INTRODUCTION
Campylobacter spp. are a widespread and major cause of foodborne disease in the industrialized world [1–Reference Mølbak, Olsen and Wegener3]. The majority of reported Campylobacter cases are sporadic. Infections are frequently associated with foodborne transmission, but other routes of exposure, such as direct contact with live animals and person-to-person transmission, have also been identified [Reference Baker4–Reference Engberg7]. Identifying the most important sources of human foodborne disease is essential for prioritizing food-safety interventions and setting public health goals [Reference Pires8].
Several types of studies have been performed to identify possible sources of apparently sporadic human cases. Case-control studies are the most commonly used analytical epidemiological approach. Typically, selected case-patients and a corresponding group of asymptomatic and therefore assumed uninfected, individuals (controls) are interviewed, and the relative role of exposures is estimated by comparing the frequency of exposures among cases and controls. When cases of disease are associated with an exposure, the proportion of cases attributed to the exposure can be calculated and this measure is defined epidemiologically as the ‘population attributable fraction’ (PAF) [Reference Clayton and Hills9]. PAFs can be used to partition the human disease burden to specific sources [Reference Stafford10]. Alternatively, the relative importance of risk factors (assessed by comparing measures of association of each risk factor) can provide an indication of which sources or routes of exposure are associated with a higher risk of disease. Case-control studies are a valuable tool to identify potential risk factors for human infections, including sources and predisposing, behavioural or seasonal factors [Reference Engberg7]. In addition to individual case-control studies, a systematic review (SR) of published case-control studies of sporadic infections of a given foodborne disease can provide a comprehensive summary of the estimated measures of association and PAFs for each exposure, and this can be combined to estimate the overall burden of illness attributed to each exposure [Reference Pires8].
SRs consist of a formal process for literature review focused on a specific research question, and include the identification of relevant literature, quality assessment of relevant studies, summarization or statistical analysis of data, and conclusions [Reference Khan11, Reference Sargeant12]. The intent of SRs is to apply review methods that minimize systematic and random errors, and thus minimize the introduction of bias and provide reliable basis for the decision-making process. Meta-analysis consists of an analysis of the summarized statistics of the studies provided by the SR.
The usefulness of a SR and meta-analysis to attribute human foodborne diseases to sources has thus far not been investigated. This study aimed at comparing the relative importance of risk factors for cases of Campylobacter, thus assessing the utility of this methodology to provide information for source attribution of human campylobacteriosis and for delineation of interventions to reduce the burden of disease.
MATERIALS AND METHODS
Literature search
A literature search was conducted in February 2008, and was limited to the languages English, German, Portuguese, Spanish and Danish. No restrictions were defined for the year the study was conducted. Relevant studies were identified using a combination of key words in the databases Medline, Science Direct, Agricola, CAB International, Biosis, FSTA, and ISI Web of Science and Web of Knowledge. In addition to published peer-reviewed studies, relevant studies published as conference proceedings and in scientific reports were also searched. A combined search was performed, looking for case-control studies of Salmonella spp. and Campylobacter spp. sporadic infections.
The search was conducted using a combination of (1) general terms, related to case-control studies and risk factors, and (2) Campylobacter and Salmonella terms. Citations were collected, de-duplicated and managed in web-based software (SRS 4.0, TrialStat! Corporation, Canada).
An additional traditional literature search, using the same search terms but without assistance of SR software, was performed in February 2010, and new references were added to the previously retrieved studies.
Relevance screening
All references were independently reviewed by two reviewers, and it was sufficient that one reviewer considered it relevant for the reference to pass to the quality assessment step of the SR. Relevance of studies was assessed on the basis of specific inclusion criteria: (1) focus on human disease; (2) focus on Campylobacter or Salmonella; (3) focus on sporadic disease; (4) reference describing a case-control study.
