Besides being responsible for the digestion and absorption of nutrients, the fish gut is also an important barrier of defence against a number of aquatic pathogens and adverse environmental stimuli. It has been reported that diet-induced enteritis can dramatically affect fish production and, ultimately, threaten the sustainable development of fish cultures(Reference Zhang, Ran and Teame1). Soyabean meal-induced enteropathy (SBMIE) has been considered a limiting condition that abrogates fish feeding in several freshwater and marine fish species(Reference Kumar, Sakhawat Hossain and Ragaza2). The SBMIE in turbot is characterised by a series of histopathological changes, including (i) reduction of the mucosal folds, (ii) bulging of the subepithelial mucosa and lamina propria, (iii) prominent infiltration of pro-inflammatory cells, (iv) induction of IL and TNF-α, (v) down-regulation of antioxidant enzymes (due to oxidative stress) and (vi) increased apoptosis of intestinal epithelial cells(Reference Gu, Bai and Zhang3,Reference Tan, Zhou and Wang4) .
Secreted pro-inflammatory cytokines and reactive oxygen species, produced by recruited immune cells in mammals, can promote apoptosis of intestinal epithelial cells and therefore compromise the integrity of the intestinal epithelial barrier as well as exacerbate the mucosal inflammatory response in inflammatory bowel disease(Reference Maloy and Powrie5). The process of inflammatory response can activate a subset of transcription factors and related signalling cascades, including activator protein-1 (AP-1), mitogen-activated protein kinases (MAPK) and NF-кB, which together contribute to the expressions of many pro-inflammatory genes(Reference Broom, Widjaya and Troelsen6–Reference Yeung, Aziz and Guerrero-Castilla8). Hence, it would be appealing to investigate possible bioactive agents that can attenuate the induction of cytokines, down-regulate pro-inflammatory cascades (i.e. AP-1, MAPK and NF-кB) and promote an oxidative homoeostasis under certain inflammatory conditions(Reference Yeung, Aziz and Guerrero-Castilla8,Reference Yadav, Varum and Bravo9) .
Chitosan (CTS), the deacetylated derivative of chitin, is a linear polymer of β-(1,4)-linked D-glucosamine. Chitooligosaccharides (COS) correspond to a depolymerised form of CTS, generated by either chemical or enzymatic hydrolysis(Reference Hamed, Özogul and Regenstein10). Over the past decades, both CTS and COS have received considerable attention because of their anti-inflammatory, antioxidant, immunomodulatory and prebiotic properties. These functions have been related to their regulatory impact on several signalling pathways(Reference Park and Kim11,Reference Lodhi, Kim and Hwang12) . Similarly, CTS and COS have also been utilised as functional additives for aquatic animals(Reference Caipang and Lazado13–Reference Abdel-Ghany and Salem15) and proved to improve the morphological structure of intestine of fish and shrimp(Reference Kamali Najafabad, Imanpoor and Taghizadeh16–Reference Rahimnejad, Yuan and Wang19). Specifically, these studies have suggested that CTS and COS may potentially attenuate SBMIE. However, other reports have also shown some differences on the bioactivity of CTS and COS. Hu et al. (Reference Hu, Jou and Yang20) and Seyfarth et al. (Reference Seyfarth, Schliemann and Elsner21) have shown that the antibacterial and antifungal activities of CTS and its derivatives are significantly decreased by a decline on its molecular mass. Chiu et al. have also reported that CTS can greatly improve the lipid metabolism of high-fat-diet-fed rats when compared with COS(Reference Chiu, Feng and Liu22). Upon evaluation of aquatic animals, Niu et al. have demonstrated that only CTS (not COS) is capable of enhancing growth and resistance to low oxygen stress in Penaeus monodon (tiger shrimp)(Reference Niu, Lin and Jiang23). Thus, the effects of CTS or COS in fish affected by SBMIE, particularly involving the intestinal homoeostasis, require further investigations.
Due to the high quality of its meat and rapid growth, Scophthalmus maximus (turbot) has become the most prominent cultured flatfish in Asia and Europe, reaching a global production of about 57 000 tons in 2017(24). Hence, turbot could be utilised as a model for causal and mechanistic studies related to SBMIE and for the mitigation of technology development(Reference Gu, Bai and Zhang3). In this research, we examined and also compared the potential positive effects of CTS and COS on the growth, histological structure, inflammation-related cytokines expression, immune and anti-oxidative parameters as well as mucosal microbiota, in the distal intestine (DI) of juvenile turbot fed with soyabean meal (SBM) diet. Functionally, we also explored related mechanisms by examining the expression and activity of NF-кB, AP-1 and MAPK-related genes.
