illness might vary. According to these inclusion criteria, our review did not include studies that examined the efficacy of hand hygiene against broader respiratory illness outcomes, but these studies did identify reasonable efficacy of hand hygiene interventions [Reference Luby46, Reference Roberts54–Reference Sandora56]. For this reason, this meta-analysis goes beyond three formerly published reviews [Reference Aiello and Larson19–Reference Rabie and Curtis21] by focusing on influenza virus infections rather than any respiratory illness symptoms, and by exploring the hypothesis that modes of transmission may vary from region to region. In our meta-regression model, although we did not find any significant effects, we noted evidence for effects of all three covariates particularly from relative humidity. The insignificant result may due to relatively low sample size.
There are several noteworthy limitations in this review. The greatest limitation is the small number of RCTs that have been conducted to date on the efficacy of hand hygiene to control influenza. Since there are only a few studies involving the same hand hygiene interventions among the included studies, we are unable to provide intervention-specific pooled estimates. The efficacy of individual hand hygiene interventions, hence, cannot be compared. The heterogeneity across studies is another limitation and to address this we performed separate analyses for developed and developing countries' data and meta-regression for hand hygiene only laboratory-confirmed influenza outcome. Although we cannot exclude the possible role of other covariates, we minimized the variations of different study design characteristics by including only RCTs. The variations associated with different settings and different hand hygiene interventions, however, cannot be ignored. The possible clustering effect may also be a limitation in our review. Since we did not adjust for clustering in the analysis, this may lead to skewed results with possibly higher risk of type I error and narrower confidence intervals. However, one previous study suggested that clustering effect did not have a significant effect on heterogeneity or overall pooled estimates from their meta-analysis assessing the effectiveness of hand hygiene interventions on infectious disease risk in the community setting [Reference Aiello20].
The findings of this review have implications for the recommendations and guidelines of hand hygiene and facemask use in the future. Given the lack of substantial efficacy of hand hygiene identified in our review (Fig. 3), and the increasing evidence supporting a role of aerosol as a mode of influenza virus transmission [Reference Cowling75–Reference Bischoff78], further public health initiatives may need to re-examine the control measures for aerosol transmission. In particular, measures such as hand hygiene that focus on reducing one mode of transmission (i.e. contact) may not be sufficient to control transmission. Measures that may require more detailed consideration include N95-type respirators, improved indoor ventilation, quarantining of infected individuals, and even the use of air humidifiers, given the potential role of humidity in reducing viability of aerosols [Reference Lowen and Palese16, Reference Yang and Marr17]. While elucidating the possible influence of humidity in influenza transmission among human populations further confirms its contribution on influenza seasonality, particularly in temperate regions, the detailed mechanisms have yet to be explored.

Fig. 3. Risk ratios for the effect of hand hygiene interventions with or without facemask on influenza-like illness in studies conducted in developed countries.
The insignificant findings from hand hygiene intervention alone and subgroup analyses from developing countries' data does not necessarily indicate that hand hygiene is an ineffective measure for preventing influenza virus transmission. Rather, the non-significant results for hand hygiene alone could raise questions on compliance with existing recommendations on hand hygiene in the community. Indeed, hand washing and sanitizing needs to be practised properly and after all potential critical contamination events that might occur throughout the day. The CDC recommends that individuals wash their hands with soap and water for at least 20 seconds, properly lathering hands, washing soap off, and drying hands completely or if a sink is not available, to use hand sanitizer when hands are not visibly soiled [Reference Boyce15]. These recommendation are rarely carried out with high compliance in the general population [79]. Clearly, hand hygiene interventions not only need to be proven effective, but they also need to be widely adopted by most of the population if they are to mitigate influenza transmission effectively. Given the existing public health recommendations and guidelines on using hand hygiene interventions in preventing influenza transmission [Reference Smith11, 80, 81], the compliance rate in the community has not yet been well established. To our knowledge, there are only a few studies exploring interventions to promote hand hygiene practice in the community [Reference Yardley82–Reference Zomer86]. Further studies, in this regard, are warranted in relation to compliance rates of hand hygiene interventions and the possible interventions to promote such practices in the community.
In conclusion, hand hygiene interventions have been, and will continue to be an important component of the public health response to seasonal and pandemic influenza. However, expectations on the impact of such measures may need to be limited, given the results of our review indicating only potentially modest effects of this specific intervention. Variation in the importance of aerosol transmission in different regions is an intriguing possibility, and could imply the need for greater focus on alternative control measures particularly in temperate zones.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S095026881400003X.
ACKNOWLEDGEMENTS
This work was supported by the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558), and the Area of Excellence Scheme of the Hong Kong University Grants Committee (grant no. AoE/M-12/06).
DECLARATION OF INTEREST
B.J.C. received research funding from MedImmune Inc., and consults for Crucell NV. A.E.A. consults for SCA Tork as part of the Tork Green Hygiene Council.