Quality assessment
Methodological soundness was assessed by two reviewers on the basis of the following study quality criteria: (1) statistical power above 80%, if information was available (if the power of the study was not mentioned, the reviewers were asked to evaluate the reference based on the other criteria); (2) case definition implying laboratory confirmation of the diagnosis; (3) random selection of controls; (4) comparability of cases and controls; (5) control for potential confounding factors (control for matching); (6) acceptable matching criteria for matched study designs (e.g. age and gender); (7) exposure window for cases and controls acceptable (maximum 10 days) and comparable; (8) response rates for cases and controls acceptable; (9) appropriate statistics; (10) the studies provided the odd ratio (OR) with the 95% confidence interval (CI) of the effect of each exposure based on logistic regression or conditional logistic regression (if a matched study); (11) acceptable study design (overall quality assessment made by reviewers).
Non-compliance with a single criterion was not sufficient to reject a study. Instead, the reviewer was asked to do an overall assessment based on all criteria, and studies not fulfilling two or more criteria were excluded. If the two reviewers disagreed on the acceptance of a study, a third reviewer was consulted.
Data extraction
Data from studies that passed the previous steps were manually extracted by one of two reviewers using a standardized form. The data extracted included country and time period of the study, age stratification of the population, study design parameters (e.g. matched or unmatched study), and outcome of the study (ORs for specific risk factors together with the 95% CI).
Data analysis
A meta-analysis was performed to compare and combine information from different studies. All risk factors were stratified according to source-categorization schemes, location of exposures and, if appropriate, frequency of exposure.
Source categorization
Exposures were categorized in six main groups: food, direct contact with animals, environment, person-to-person, predisposition and travel. Additionally, food preparation risk factors were included for specific food routes with the purpose of distinguishing between the impact of exposure through consumption of foods and through handling of food items.
Risk factors from the main groups food (Fig. 1) and direct contact were categorized in a hierarchical scheme of mutually exclusive categories. Environmental transmission routes included drinking water, exposure to recreational waters, and exposure to contaminated environments (e.g. playgrounds) or objects (e.g. bottles). In general, categorizations were based on (1) main reservoirs of the pathogen, (2) main routes of transmission from the reservoir to the susceptible population, and (3) important predisposing and behavioural factors for human exposure (e.g. occupational exposure to farm animals or daily contact with pets). The main groups (person-to-person, predisposition and travel) were not sub-categorized. Predisposing factors included previous intake of drugs (e.g. antimicrobials and anti-acids), or pre-existent chronic disease, and were analysed individually.
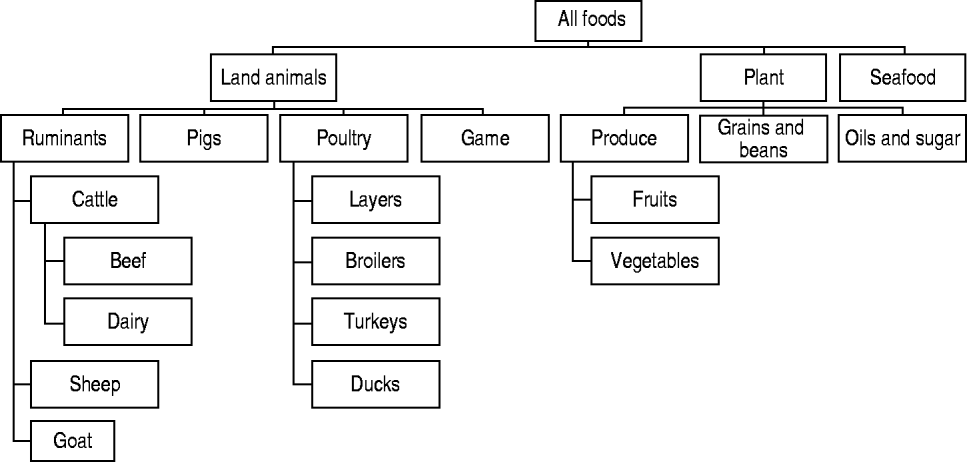
Fig. 1. Categorization of foods (based on [Reference Painter35]).