Materials and methods
Feed content and formulation
A SBM-based diet was prepared as previously(Reference Gu, Bai and Zhang3). This diet contained 48 % protein and 12 % lipids, which included soyabean and fishmeal. Wheat flour was used as carbohydrate source, while fish and soyabean oil were added as lipid sources (Table 1). Two isolipidic and isonitrogenous diets were also designed by supplementing the SBM diet (control) with 7·5 g/kg CTS (CTS diet) or 2 g/kg COS (COS diet)(Reference Niu, Lin and Jiang23,Reference Chen, Zhu and Yang25–Reference Su, Han and Jiang28) . All formulations were designed to meet the essential amino acid requirements for juvenile turbot, according to the whole-body amino acid profiling(Reference Kaushik29,Reference Peres, Oliva-Teles and Kaushik30) . Standard methods were used to evaluate the nutritional values of these diets(31). Ash and moisture contents were gravimetrically determined after heating procedures (550 °C and 105 °C, respectively). Crude lipid content was also gravimetrically analysed following ethyl ether extraction (Extraction System B-811, BUCHI). According to the Kjeldahl method, the content of crude protein was evaluated by a Kjeltec 2300 Autoanalyzer (FOSS), with the use of boric acid to trap the released ammonia. Gross energy was examined using a calorimetric pump (Parr). Amino acid profiles related to both ingredients and whole diet were determined accordingly (S-433D, Sykam). The preparation and storage of respective diet formulations were conducted as previously(Reference Gu, Bai and Kortner32). All ingredients except fish oil, soyabean oil and soyabean lecithin were first ground into fine powder through 180-μm mesh and were mixed thoroughly. Fish oil, soyabean oil and soyabean lecithin were also mixed. The oil and water were mixed with other ingredients thoroughly to produce stiff dough. Finally, the dough was pelleted by experimental feed mill (SKJ120, Shandong Minglun machinery factory) at the length and diameter of 2 mm and dried for about 12 h at 45 °C. The dried feed was stored in a freezer at −20 °C until used.
Table 1. Ingredients and composition of the experimental diets (DM basis)

* SBM, a basal diet containing 400 g/kg of soyabean meal; CTS, inclusion of 7·5 g/kg of chitosan in SBM diet; COS, inclusion of 2 g/kg of chitooligosaccharide in SBM diet.
† Steam-dried fish-meal (COPENCA Group).
‡ Vitamin premix consisted of the following compounds (mg kg−1 diet): retinyl acetate, 32; vitamin D3, 5; dl-α-tocopherol acetate, 240; vitamin K3, 10; thiamin, 25; riboflavin (80 %), 45; pyridoxine hydrochloride, 20; vitamin B12 (1 %), 10; L-ascorbyl-2-monophosphate-Na (35 %), 2000; calcium pantothenate, 60; nicotinic acid, 200; inositol, 800; biotin (2 %), 60; folic acid, 20; and cellulose, 11 473. Mineral premix composed of the following ingredients (mg/kg diet): FeSO4·H2O, 80; ZnSO4·H2O, 50; CuSO4·5H2O, 10; MnSO4·H2O, 45; KI, 60; CoCl2·6H2O (1 %), 50; Na2SeO3 (1 %), 20; MgSO4·7H2O, 1200; and zeolite, 8485.
§ Procured from Sigma-Aldrich; No. 417963.
|| Procured from Sigma-Aldrich; No. 523682.
Fish storage and culture
Procedures of fish manipulation were initially approved by the Ethical Scientific Committee for Animal Experimentation of the Shandong University and performed in compliance with the European directive 2010/63/UE.
Healthy juvenile turbots were supplied by a commercial farm in Haiyang (China). The fish were allocated into an indoor flow-through water system (Haiyang Yellow Sea Aquatic Product Co. Ltd), acclimated accordingly and fed with a commercial diet for 2 weeks. Subsequently, turbots with an initial body weight of about 11·7 g were randomly placed into nine tanks (thirty-five fish in each tank; 300 litres of seawater per tank). For seawater acquisition, adjacent coastal water was filtered by a sand filter and then transferred to each fish tank at the flow rate of about 2·0 litres/min. Respective fish diet (total of 3) was randomly administered to each three tanks. The fish were fed twice a day, at 07.00 and 06.00 hours. Feed consumption and feed intake were recorded accordingly. Water temperature was adjusted between 12 and 16 °C, pH was in the range of 7·8–8·2 and the salinity was at 28–30 g/l.
Fish sampling
After 56 d of feeding, all fish were anaesthetised with eugenol (1:10 000 dilution; Shanghai Reagent Co.) and then weighted. Afterwards, six fish were selected from each tank and further dissected. For this, both liver and intestine were removed (cleared of any mesenteric and adipose tissues) and then washed with cold PBS to eliminate any remaining gut content. Body length and weight, as well as intestine and liver weight, were measured and recorded for further calculation of condition factor, hepatosomatic index and intestosomatic index. To ensure dietary exposure, only the fish with digested food along the intestinal tract were sampled. Four fish were randomly selected from the six selected fish, followed by the removal of their respective DI. Tissues were then divided into two sections to allow both histological and gene expression analyses. A section of DI was added into 4 % formaldehyde in PBS for 24 hours and, thereafter, kept in 70 % ethanol until microscopy analyses. The other tissue section was placed in RNAlater (Ambion) and stored at −80 °C before expression analysis. For both analyses, tissues were treated, stored and coded individually. To assess their enzymatic activity, sections of dissected DI from another fish (four per tank) were frozen in liquid N2 and stored at −80 °C until further use.
For autochthonous microbiota analysis, the remaining fish were starved for 24 h and then sampled as previously reported(Reference Gajardo, Rodiles and Kortner33). For this, fish were anaesthetised and then cleaned with 70 % ethanol before assessing their abdomen at the ventral midline. Whole intestines were then removed, under aseptic conditions, from the abdominal cavity. Fish intestine was opened longitudinally, and the mucosa-associated microbiota was further extracted by scraping the mucosal layer using a sterile scalpel. Mucosal layers (from at least twenty fish per tank) were pooled as single samples, frozen in liquid N2 and stored at −80 °C until further analysis. During the feeding, sampling and the following analysis and data statistics, the researchers excluding corresponding author were blinded for the sampling sources.
