Significant outcomes
Cytokines were related to level of mental distress in psychiatric inpatients.
Cytokines were not related to the progression of mental distress in treatment over time.
Anti-inflammatory drugs seemed to modify the relationship between cytokines and mental distress.
Limitations
Body mass index and smoking status were not assessed.
The sample size was small and raises the question of type II error.
Caution should be used in interpreting the results, as some patients had serum levels under detectable limit.
Patients were not fasting before blood collection.
Introduction
Several studies have found an association between cytokine levels and severity of mental distress (Maes, Reference Maes1995; Furtado & Katzman, Reference Furtado and Katzman2015; Miller & Raison, Reference Miller and Raison2016; Dalton et al., Reference Dalton, Bartholdy, Robinson, Solmi, Ibrahim, Breen, Schmidt and Himmerich2018). Meta-analyses and systematic reviews of cross-sectional studies have shown that circulating pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, and tumour necrosis factor-alpha (TNF-α), are significantly elevated in depressed individuals as well as in a broad spectre of other psychiatric diagnoses compared to healthy controls (Dowlati et al., Reference Dowlati, Herrmann, Swardfager, Liu, Sham, Reim and Lanctôt2010; Passos et al., Reference Passos, Vasconcelos-Moreno, Costa, Kunz, Brietzke, Quevedo, Salum, Magalhaes, Kapczinski and Kauer-Sant’anna2015; Dalton et al., Reference Dalton, Bartholdy, Robinson, Solmi, Ibrahim, Breen, Schmidt and Himmerich2018). We have previously found both higher levels and a distinct escalation during treatment of pro-inflammatory and anti-inflammatory cytokines in patients with post-traumatic stress disorder (PTSD), but not for other psychiatric disorders (Toft et al., Reference Toft, Bramness, Lien, Abebe, Wampold, Tilden, Hestad and Neupane2018). There is evidence of a causal pathway from low-grade inflammation to depression, as raised inflammatory markers have been found to precede depressive symptoms in longitudinal studies (Valkanova et al., Reference Valkanova, Ebmeier and Allan2013). There is, however, also evidence of an opposite direction, that is, mental distress preceding an inflammatory response (Wang et al., Reference Wang, Caughron and Young2017).
The notion that low-grade inflammation may lead to depression has been supported by a longitudinal study in the general population reporting that elevated levels of IL-6 was associated with psychological distress, and where low levels at the first measurement and follow-up after 6 years were associated with being symptom free (Virtanen et al., Reference Virtanen, Shipley, Batty, Hamer, Allan, Lowe, Ebmeier, Akbaraly, Alenius, Haapakoski, Singh-Manoux and Kivimäki2015). Other studies have shown that patients with inflammatory diseases have greater risk of depression (Maes, Reference Maes2011; Miller & Raison, Reference Miller and Raison2016), that repeated exposure to systematic inflammation increased the risk of future depressive symptoms among women (Bell et al., Reference Bell, Kivimaki, Bullmore, Steptoe, Consortium and Carvalho2017), and pro-inflammatory agents are known to induce depression as a side effect (Schiepers et al., Reference Schiepers, Wichers and Maes2005; Köhler et al., Reference Köhler, Krogh, Mors and Benros2016). This supports the notion of causality. Furthermore, cytokines and psychiatric symptoms in outpatients have been found to decline during treatment (Dahl et al., Reference Dahl, Ormstad, Aass, Sandvik, Malt and Andreassen2016), and also to play a role in the progression and severity of established depressive disorders in various populations (Young et al., Reference Young, Bruno and Pomara2014).
