A 64-year-old woman underwent a left ulnar nerve sub-muscular transposition for 18 months of symptoms attributed to cubital tunnel syndrome. She was referred 6 months postoperatively indicating excruciating pain in the ulnar distribution starting immediately after surgery. She also complained of hand dysfunction and numbness of the ring and small fingers. She was assessed and found to have marked atrophy of left hand ulnar innervated intrinsic muscles. Strength on manual muscle testing was flexor carpi ulnaris (FCU) 3/5, flexor digitorum profundus (FDP) (digits 4 and 5) 3/5, first dorsal interosseous (FDI) 1/5, abductor digiti minimi (ADM) 1/5. Median and radial innervated muscles in the limb were normal. Sensation was markedly reduced in the ulnar distribution in the hand, including both palmar and dorsal ulnar cutaneous distributions. Past medical history was significant for diabetes mellitus controlled with Jardiance and Tradjenta and a 50 pack-year smoking history. Ulnar motor conduction studies demonstrated (Figure 1) small amplitude responses of 0.7 mV (ADM) and 0.2 mV (FDI) and slowing across the elbow (38 m/s ADM, 40 m/s FDI); distal latencies were normal. Ulnar sensory responses to the small finger were absent. Based on these amplitudes, it was concluded that the patient had marked axon loss in the ulnar distribution.
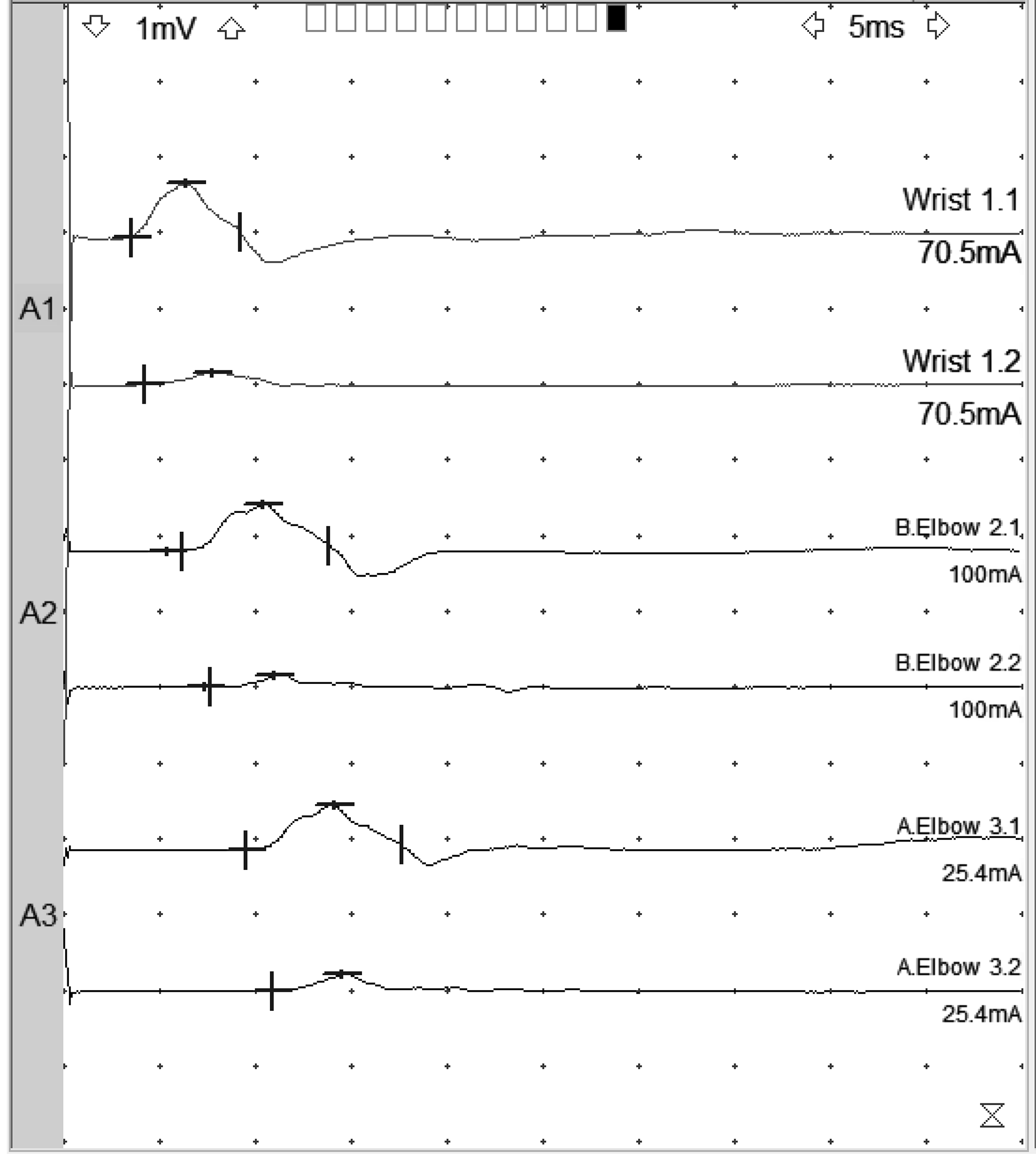
Figure 1: Ulnar motor responses of May 2021 recorded from ADM, top tracing of each stimulation site, and FDI, bottom tracing of each stimulation site. Note small distal CMAP of 0.7 mV for ADM and 0.2 mV for FDI. Distal latencies are 3.5 ms for ADM and 4.2 ms for FDI. Forearm NCV is 62 m/s (ADM) and 49 m/s (FDI). Across elbow, NCV is 38 m/s (ADM) and 40 m/s (FDI).