Histological analysis
Fixed tissue samples were prepared accordingly before staining with haematoxylin–eosin. Tissue examination was performed blindly using a light microscope. According to Penn et al. (Reference Penn, Bendiksen and Campbell34), a progressing scoring scale (0–10) was defined. For this, the following histological characteristics were considered: (i) fusion and extension of the mucosal folds, (ii) cell infiltration and width along the submucosa and lamina propria (iii), enterocyte vacuolisation and (iv) position of the nucleus within enterocytes.
Quantitative real-time PCR
Total RNA was isolated from DI tissues (about 50 mg per sample) by following the manufacturer’s instructions (RNeasy Protect Mini Kit, catalogue no. 74126, Qiagen, GmbH, Hilden). Purified RNA was further quantified using a NanoDrop® ND-1000 spectrophotometer (Nano-Drop Technologies). RNA quality was examined using an Agilent Bio-Analyzer (Agilent Technologies). First-strand cDNA synthesis was conducted using one microgram of RNA/sample, as indicated by the manufacturer’s protocol (QuantiTect Reverse Transcription Kit, catalogue no. 205311, Qiagen, GmbH).
The expression profiles of selected genes, including AP-1, IL-1β, IL-8, NF-кB, TNF-α, p38 mitogen-activated protein kinase (p38), c-Jun N-terminal kinase (JNK) and extracellular regulated kinase (ERK) were determined by quantitative PCR. The expression ribosomal protein S4 was utilised for data normalisation. Validated gene-specific primers were provided by Gu et al. (Reference Gu, Bai and Zhang3) and Zhao et al. (Reference Zhao, Chen and Zheng35). QPCR reactions were conducted as described previously(Reference Gu, Bai and Kortner32) in 25 μl reaction volume including 12·5 μl of SYBR Green PCR Master Mix (QuantiTect SYBR Green RT-PCR Kit, Qiagen, 204243), 10·5 μl of ultrapure water (Sigma-Aldrich), 1·0 μl of each specific primer (10 μm) and 1·0 μl of cDNA template. The thermal profile was 95 °C for 20 s, followed by 40 cycles of 95 °C for 5 s, 56 °C for 30 s and 72 °C for 30 s. At the end of each cycle, fluorescence readings were recorded to estimate quantification cycle values (Cq). Melting curve analysis was performed to verify that only one PCR product was present in each reaction. Raw Cq values were normalised to ribosomal protein S4 using a relative quantitative method (2−ΔΔCT) and expressed as fold change.
Intestinal biochemical analysis
Gut samples were homogenised in cold saline solution (10 volumes, w/v) and then centrifuged at 5000 × g for 20 mins at 4 °C. Thereafter, supernatants were separated and split into twenty pieces. Respective solutions were kept at –80 °C until further analysis.
The concentrations of malondialdehyde (MDA) in the gut were assessed as an indicator of lipid peroxidation. The activities of other enzymes, such as acid phosphatase, catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPX), lysozyme and superoxide dismutase (SOD), were also assayed. Additionally, the levels of intestinal glutathione (GSH), complement 3, complement 4 and IgM were also measured accordingly(Reference Bai, Gu and Liu36). All the analysis was conducted by the kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China; Product code: MDA, A003-1-1; acid phosphatase, A060-1-1; CAT, A007-1-1; GR, A062-1-1; GPX, A005-1-1; lysozyme, A050-1-1; SOD, A001–1-1; GSH, A006-1-1; complement 3, H186-1; complement 4, H186-2; IgM, H109).
Analysis of autochthonous microbial community in the fish gut
DNA sequencing
Based on our previous work(Reference Bai, Gu and Xu37), bacterial DNA was extracted using the CTAB/SDS method. The DNA quality was monitored on a 1 % agarose gel electrophoresis, and the concentration was determined using a NanoDrop® ND-1000 spectrophotometer (Nano-Drop Technologies). After diluting to 1 ng/μl with sterile water, DNA samples were subjected to amplification using 341F and 806R primers with barcode specific for 16SV3-V4 regions. All PCR were carried out with Phusion® High-Fidelity PCR Master Mix (New England Biolabs). Thermal cycling consisted of initial denaturation at 98 °C for 1 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s and elongation at 72 °C for 30 s. The PCR products were mixed with same volume of 1 × loading buffer (contained SYBR Green), and the quantification and qualification were assayed in electrophoresis on 2 % agarose gel for detection of bands between 400 and 450 bp. After mixing in equidensity ratios, PCR products from all samples were purified with a Qiagen Gel Extraction Kit (Qiagen). Sequencing libraries were generated using a TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina), and index codes were added. The library quality was assessed on the Qubit@ 2·0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. DNA sequencing library was constructed using an Illumina HiSeq 2500 platform, upon generation of 250-bp paired-end reads.