Location of exposures
Risk factors were further sub-classified as household or outside the household, according to the setting of the exposure. The location of exposure corresponds to where the food was consumed or exposure occurred (e.g. cafe/restaurant, institution, home).
Meta-analysis procedure
Outcome parameters
The ORs and 95% CIs per risk factor from each study were pooled in a meta-analysis using commercial software [13]. Some studies presented more than one risk factor that could be integrated in the same categorization stratum (e.g. ‘eating beef pink inside’ and ‘eating beef undercooked’). For these cases, a combined effect was calculated per study [13] so that a study with several risk factors in the same stratum did not have more influence on the total effect. When a study had more than one risk factor in the same main category (e.g. the food category chicken) but was classified in a different location category (e.g. ‘eating chicken at home’ and ‘eating chicken outside home’), each factor was treated individually. A random-effects model was used to calculate the pooled ORs [Reference Borenstein14].
The meta-analysis was designed to assess the influence of the factors age of the study population, geographical region, study period and serotype in the final outcome. Regional analyses were performed according to the United Nations regions (http://www.un.org/depts/dhl/maplib/worldregions.htm). For each stratum, we calculated (1) a pooled OR and 95% CI per group (age, region, time of the study and serotype, if information was available), and (2) a total pooled OR and 95% CI based on all groups [13]. The meta-analysis was performed only when at least four studies were available [Reference Halasa15] for each stratum.
Publication bias
The publication bias was assessed using Duval & Tweedie's trim-and-fill method [Reference Duval and Tweedie16], Begg & Mazumdar's rank correlation test [Reference Begg and Mazumdar17] and Egger's regression test [Reference Egger18]. When significant publication bias and change in the estimated pooled ORs were detected, the number of studies necessary to reverse the overall pooled effect was calculated using Orwin's fail-safe N method [Reference Orwin19]. The influence of a single study was also examined using the one study removed method [Reference Dohoo, Martin and Stryhn20]. If significant publication bias existed, the pooled ORs were estimated after correcting for the bias, based on Duval & Tweedie's trim-and-fill method.
A significant publication bias was considered to exist when adjustment for the bias altered a previous conclusion or when the confidence limits of the unadjusted and the adjusted ORs did not overlap.
RESULTS
Systematic review
From 1295 identified references, 131 passed the relevant screening, 72 passed the quality assessment stage, and data was extracted from 71. Full text references could not be found for 13 references, which therefore did not pass to the data extraction phase. One reference was added after a posterior non-SR. Results of the SR process are summarized in Table 1.
Table 1. Systematic review statistics
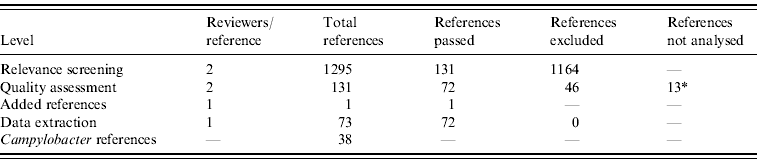
* Full text references could not be found, and a proper quality assessment was not possible.