Two months later, the patient underwent exploration of the ulnar nerve at the arm and proximal forearm, decompression of the ulnar nerve at Guyon’s canal, and pronator quadratus anterior interosseous nerve motor branch transfer to the ulnar nerve. At the area of the previous surgery, the ulnar nerve passed underneath the flexor-pronator mass initially, it then became superficial and then turned once again under a portion of the FCU. At two levels, it was quite tight. First underneath the flexor-pronator mass at the distal edge of this prior transposition as well it took a very sharp turn through a portion of the FCU muscle, what appeared to be an unreleased head of the FCU from the common flexor origin. The nerve was completely released from all of these structures.
Only 2 months postoperatively, the patient demonstrated marked improvement. Strength was measured as 4/5 in ulnar intrinsic muscles of the hand, and there was profound recovery of intrinsic muscle bulk. Repeat ulnar motor conduction studies were performed 5 months after surgery. These demonstrated marked improvement in distal CMAPs of 2.0 mV (ADM) and 1.4 mV (FDI), although slowing persisted across the elbow (Figure 2). There was no response from ADM or FDI with median nerve stimulation at the elbow (other than volume conduction). The ulnar sensory response was present with a small amplitude of 2 µV.
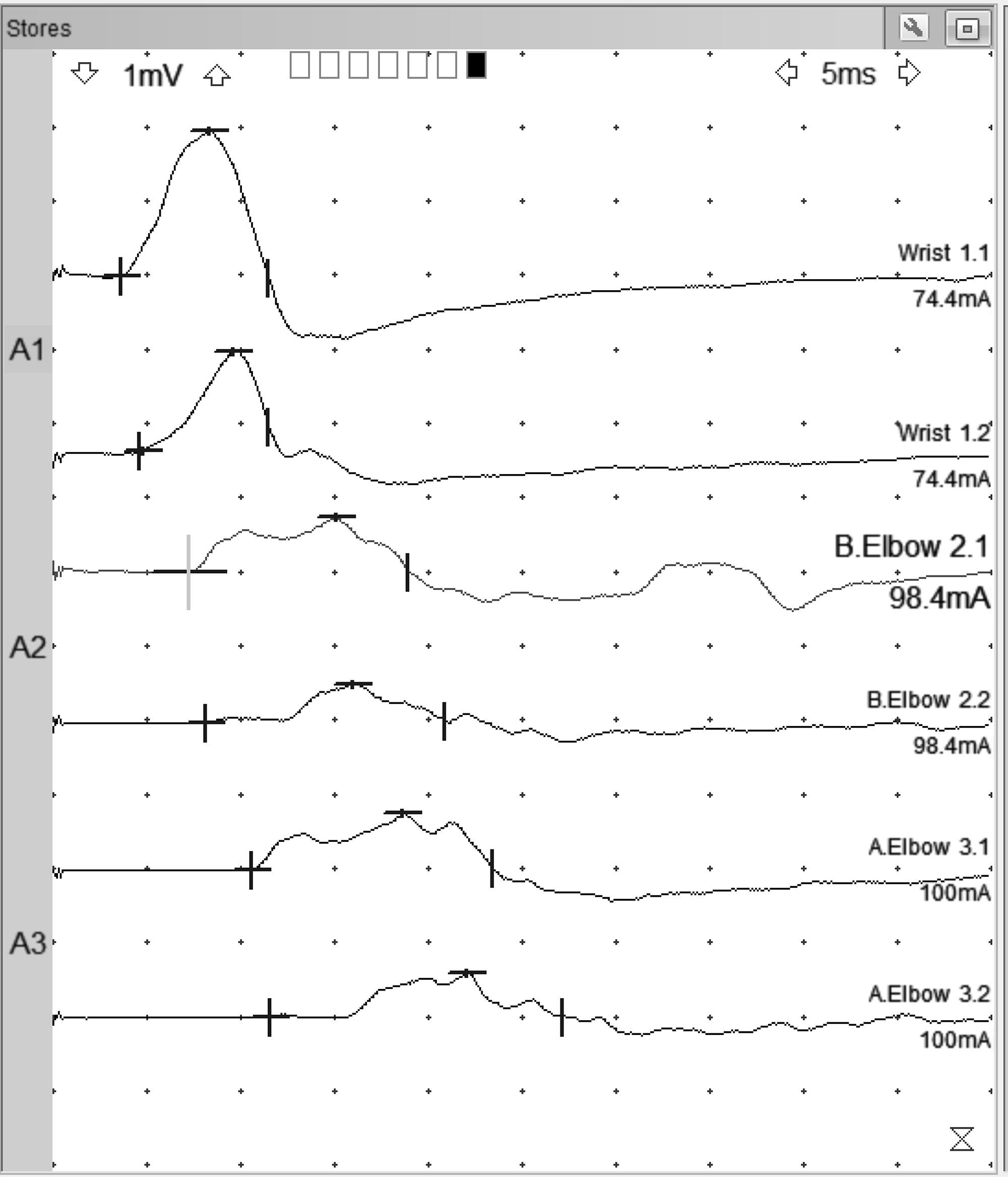
Figure 2: Ulnar motor responses of December 2021 recorded from ADM, top tracing of each stimulation site, and FDI, bottom tracing of each stimulation site. Note distal CMAP of 2.0 mV for ADM and 1.4 mV for FDI. Distal latencies are 3.6 ms for ADM and 4.6 ms for FDI. Forearm NCV is 48 m/s (ADM) and 48 m/s (FDI). Across elbow, NCV is 34 m/s (ADM) and 34 m/s (FDI). Note: cursors set on higher sensitivity to detect initial deflection from baseline. No response from FDI or ADM, aside from volume conduction, was seen with median nerve stimulation at the elbow.