Data analysis
The assembly and quality control of respective paired-end reads as well as species annotation and OTU-based clustering were carried out as previously(Reference Bai, Gu and Xu37). Paired-end reads were merged using FLASH (http://ccb.jhu.edu/software/FLASH/; version 1.2.7). High-quality clean tags were obtained by QIIME (http://qiime.org/index.html; version 1.7.0). Effective tags were obtained by removing chimera sequences detected UCHIME algorithm (http://www.drive5.com/usearch/manual/uchime_algo.html). Sequences were analysed by Uparse (http://drive5.com/uparse/; version 7.0.1001), and sequences with ≥97 % similarity were assigned to the same OTU. Representative sequence for each OUT was annotation using the Green Gene Database (http://greengenes.lbl.gov/cgi-bin/nph-index.cgi) based on RDP 3 classifier (http://sourceforge.net/projects/rdpclassifier/; version 2.2). Multiple sequence alignment was conducted using the MUSCLE software (http://www.drive5.com/muscle/; version 3.8.31). OTU abundance information was normalised using a standard of sequence number corresponding to the sample with the least sequences. Alpha- and beta-diversities were further analysed according to the normalised output data. Alpha diversity metrics, such as Chao 1 richness and Shannon diversity index, were analysed by QIIME (version 1.7.0) and then interpreted using R software (version 2.15.3). In the context of the beta-diversity, a cluster analysis was performed by nonmetric multidimensional scaling using the vegan package in R language (https://www.r-project.org/; version R-2.15.3).
Calculation and statistics
Growth performance and food acquisition were quantified by calculating the respective feed efficiency ratio and specific growth rate as follows:
 $${\rm{Intestosomatic}}\ {\rm{index}}\ \left( \% \right) = \left( {{\rm{intestine}}\ {\rm{weight}}/{\rm{body}}\ {\rm{weight}}} \right) \times {\rm{1}}00$$
$${\rm{Intestosomatic}}\ {\rm{index}}\ \left( \% \right) = \left( {{\rm{intestine}}\ {\rm{weight}}/{\rm{body}}\ {\rm{weight}}} \right) \times {\rm{1}}00$$
Statistical analysis was performed using SPSS 11.0. All data (except the light microscopy and quantitative PCR assay) were analysed by the one-way ANOVA test. The difference among the means was examined using Duncan’s multiple range test. P-value of <0·05 was deemed statistically significant. Light microscopy analysis and quantitative PCR data were analysed by Kruskal–Wallis/Wilcoxon test, followed by the tech post hoc Wilcoxon method for comparison of the means.
Results
Dietary impact on turbot growth and biometric parameters
As indicated in Table 2, the final body weight, specific growth rate, feed efficiency ratio and condition factor of fish fed with CTS or COS diets were remarkably increased compared with those of fish fed with SBM diet only (P < 0·05), but no obvious difference was found between these two groups (P > 0·05). In addition, no significant differences were found among the three groups in regard to FI, intestosomatic index and hepatosomatic index (P > 0·05).
Table 2. Growth performance and biological parameters of the turbots fed the experimental diets for 8 weeks*
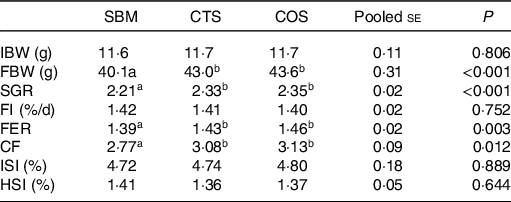
CF, condition factor; FBW, final body weight; FER, feed efficiency ratio; FI, feed intake; HSI, hepatosomatic index; IBW, initial body weight; ISI, intestosomatic index; SGR, specific growth rate; SBM, a basal diet containing 400 g/kg of soyabean meal; CTS, inclusion of 7·5 g/kg of chitosan in SBM diet; and COS, inclusion of 2 g/kg of chitooligosaccharide in SBM diet.
* All values are expressed as means of three replicate measurements. For IBW, FMW, SGR, FI and FER, the result of a single replicate measurement was calculated by the body weight data and feed consumption data of all thirty-five fish in one tank. For CF, ISI and HIS, the result of a single replicate measurement was calculated by the average of six randomly selected fish in one tank.
a,bMean values in the same row with different superscript letters denote a statistically significant difference (P < 0·05).
Distal intestinal inflammation
Morphological characteristics of the distal intestine
According to our previous studies, here we confirmed that dietary SBM was capable of inducing SBMIE(Reference Gu, Bai and Zhang3). As demonstrated in Fig. 1 and Table 3, the fish fed with CTS or COS diets presented a significant increase on the height of mucosal folds and enterocyte nucleus, as well as a decrease on the width and cellular (leucocyte-based) infiltration in both submucosa and lamina propria when compared with those fed with SBM diet only (P < 0·05). The fusion of the mucosal folds was also markedly reduced in CTS or COS diet groups v. SBM diet only group (P < 0·05). In regard to all evaluated parameters, no noticeable differences were observed when comparing both CTS and COS groups (P > 0·05).

Fig. 1. Representative images of H&E-stained distal intestinal sections from turbots fed with the three diets. Abbreviations: SBM, a basal diet containing 400 g kg-1 of soybean meal; CTS, SBM diet containing 7.5 g kg-1 chitosan inclusion; COS, SBM diet containing 2 g kg-1 of chitooligosaccharides. Bar represents 500μm.
Table 3. Distal intestine tissue variable scores of the turbots fed the experimental diets for 8 weeks

LP, lamina propria; MF, mucosal fold; SM, submucosa; ent, enterocyte; SBM, a basal diet containing 400 g/kg of soyabean meal; CTS, inclusion of 7·5 g/kg of chitosan in SBM diet; and COS, inclusion of 2 g/kg of chitooligosaccharide in SBM diet.
* All values are expressed as means of twelve fish randomly selected from three replicate groups.
a,bMean values in the same row with different superscript letters indicate a statistically significant difference (P < 0·05).