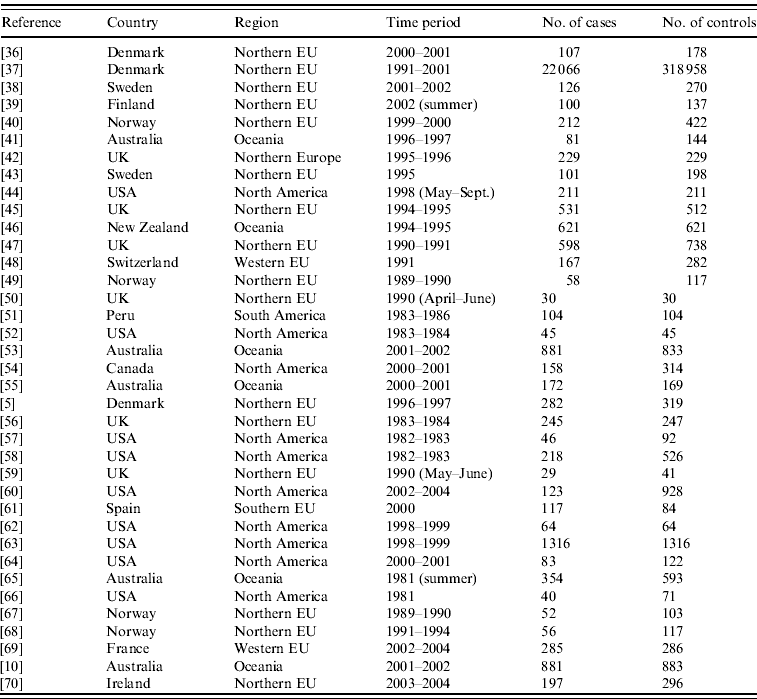
From the 72 references, 34 investigated risk factors of sporadic salmonellosis [Reference Domingues21], and 38 focused on sporadic campylobacteriosis. Campylobacter case-control studies were conducted between 1983 and 2004 in 14 different countries from three different continents. Seven studies investigated exposures in children, and two focused only on adult age groups. Most studies were designed to investigate exposures in Campylobacter in general, and only seven out of 38 investigated exposure to C. jejuni. Overall, the number of cases and controls interviewed varied between 30 (small-scale studies) and around 300 000 (community studies). All studies were published in English. The Appendix presents the complete list of Campylobacter studies collected in the SR.
Meta-analysis of risk factors of human sporadic campylobacteriosis
Results show that international travel was the most important risk factor for human campylobacteriosis in the overall studied population (OR 4·9, 95% CI 2·9–8·2), followed by consumption of undercooked chicken (OR 3·4, 95% CI 2·2–4·5), environmental exposure to Campylobacter (OR 3·2, 95% CI 2·0–5·3), and direct contact with farm animals (OR 2·6, 95% CI 2·0–3·4) (Fig. 2). Other important risk factors included pre-existent chronic disease and medication, drinking untreated water, consumption of different food products, and food preparation with poor hygiene. Among food transmission routes, consumption of undercooked chicken, eating chicken in a restaurant, eating poultry, and consuming unpasteurized dairy products were identified as the important sources for human campylobacteriosis (Table 2).
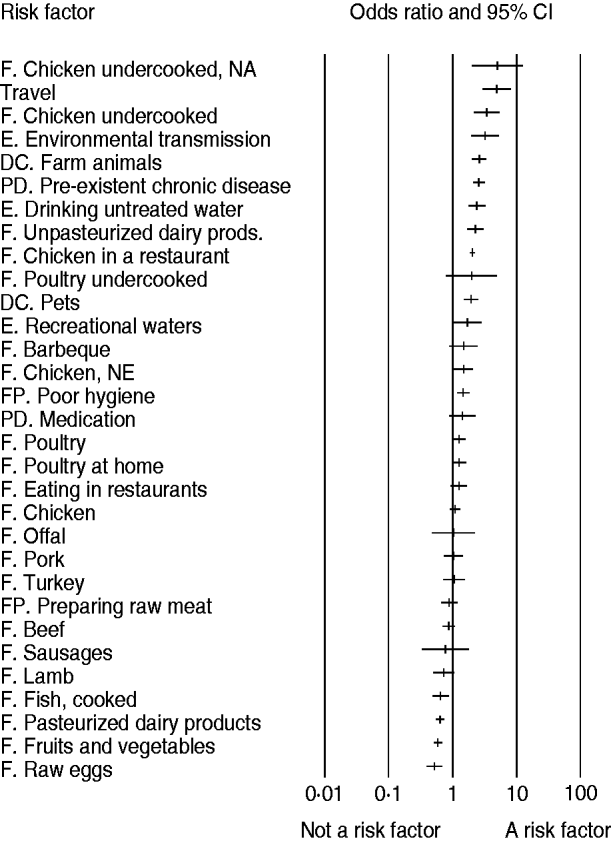
Fig. 2. Relative importance of risk factors for sporadic campylobacteriosis (odds ratio and 95% CI). F, Food; DC, direct contact; E, environmental; PD, pre-disposition; FP, food preparation; NA, North America; NE, Northern Europe.