This rapid improvement was unexpected in the face of initially very small ulnar motor CMAPs, which were interpreted as indicating severe axon loss. We do not believe axon regrowth from the site of compression could occur this quickly, especially in a 64-y-o smoker with diabetes. We also do not believe the nerve transfer from the anterior interosseous branch that is responsible for recovery, both because the timing is too early and we could not demonstrate a CMAP from ulnar innervated muscles when stimulating the median nerve at the elbow. Finally, it is unlikely that there was a significant compression at the wrist because the ulnar motor distal latencies were normal.
Rather we believe this is a case of a “silent synapse” as described by McComas, Reference McComas1 that is “sick” motor axons that were viable but not healthy enough to generate a motor response with distal stimulation. McComas postulated a transient interruption of neuromuscular transmission (silent synapse) related to interference of axoplasmic transport. Reference McComas1 He based this upon observed rapid increases in motor unit number estimates (MUNE) that could not be explained based on the distance of regeneration required to reach the target muscles. We postulate in this case that the very sharp angulation noted at the time of decompression may have blocked axoplasmic flow and created silent synapses, which then quickly recovered upon decompression. A somewhat similar case, though not treated surgically, was reported by McComas and White. Reference McComas and White2 Reports from Thesleff Reference Thesleff3 indicate that denervated muscle fibres may sustain a fall in the resting membrane potential and a fall in the action potential amplitude; the action potential rise time is also reduced. It is possible that similar changes occur in axons subjected to blocked axoplasmic flow.
While likely a rare event, this case is an important indicator that a small distal CMAP does not always indicate severe axon loss. It may, rather, represent interruptions in axoplasmic flow and sick motor axons that are unable to generate a synaptic response. It is possible, in cases such as this, to underestimate the number of surviving axons and thus indicate an erroneously poor prognosis.
Conflict of Interest
None of the authors has any conflict of interest to disclose.
Statement of Authorship
Both authors participated substantively in the writing of this manuscript.