Gene expression analysis
Compared with SBM-fed fish (control), fish receiving either CTS or COS diets showed significantly lower expression levels of IL-1β, IL-8, AP-1, NF-кB, TNF-α, p38, JNK and ERK (P < 0·05; Fig. 2). Although the expression levels of all genes presently tested in COS group were slightly up-regulated compared with CTS group, such difference was not statistically significant (P > 0·05).
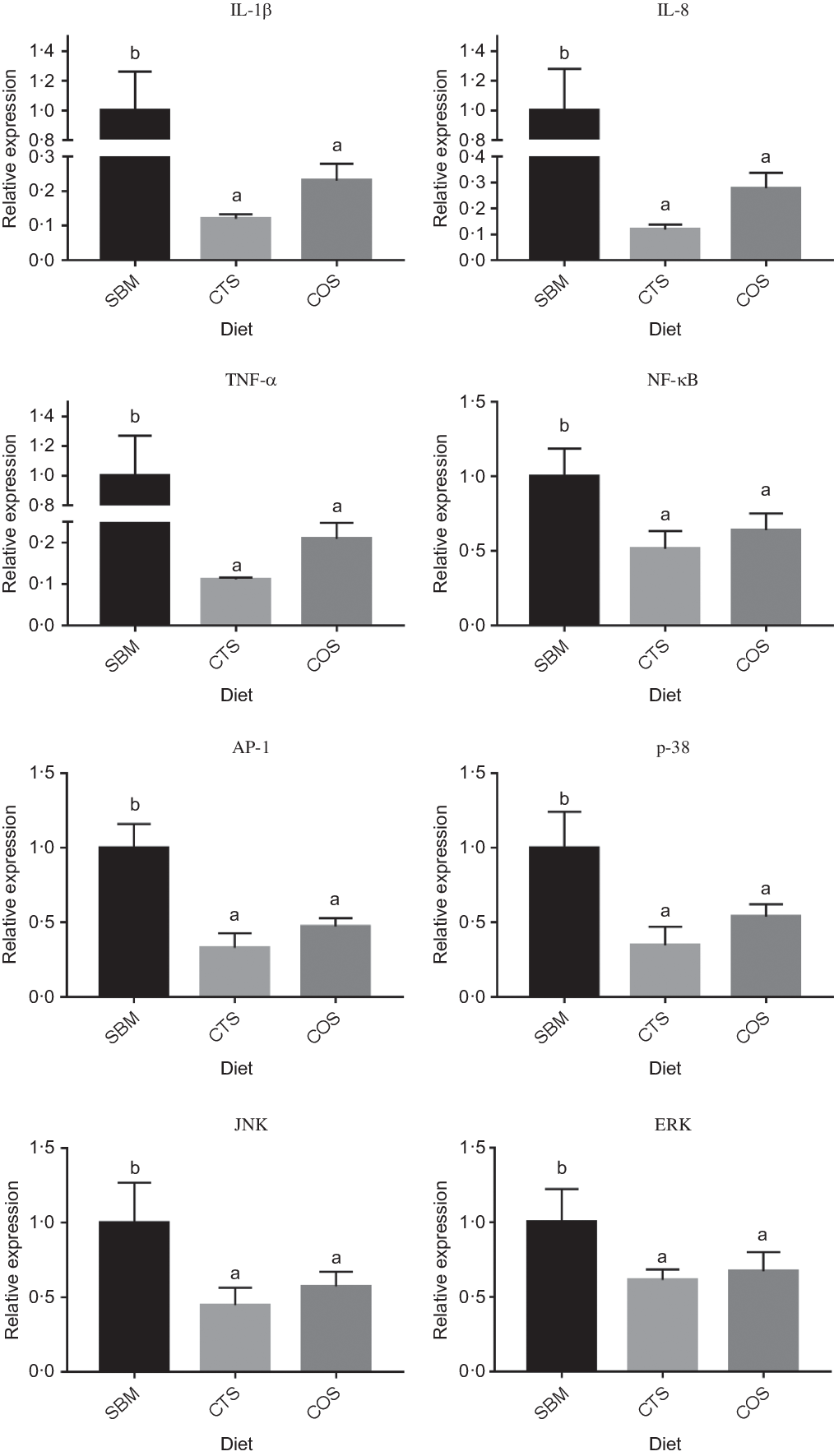
Fig. 2. Relative expression of three inflammation-related genes and five pathway regulatory molecule genes in the intestine of turbots fed with the three diets. Data are presented as means and standard error from three replicates. Data of one replicate was measured from the intestines of four random selected fish in one tank. Mean values for the same gene (with different letters) were significantly different (P<0.05). Abbreviations: SBM, a basal diet containing 400 g kg-1 of soybean meal; CTS, SBM diet containing 7.5 g kg-1 chitosan inclusion; COS, SBM diet containing 2 g kg-1 of chitooligosaccharides; AP-1, activator protein-1; IL-1β, interleukin-1 beta; IL-8, interleukin 8; NF-κB, nuclear transcription factor-kappa B; TNF-α, tumor necrosis factor alpha; p38, p38 mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase; ERK, extracellular regulated kinase.