Table 2. Relative importance of risk factors for sporadic campylobacteriosis in the overall population with odds ratio (OR) and 95% confidence interval (CI)
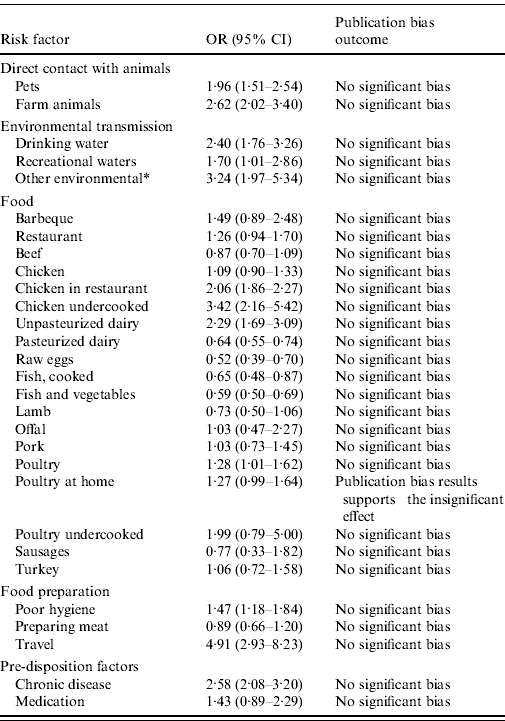
* Other environmental exposures refer to contact with bird droppings.
The analyses by age, region and location categories revealed differences in the impact of some sources of exposure in these subgroups. Direct contact with farm animals was estimated to be a more important risk factor for campylobacteriosis in children compared to the overall population (Fig. 3). However, there were only three studies focusing on children, which did not fulfil the minimum criterion for inclusion in the overall analysis, and thus results are not presented in the overall results, tables and figures. No significant differences in the impact of other sources in children and the overall population were found. Results of the sub-analyses by location showed that consumption of beef or pork in restaurants was a risk factor for campylobacteriosis, whereas eating beef or pork at home was not estimated to be important for infection (results not shown). Once again, due to the low number of studies focusing on eating pork or beef in a restaurant (n=3), the impact of different locations could not be confirmed. Relevant differences between regions were found in the impact of chicken products. The consumption of chicken, with no information on the method or degree of cooking, was a significant risk factor only in Northern Europe (OR 1·5, 95% CI 1·1–2·1), and consumption of undercooked chicken was shown to be important for human campylobacteriosis only in North America (OR 5·05, 95% CI 1·99–12·79).
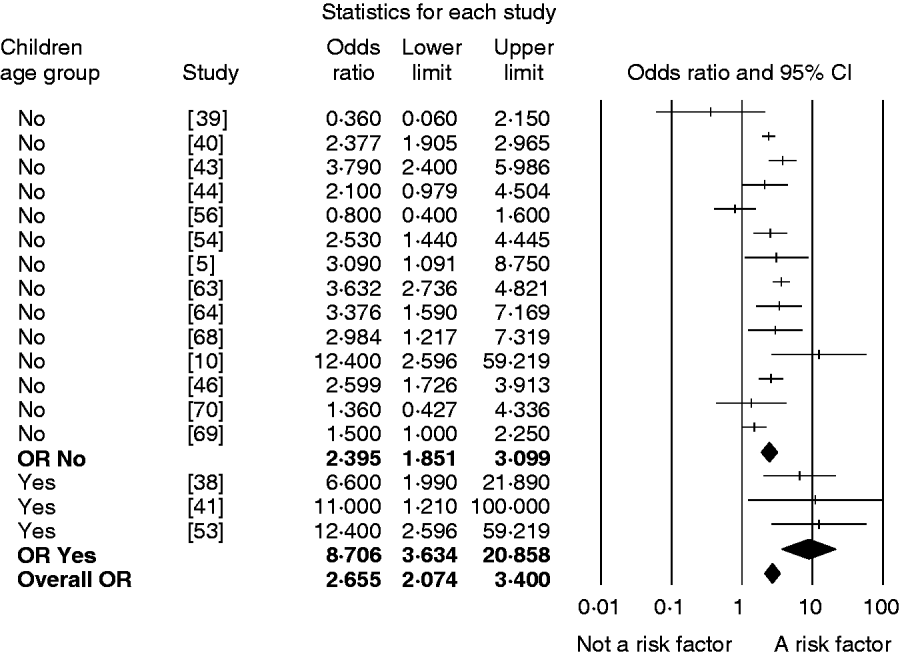
Fig. 3. Forest plot of the odd ratio (OR) of the risk of human campylobacteriosis after direct contact with farm animals for all ages (children age group=no) and only children (children age group=yes) for each of the 16 studies investigating this risk factor, the pooled OR per age group and the overall pooled OR together with the 95% confidence interval (CI).