The growing understanding of inflammatory processes being related to psychiatric disorders has led to clinical trials using anti-inflammatory drugs in treatment of depressed patients. In such studies, the use of non-steroidal anti-inflammatory drugs (NSAIDs) has been associated with improved antidepressant treatment response, suggesting an antidepressant effect of anti-inflammatory treatment (Köhler et al., Reference Köhler, Benros, Nordentoft, Farkouh, Iyengar, Mors and Krogh2014; Miller & Raison, Reference Miller and Raison2016). It has been speculated that some patients have low-grade inflammation as a trait and may benefit from anti-inflammatory drugs (Morch et al., Reference Morch, Dieset, Faerden, Hope, Aas, Nerhus, Gardsjord, Haram, Falk, Joa, Morken, Agartz, Aukrust, Djurovic, Melle, Ueland and Andreassen2017). However, anti-inflammatory drugs alone have shown a low to negligible effect on psychopathology (Eyre et al., Reference Eyre, Air, Proctor, Rositano and Baune2015), and may even contribute to more severe depression symptoms in patients treated with selective serotonin reuptake inhibitors (SSRI), seemingly attenuating the central effect of the SSRI treatment (Warner-Schmidt et al., Reference Warner-Schmidt, Chen, Marshall, Greengard and Vanover2011). Such results underline the necessity that anti-inflammatory drugs are taken into consideration when assessing cytokines and mental health.
Even if the effects of anti-inflammatory drugs on mental distress seem to be ambiguous, it is probable that the use of such drugs may influence the pathomechanism between inflammatory processes and mental distress, as both NSAIDs (Müller et al., Reference Müller, Schwarz, Dehning, Douhe, Cerovecki, Goldstein-Müller, Spellmann, Hetzel, Maino, Kleindienst, Möller, Arolt and Riedel2006) and immuno-suppressants (Köhler et al., Reference Köhler, Benros, Nordentoft, Farkouh, Iyengar, Mors and Krogh2014) inhibit the production of pro-inflammatory cytokines. The use of anti-viral drugs may also interfere with cytokine levels (Canivet et al., Reference Canivet, Menasria, Rheaume, Piret and Boivin2015).
A 5-year follow-up study found higher levels of IL-1β, IL-1RA, and TNF-α at the first measurement to be related to depressive symptoms over time (van den Biggelaar et al., Reference Van Den Biggelaar, Gussekloo, De Craen, Frolich, Stek, Van Der Mast and Westendorp2007), a finding supported by a literature review (Dantzer et al., Reference Dantzer, O’connor, Freund, Johnson and Kelley2008). There is also evidence on cytokines being related to development of mental disorders once it has already been established (Kim et al., Reference Kim, Amidfar and Won2019). However, more research is needed as studies on the level of mental distress seen in light of inflammatory biomarkers over time are still scarce, with inconsistent findings (Eyre et al., Reference Eyre, Air, Proctor, Rositano and Baune2015). In the present study we hypothesised that there is an association between elevated cytokine levels and mental distress in patients undergoing treatment. Thus, we aimed to investigate the relationship between cytokines levels and development of mental distress and the potential moderating role of anti-inflammatory drugs.
Material and methods
Study participants and recruitment procedure
Patients were recruited from Modum Bad Psychiatric Center, a specialised psychiatric centre in Norway, treating patients with long-standing or treatment-resistant trauma, anxiety, eating and depressive disorders. Patients with severe self-destructive behaviour or psychotic disorders were not eligible for admission. The facility offered group and individual therapies in a 12-week inpatient treatment programme. Therapy was paid by public insurance, and patients in work were entitled to sick leave while in treatment. Data were collected from March 2015 through April 2016.
Patients were recruited from the Depression, the Eating, the Anxiety, and the Trauma departments. The patients were admitted in groups of eight at a time. They were given a 15 min presentation during group therapy by the first author about the study during one of the first days of their stay. Written information was handed out, explaining the aim of the study and the procedures involved. A consent form was also distributed to each potential participant. Altogether 148 (59% of the total 249 patients approached) gave their written consent. One individual withdrew her consent 2 weeks later. We excluded 19 patients from the data material due to their having extreme cytokine levels above the 95th percentile, indicating possible acute infections. These 95th percentile limits were 436.6 pg/ml for IL-1RA and 216.3 pg/ml for MCP-1. One patient had missing values in cytokine data due to failed venipuncture. Altogether the present study comprised data provided by 92 women (72%, mean age 39.04 years, SD 11.26) and 36 men (28%, mean age 49.06 years, SD 9.36), giving a total of 128 patients. The 102 patients who did not participate comprised 81 women (79%, mean age 35.96, SD 11.77) and 21 men (21%, mean age 44.52, SD 8.58).