Intestinal oxidant and antioxidant parameters
As presented in Table 4, MDA concentrations in the fish fed with CTS and COS diets were remarkably lower than those fed with SBM diet only (P < 0·05). In contrast, the levels of CAT, GPX, GR, GSH and SOD in fish fed with CTS and COS diets were markedly higher than those receiving SBM diet only (P < 0·05). However, no remarkable differences in the levels of MDA, SOD, CAT, GSH and GR was detected upon comparing the CTS and COS groups (P > 0·05). Nevertheless, the activity of GPX in COS group was obviously increased when compared with CTS group (P < 0·05).
Table 4. Intestinal oxidant and antioxidant parameters of the turbots fed the experimental diets for 8 weeks

CAT, catalase; GPX, glutathione peroxidase; GR, glutathione reductase; GSH, glutathione; MDA, malondialdehyde; SOD, superoxide dismutase; SBM, a basal diet containing 400 g/kg of soyabean meal; CTS, inclusion of 7·5 g/kg of chitosan in SBM diet; COS, inclusion of 2 g/kg of chitooligosaccharide in SBM diet.
* All values are expressed as means of three replicates. The data of one replicate were measured from the distal intestines of four randomly selected fish in one tank.
a,bMean values in the same row with different superscript letters denote a statistically significant difference (P < 0·05).
Immune-related parameters
Fish fed with either CTS or COS diets presented a significant increase on lysozyme and acid phosphatase activities as well as IgM concentration when compared with those fed with SBM diet only (P < 0·05; Table 5). In particular, the acid phosphatase activity of fish was markedly higher in COS diet group than in CTS diet group (P < 0·05). There were no obvious differences noted among the three groups at complement 3 and complement 4 concentrations (P > 0·05).
Table 5. Intestinal immune parameters of the turbots fed the experimental diets for 8 weeks*

ACP, acid phosphatase; C3, complement 3; C4, complement 4; LZM, lysozyme; SBM, a basal diet containing 400 g/kg of soyabean meal; CTS, inclusion of 7·5 g/kg of chitosan in SBM diet; COS, inclusion of 2 g/kg of chitooligosaccharide in SBM diet.
* All values are expressed as means of three replicates. The data of one replicate were measured from the distal intestines of four randomly selected fish in one tank.
a,bMean values in the same row with different superscript letters indicate a statistically significant difference (P < 0·05).
Mucosal microbiota in the intestine
Phylotype coverage
After quality control analysis, 467 436 reads were obtained, covering 2034 OTU (97 % of similarity) in a total of nine samples. The average number of reads per treatment was 52 011, 51 435 and 52 366 reads. The Good’s coverage of all samples was 0·999, implying that the sequence depth was sufficient.
Bacterial diversity in the intestine mucosa
Changes in the bacterial richness (estimated with the Chao1 index) and diversity (estimated with the Shannon index) of fish fed with CTS or COS diet are presented in Fig. 3(a) and (b). Fish fed with either CTS or COS diets had a significantly lower Chao1 and Shannon indices than those receiving SBM diet only (P < 0·05). The beta diversity among fish groups was evaluated by nonmetric multidimensional scaling. Three diet-related nonmetric multidimensional scaling clusters were distinctively separated, suggesting a differential response model of the intestinal microbiota towards each respective diet (Fig. 3(c)). In addition, the relative abundances of top fifty most abundant bacterial genera were analysed (online Supplementary Table S1) and the relative abundance of Bacillus, Lactobacillus and Pseudomonas was compared among groups. Consistently, CTS or COS treatment markedly elevated the relative abundances of all three bacteria genera (P < 0·05; Fig. 3(d), (e) and (f)). However, no noticeable differences in Chao1 and Shannon indices as well as Lactobacillus, Bacillus and Pseudomonas abundances were observed between CTS and COS groups (P > 0·05).

Fig. 3. General structural changes of gut microbiota and relative abundance of selected bacterial genus in turbots fed with the three diets. Chao1 (A) and Shannon (B) indices were used to estimate the richness and community diversity of the gut microbiota. Non-metric multi-dimensional scaling (NMDS) was calculated according to the relative abundances of OUT of turbots in each diet group. Each point represents the microbiota composition in, at least, 20 fish from a single tank (C). Determination of the relative abundance of genus of Lactobacillus (D), Bacillus (E) and Pseudomonas (F). Mean values for the same genus (with different letters) are significantly different (P<0.05). Abbreviations: SBM, a basal diet containing 400 g kg-1 of soybean meal; CTS, SBM diet containing 7.5 g kg-1 chitosan inclusion; COS, SBM diet containing 2 g kg-1 of chitooligosaccharides.
Discussion
Although some reports have shown differences on the bioactivity of CTS and COS, our present work did not identify any difference between these additives in regard to growth promotion or anti-inflammatory effects in turbots affected by SBMIE. CTS-mediated growth has been demonstrated in other fish models, like Carassius auratus gibelio (gibel carp)(Reference Chen, Zhu and Yang25), Cyprinus carpio (common carp)(Reference Gopalakannan and Arul38), Dicentrarchus labrax (sea bass)(Reference Zaki, Salem and Gaber17), Misgurnus anguillicaudatus (loach)(Reference Yan, Guo and Dawood39), Mugil cephalus (grey mullet)(Reference Akbary and Younesi40), Oreochromis niloticus (Nile tilapia)(Reference Abd El-Naby, Naiel and Al-Sagheer41,Reference Fadl, El-Gammal and Abdo42) , Rachycentron canadum (cobia)(Reference Geng, Dong and Tan43) and shrimps including Litopenaeus vannamei (white shrimp)(Reference Niu, Liu and Lin44) and P. monodon (tiger shrimp)(Reference Niu, Lin and Jiang23,Reference Niu, Li and Tian45) . Similarly, COS can also enhance the growth performance of numerous fish species, such as Cyprinus carpio koi (koi)(Reference Lin, Mao and Guan26), Micropterus salmoides (largemouth bass)(Reference Lin, Jiang and Chen27), O. niloticus (Nile tilapia)(Reference Meng, Wang and Wan46,Reference Guan, Sun and Meng47) , Pangasianodon hypophthalmus (striped catfish)(Reference Nguyen, Van Dang and Le48), Paramisgurnus dabryanus (loach)(Reference Zhang49), Takifugu rubripes (tiger puffer)(Reference Su, Han and Jiang28) and Trachinotus ovatus (pompano)(Reference Lin, Mao and Guan50). Here, we were able to confirm that both CTS and COS can actively promote fish growth. This enhanced growth performance appears to translate into better intestinal health status, in response to CTS and COS supplementation.