The publication bias tests indicated the absence of potential significant publication bias in the analysis. Frequently, the funnel plot showed a lack of complete symmetry around the estimated pooled OR. However, adjusting for this effect did not change the results significantly. As an example, in the investigation of the effect of direct contact with a pet on the risk of campylobacteriosis, Duval & Tweedie's trim-and-fill method suggested adding three studies (solid symbols, •) to the left side of the funnel plot (Fig. 4). Adding these three missing studies would complete the symmetry around the pooled OR, and result in a slight shift of the pooled OR with its 95% CI to the left side (the black diamond at the bottom of the plot). This shift indicates a reduction of the magnitude of direct contact with a pet as a risk factor for campylobacteriosis. This lower magnitude was supported by the finding of Egger's regression test that suggested a significant association between study size and effect (intercept=2·6, s.e.=0·93, P=0·01). Furthermore, Begg & Mazumdar's test suggested a significant correlation between study size and effect (tau=0·3, P=0·04). Removing one study and re-calculating the effect using the one removed study method suggested the absence of influential studies. Nonetheless, adjusting for this bias by including the three missing studies as shown in Figure 3 only resulted in a slight shift of the pooled OR, without a significant change.
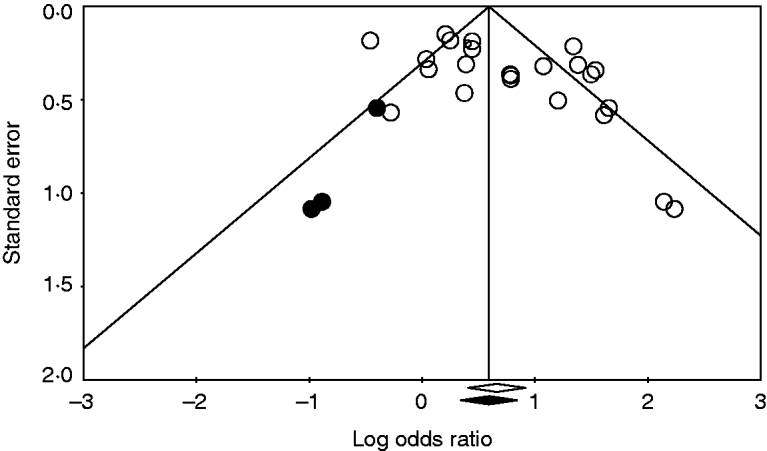
Fig. 4. Funnel plot of the logarithm pooled odds ratio (OR) of 24 studies (○) quantifying the effect of direct contact with a pet on the risk of human campylobacteriosis. The solid symbols (•) are the potential missing studies according to Duval & Tweedie's trim-and-fill method (if they had existed, the pooled effect would have shifted slightly towards the null effect; the black diamond under the x-axis).