The material comprised venous blood samples and psychometric data. The blood samples and Hopkins Symptom Checklist 90 Revised (HSCL-90R) questionnaires were submitted within 1 week after entering treatment (T 0), at halfway (T 1), and a few days before discharge (T 2). The study was approved by the Norwegian Regional Ethics Committee prior to data collection (reference number 2014/2189).
Methods for clinical data
All patients were interviewed by trained psychologists or psychiatrists using the MINI clinical interview (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998). The MINI interview gives diagnoses within the 10th revision of the International Classification of Diseases and Related Health Problems (ICD-10). The staff used a combination of psychometric questionnaires and clinical judgement taken into consideration when disorders were assessed. All disorders were in the spectrum of depression, anxiety, and eating disorders. Fifty-four patients had only one disorder, and 59 had two or more disorders. Thirty-two patients had PTSD as primary diagnosis, some with one or even two additional disorders. Fifteen patients had no registered diagnosis due to missing data. An overview of diagnoses and frequencies of the diagnoses are included in Supplementary Table S1. Patients completed the self-reporting questionnaire HSCL-90R either on a computer or on a digital tablet. The HSCL-90R is a 90-item questionnaire measuring levels of psychological distress during the past 7 days, each item ranging from 0 to 4, giving a mean score of all responses as the Global Severity Index (GSI) (Derogatis et al., Reference Derogatis, Lipman and Covi1973). The questionnaire assesses symptoms of somatisation, obsession and compulsion, depression, anxiety, and hostility. The HSCL-90R has been shown to provide a psychometrically valid evaluation of psychiatric patients regarding the severity of depression, specific anxiety, and interpersonal sensitivity (Bech et al., Reference Bech, Bille, Moller, Hellstrom and Ostergaard2014). A cut-off for caseness was set to a GSI score at 0.85 (Pedersen & Karterud, Reference Pedersen and Karterud2004). In comparison, a previous study has found the GSI to be 0.49 in healthy controls (Rytila-Manninen et al., Reference Rytila-Manninen, Frojd, Haravuori, Lindberg, Marttunen, Kettunen and Therman2016). The GSI is an overall scale taking various symptoms into account, thus tapping into symptoms common to a heterogeneous psychiatric patient sample (Rytila-Manninen et al., Reference Rytila-Manninen, Frojd, Haravuori, Lindberg, Marttunen, Kettunen and Therman2016). At T 0, all patients successfully submitted their GSI scores, but at T 1, data were missing on 6 patients and at T 2, on 13 patients. We also explored three subscales of the HSCL-90R. These were the scales for somatisation, anxiety, and depression. The pattern of missing values was the same as in the GSI.
Anti-inflammatory drugs were NSAIDS, anti-viral drugs, and immuno-suppressants. We do not know the exact indications for which these drugs were given. The dichotomous anti-inflammatory drugs variable was used to stratify the patients in two groups. An overview of the anti-inflammatory drugs is included in Supplementary Table S2. Patients were recorded as users of these drugs if they had used such drugs at three occasions or more during the treatment period. The drugs categorised as anti-depressants were SSRIs, norepinephrine-dopamine reuptake inhibitors (NDRI), tricyclic anti-depressives (TCAs), and serotonin-norepinephrine reuptake inhibitors (SNRIs). The drugs in use were recorded by looking into each patient’s medical charts.
Blood collection and serum preparation
The blood samples were drawn at T 0, T 1, and T 2. They were collected between 08:00 a.m. and 09:00 a.m., except for 16 patients from the depression ward, who had their blood drawn between 12:00 am and 03:00 pm. Patients were not fasting and sat in an upright position when blood was collected. Vacuette 8 ml serum containers were used for blood collection. These were turned upside-down 8–10 times immediately after the blood was collected, and allowed to clot for 30–60 min. The samples were then spun at 1917 g for 10 min at room temperature. Separated serum samples were immediately put to freeze at −80°C until assay.