SBM-mediated inflammation of fish DI is characterised by swelling of the subepithelial mucosa and lamina propria, as well as by strong infiltration of various pro-inflammatory cells and overexpression of inflammation-related cytokines(Reference Sirri, Sarli and Bianco51,Reference Trushenski52) . In turbot, the infiltration of inflammatory cells and expression of a subset of inflammation-related cytokines were promoted by SBM in a dose-dependent fashion, as indicated previously(Reference Gu, Bai and Zhang3). In this study, fish fed with CTS- or COS-supplemented diet showed a lower content of pro-inflammatory cell infiltration as well as down-regulated gene expression of prototypical cytokines, such as IL-1β, IL-8 and TNF-α, which clearly indicate the protective effects of these additives against SBM-induced inflammation in turbot DI. To our knowledge, the present study inaugurally demonstrates the protective role of CTS and COS against intestinal inflammatory in fish. Based on the present work, other fish species suffering from SBMIE or other kind of inflammation could utilise CTS and COS as treatment strategy.
Compelling investigations in humans and other higher organisms have shown that the secretion of pro-inflammatory cytokines can play an essential role in the progression of inflammatory bowel disease(Reference Friedrich, Pohin and Powrie53). It has been indicated that both CTS and COS supplementation can attenuate intestinal inflammation by suppressing the expression of certain cytokines as well as the production of inflammatory regulators both in vivo (Reference Azuma, Osaki and Kurozumi54–Reference Huang, Xiao and Tan56) and in vitro (Reference Tu, Xu and Xu57,Reference Yang, Tong and Luo58) . Hence, according to our present study, it is reasonable that CTS or COS supplementation in fish diet can attenuate SBM-induced inflammatory response in turbot, partially by suppressing the production of pro-inflammatory cytokines.
AP-1 and NF-кB are two well-studied transcription factors which are capable of inducing the production of inflammatory cytokines such as IL-1β, IL-8 and TNF-α (Reference Jimi, Takakura and Hiura59,Reference Zenz, Eferl and Scheinecker60) . It has been shown that both CTS and COS may reduce an inflammatory response by abrogating AP-1 and NF-кB activation both in vivo and in vitro (Reference Yousef, Pichyangkura and Soodvilai55–Reference Yang, Tong and Luo58,Reference Liu, Huang and Ma61–Reference Ahn, Jung and Park66) . In the present study, dietary CTS or COS could down-regulate the mRNA expression of inflammation-related genes, from which we speculate that the inhibitory effect of these additives towards cytokine production in SBM-fed fish may be due to the suppression of NF-кB and AP-1 activation. The activation of related pathways is modulated by MAPK(Reference Franklin and Kraft67). LPS-mediated activation of three MAPK, such as p38 MAPK, extracellular (ERK) and c-Jun N-terminal kinase/stress-activated protein (JNK), has been shown to promote gene expression related to an inflammatory response(Reference Kaminska68). Activated p38 MAPK can affect cytokine levels by regulating NF-кB-dependent gene expression(Reference Kaminska68). Activated JNK initiates the activation of c-Jun that constitutes AP-1, while ERK can act by inducing AP-1 expression(Reference Uto, Fujii and Hou69,Reference Surh, Chun and Cha70) . In this context, it has been reported that CTS and COS can suppress LPS-induced phosphorylation of p38 MAPK, ERK and JNK(Reference Liu, Huang and Ma61,Reference Ahn, Jung and Park66,Reference Pangestuti, Bak and Kim71) . Taken altogether, our findings suggest that CTS and COS may block SBM-induced signal transduction of inflammatory cytokines by suppressing the mRNA levels of genes involved in NF-кB, AP-1 and MAPK pathways. Nevertheless, further investigations are still necessary to clarify the precise mechanism involved in these events.