DISCUSSION
This SR followed a rigorous search strategy to identify all potentially relevant peer-review case-control studies of sporadic campylobacteriosis. Collected studies were conducted in a wide variety of countries and time periods, designed with different settings, and sometimes focused on specific age groups within the population. The quality of the studies also varied, and was evaluated on the basis of defined methodological criteria during the formal process of the SR, and not judged on an individual basis by the reviewers.
The risk factors extracted from individual studies were categorized according to main source-classification schemes, and the meta-analyses of collected data were carried out per risk factor stratum, analysing information from all references that assessed the impact of that specific factor on the risk of disease. This categorization implied the harmonization of risk-factor labelling, which may have resulted in loss of information from individual studies, but which allowed for the integration and meta-analysis of results from all collected studies. Additionally, risk factors were included in the analysis only if they were investigated in four or more case-control studies. This criterion resulted in the exclusion of risk factors from the meta-analyisis, which may also have resulted in the loss of evidence and potentially biased estimates. For all analyses, risk factors that did not show any significant result were not described to avoid extending the length of the paper.
The meta-analysis of sporadic campylobacteriosis studies showed that travel, eating undercooked chicken, exposure through environmental routes, direct contact with farm animals and having a pre-existent chronic disease were the most important risk factors for infection in the overall population. International travel is not considered as a route of transmission by itself, since individuals may be exposed through the same routes as in the country of origin, but it is often considered separately for the purpose of source attribution [Reference Pires8]. The estimation of the impact of travel in the burden of foodborne diseases is important for the risk management process, since it allows for the estimation of the proportion of cases that are caused by domestic sources, and constitutes valuable information to raise awareness in the population on how to prevent foodborne infections when travelling abroad. Previous studies have identified travel as an important cause of infection with pathogens commonly transmitted through foods, including Campylobacter [Reference Ekdahl22, Reference Havelaar23]. Important environmental transmission routes include contact with objects contaminated with wild birds' droppings (e.g. bottles), drinking untreated water and swimming in recreational facilities, and these routes have been previously identified as sources of Campylobacter [Reference Mullner24, Reference Wilson25]. Supporting our results, chicken and poultry products have been identified as the most important food source of Campylobacter infections in all source attribution studies conducted in several countries worldwide (see e.g. [Reference Mullner24–Reference Evers26]). The category poultry may include chicken and other poultry meats (e.g. turkey and duck meats). The role of poultry in human campylobacteriosis has also been demonstrated in Belgium in 1999, when the discovery of high dioxin levels in feed led to a temporary withdrawal of poultry and eggs from retail outlets. This resulted in an estimated 40% reduction in the number of reported campylobacteriosis cases; however, reported disease returned to previous levels when consumption of poultry resumed [Reference Vellinga and Van27]. A similar phenomenon was observed in The Netherlands in 2003, when the threat of avian influenza led to the depopulation of 30 million birds [Reference Rosenquist28, Reference Van Pelt29]. These estimates provide a strong argument for the continuation of government and industry efforts focused on the reduction of the level of contamination of Campylobacter in broiler flocks and chicken carcasses.
When focusing on children, direct contact with farm animals was revealed to be an important risk factor, suggesting that this transmission route can be responsible for a high number of infections in children visiting a farm. This finding is supported by results of previous source attribution studies [Reference Evers26, Reference Mullner30], and may be explained by potential close contact with contaminated animal fur, fences or surrounding environments in a farm. Additionally, children are less likely to be careful with hand hygiene during and after a farm visit, are in general expected to be exposed to farm animals infrequently (e.g. during school visits), and may have less developed immunity to pathogens potentially transmitted through direct contact. However, only three studies have estimated this impact, and therefore further research is necessary to confirm or reject this hypothesis. No significant differences in the impact of other routes of transmission on the burden of disease in children and the overall population were found. Among direct contact with animals' routes, pets were also estimated to be an important source of exposure for the general population.