Many studies have shown that oxidative stress is largely accompanied by inflammation, thus leading to tissue damage(Reference Balmus, Ciobica and Trifan72). In regard to SBMIE-affected turbots, Chen et al. (Reference Chen, Zhao and Liu73) and Tan et al. (Reference Tan, Zhou and Wang4) have reported that SBM may induce oxidative stress in the turbot intestine by elevating the intestinal MDA content and also decreasing the intestinal total antioxidant capacity and the transcript levels of antioxidant enzymes, including SOD, GPx, heme oxygenase 1 and peroxiredoxin 6. It has been considered that these antioxidant enzymes are consumed upon defence against the oxidation caused by SBM(Reference Tan, Zhou and Wang4). Due to their free radical scavenging activities and proton donation ability, CTS and COS act as efficient additives capable of maintaining expected antioxidant levels(Reference Kim74). In the present work, the administration of CTS or COS appears to prevent SBM-induced lipid peroxidation and, at the same time, to preserve anti-oxidant substrates (GSH) and anti-oxidative enzyme activity (SOD, CAT, GPX and GR). Here, we validated that either CTS or COS behaves as efficient antioxidants in turbot, similarly to M. anguillicaudatus (dojo loach)(Reference Yan, Guo and Dawood39), O. niloticus (Nile tilapia)(Reference Abu-Elala, Mohamed and Zaki75), P. dabryanus (loach)(Reference Zhang49) and P. monodon (tiger shrimp)(Reference Niu, Lin and Jiang23).
Wang and co-workers have demonstrated that a substitution of fishmeal for SBM diminishes the immunity of turbot(Reference Wang, Zhou and He76). This effect might be attributed to nutritional imbalances as well as anti-nutritional substances present in SBM(Reference Wang, Zhou and He76–Reference Krogdahl, Penn and Thorsen79). The immunostimulating effects of CTS have been widely studied in several aquatic animals (as reviewed by Abdel-Ghany & Salem(Reference Abdel-Ghany and Salem15)). Similarly, COS can also increase the immunity of C. carpio koi (koi)(Reference Lin, Mao and Guan26), L. vannamei (white shrimp)(Reference Rahimnejad, Yuan and Wang80), M. salmoides (largemouth bass)(Reference Lin, Jiang and Chen27), P. hypophthalmus (striped catfish)(Reference Nguyen, Van Dang and Le48) and T. ovatus (pompano)(Reference Lin, Mao and Guan50). Our work validates the fact that CTS and COS can improve the intestinal immunity of turbot, thus playing pivotal roles in the intestinal homoeostasis. Still, contrarily to results obtained in C. auratus gibelio (gibel carp)(Reference Chen, Zhu and Yang25) and C. mrigala (mrigal carp)(Reference Shanthi Mari, Jagruthi and Anbazahan81), no positive effects of CTS towards complement activity were detected in the present study. The exact underlying mechanism(s) on how CTS or COS may impact the immune function in SBM-fed fish remains to be further elucidated.
The intestinal microbiota plays important roles in fish health, affecting the gut morphology, nutritional status, disease resistance and immune response(Reference Nayak82). Previous studies have shown that SBM supplementation increases the bacteria diversity and alters the composition of the autochthonous bacterial communities in turbot intestine(Reference Bai, Gu and Xu37,Reference Gu, Bai and Xu83) . Similar results were also obtained in Salmo salar (Atlantic salmon)(Reference Gajardo, Rodiles and Kortner33,Reference Bakke-McKellep, Penn and Salas84) and Oncorhynchus mykiss (rainbow trout)(Reference Desai, Links and Collins85) upon supplementation with plant protein sources. According to our high-throughput sequencing data, CTS and COS appear to decrease the diversity and the composition of the intestinal microbiota in SBM-fed fish. This result parallels the findings in T. rubripes (tiger puffer), where the microbial abundance and species diversity in fish fed with COS-supplemented diet were lower than those fed with non-supplemented diet(Reference Su, Han and Jiang28). One possible explanation for this effect is that either CTS or COS can foster an ecological niche through secreted materials, thus leading to modifications in the microbiota composition(Reference Wang, Yoo and Kim86). However, some direct evidence that may support this hypothesis is still missing. We presently observed that both CTS and COS can significantly increase the relative abundance of these three distinct genera (i.e. Lactobacillus, Bacillus and Pseudomonas) with probiotic roles in fish(Reference Nayak82,Reference Ringø and Song87,Reference Ringø, Zhou and Vecino88) . This observation reinforces the beneficial effects of these two additives towards fish intestinal microbiota. Similar findings have also been reported in C. auratus gibelio (gibel carp) fed with CTS-based diet(Reference Chen, Zhu and Yang25) and T. rubripes (tiger puffer) fed with COS-supplemented sources(Reference Su, Han and Jiang28).
In conclusion, this study indicates that dietary CTS and COS can improve the performance of fish growth and intestinal immunity, eliciting anti-oxidant effects and ameliorating intestinal microbiota in SBMIE-affected turbot. Furthermore, these two prebiotics are beneficial against SBM-induced inflammation, since they enable a decrease on the expression of inflammatory cytokines by possibly modulating NF-кB, AP-1 and MAPK pathways.
Acknowledgements
This study was supported by the National Key R&D Program of China (2019YFD0900200); National Science Foundation of China (grant 31702363); China Postdoctoral Science Foundation (grant no. 2017M610428, 2018M632678 and 2019T120592); Function Laboratory for Marine Fisheries Science and Food Production Processes, P. R. China (grant no. 2019-ST-A01); Key Laboratory of Mariculture (KLM), Ministry of Education, Ocean University of China (grant no. KLM2018001); and Young Scholars Program of Shandong University, Weihai (grant no. 2016WHWLJH04 and 2018WHWLJH). All funders had no role in the design, analysis or writing of this article.
The entire experiment was designed by M. G. and N. B. The experiment was conducted by M. G., S. P., Q. L., Z. Q. and W. D. The data analysis and manuscript preparing were completed by M. G. and N. B.
The authors declared no competing interests.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521000489