The regional sub-analyses revealed significant differences in the impact of the consumption of chicken in different regions of the world. We estimated that the consumption of undercooked chicken was the most important source of campylobacteriosis in North America, while chicken, with no information on the degree of cooking, was revealed to be an important source in Northern Europe, but with a lower estimate. It is widely recognized that contaminated chicken meat may be a vehicle for exposure to the pathogen, but that appropriate cooking times and temperatures allow for the elimination of the pathogen in foods [Reference Evers26, Reference Wallace and Hocking31]. The majority of the case-control studies conducted in Northern European countries included in our analysis asked participants about the consumption of chicken in general. Because the individuals interviewed in this way do not distinguish between fully cooked (and consequently more likely to be safe) and undercooked chicken products, this may have lead to a lower estimate of the OR. No other source or route of exposure was estimated to have a specific public health impact in different world regions. The lack of identified regional differences is probably explained by the low number or absence of studies available from some regions, namely Southern Europe (one study available), South America (one study available), and Asia (no studies available).
Our results were consistent in showing a higher impact of consumption of food products in restaurants compared to consumption in the household. Eating in a restaurant was estimated as an important risk factor by itself (Table 2), and the relative impact of eating chicken (Table 2), beef (Fig. 2) and pork (results not shown) outside the home for campylobacteriosis was higher than the estimated contribution of the consumption of the same foods at home. This may be explained by differences in the preparation of food products or respect for cooking and preservation times and temperatures between the two types of location, or could be a reflection of biased answers to the case-control studies' questionnaires.
In general, our results are supported by the findings of previous source attribution studies (see e.g. [Reference Havelaar23, Reference Evers26, Reference Hald32]). With the purpose of attributing the burden of disease to different sources, we do not draw conclusions on factors associated with a statistically significant reduced risk of disease. Main reasons include the impact of bias inherent in individual case-control studies, and thus to the final meta-analysis. While this is true for all exposures and all data that originate from interviews with patients and controls, it is particularly important when making inferences on the protective effect of specific exposures, which may eventually also be routes for infection.
The statistical analysis took into account the potential innate heterogeneity of studies by using the random-effects model [Reference Borenstein14]. A random-effects model is justified because studies are designed differently, conducted in different time periods and on different populations, which can create heterogeneous study populations [Reference Halasa33]. Moreover, if only a small number of studies for a risk factor are available, a lack of difference between groups could be apparent, but this would be a consequence of low statistical power and not due to actual lack of differences. Potential factors that could explain the heterogeneity were further investigated using classification. Publication bias results have shown the absence of significant bias. The fact that only slight changes occurred after adding missing studies to complete the symmetry in some of the analyses, as exemplified in Figure 4, indicates that we have probably not missed studies from the literature, and that even if we had, this effect would be minor and negligible. The utilized test assumes that these missing studies have been conducted, but were either missed in the search or were not published [Reference Ferguson34].
Because collected studies were conducted in different time periods and in a wide variety of regions, where the relative importance of sources for human campylobacteriosis can evidently vary, the representativeness of obtained estimates should be evaluated with care. Nonetheless, results give an indication of the most important sources of campylobacteriosis, which can be particularly useful for countries with no effective surveillance and where consequently data for other source attribution studies are not available. It is, however, important to acknowledge that one of the limitations of this method for source attribution is that it cannot be applied routinely (e.g. to investigate trends or the effect of implemented interventions), as an update of the results would require that a sufficient number of new case-control studies are conducted and published.
We conclude that a SR and meta-analysis of case-control studies is a valuable tool to collect and analyse all available information on risk factors of sporadic cases of pathogens commonly transmitted through foods. The approach is considered particularly useful for countries with no public health surveillance system in place, where the results can be used to support risk management decisions in identifying and prioritizing areas for interventions.
ACKNOWLEDGEMENTS
Financial support was provided be the EU Network of Excellence MedVetNet.
APPENDIX. Reference, country, region, time period, and number of cases and controls interviewed of case-control studies of sporadic campylobacteriosis collected in the SR
DECLARATION OF INTEREST
None.