Cytokine and chemokine measurements
We analysed seven cytokines and one chemokine based on the available literature on the neuroimmune correlates of psychiatric disorders: IL-1β, IL-1RA, IL-6, IL-10, IL-17A, IFN-y, MCP-1, and TNF-α. Serum samples were thawed on ice, vortexed, and then spun down a tube with 250 μl serum at 14 000 × g for 10 min at 4°C, before dilution (1 : 5) and further processing. The serum levels were measured in picograms per millilitre (pg/ml). The cytokine measurements were performed using Bio-Plex xMAP technology (Bio-Rad, Austin, TX, USA) with a Luminex IS 100 instrument (Bio-Rad, Hercules, CA, USA), powered using Bio-Plex Manager (version 6.0.1) software. Multiplex bead–based technologies such as Luminex allow detection and quantification of multiple cytokines with good efficiency, speed, and dynamic range at reasonable cost. The assay was performed according to the manufacturer’s instructions, but an additional standard point was included. To achieve a more reliable result, individual sets of samples from patients were run in the same assay, all samples were assayed in duplicate, and a magnetic plate washer was used during assay set-up. The StatLIA software package (ver. 3.2; Brendan Scientific, Carlsbad, CA, USA) incorporates a weighted, five-parameter logistic curve-fitting method and was used to calculate sample cytokine concentrations. One longitudinal control from each participant was used for multiple analyses on each plate to define the intra-plate CVs; IL-1RA (3.0%) and MCP-1 (4.0%). Longitudinal controls were also used in order to validate the inter-assay (i.e., between plates) coefficient of variability (CV). The CVs were 10.2% for IL-1RA and 6.7% for MCP-1. An inter-assay per cent CV of 10–12% is common (and acceptable). The mean inter-assay per cent CV for all sample plates was 8.5%. The limit of detection (LOD) was 3 pg/ml for IL-1RA and 0.76 pg/ml for MCP-1.
Choice of cytokines and imputation
We investigated cytokine IL-1RA and chemokine MCP-1 due to their robustness and very high detectability, a finding in line with other studies (Leemasawatdigul & Gappa-Fahlenkamp, Reference Leemasawatdigul and Gappa-Fahlenkamp2011; Holub et al., Reference Holub, Lawrence, Andersen, Davidová, Beran, Marešová and Chalupa2013). Also, these cytokines had fewer than 6% below LOD. Cytokine levels below the LOD were replaced with the LOD value. Taking all three blood sampling occasions into consideration, 423 samples were collected. For IL-1RA, we imputed one value (0.2%). For MCP-1, we imputed 25 values (5.9%). We excluded six of the cytokines due to a rather high number of values below LOD. There were 194 (45.9%) values below LOD for IL-1β, 148 (35.0%) for TNF-α, 200 (47.3%) for IL-6, 226 (53.4%) for IL-10, 384 (90.8%) for IL-17, and 332 (78.5%) for interferon-gamma (IFN-γ).
Statistical analyses
The Mann–Whitney U-test and Pearson’s chi square were used when analysing demographic data. Non-normally distributed cytokines were attempted normalised by log-transformation. The cytokines were, however, also skewed following log-transformation, and the non-transformed cytokine values were ultimately used. Multilevel models were used to assess the repeated measurements of cytokines and GSI (Rabe-Hesketh & Skrondal, Reference Rabe-Hesketh and Skrondal2016). Main effects of cytokines and cytokines in interaction with time were analysed. We stratified the patients on the use of anti-inflammatory drugs to specifically explore the potential effect of using such drugs. This resulted in two groups (n = 28 for users and n = 99 for non-users of such drugs). Power analysis was conducted with the software G*Power, release 3.1.9.4, to assess the achieved statistical power with the given sample size. With a standardised mean difference of d = −0.54 (Köhler et al., Reference Köhler, Benros, Nordentoft, Farkouh, Iyengar, Mors and Krogh2014), the power was found to be 0.71, giving a 29% chance of not finding an effect even if present. Further, we specifically assessed the relationship between PTSD and GSI since we have previously found the PTSD patients to differ from patients without PTSD (Toft et al., Reference Toft, Bramness, Lien, Abebe, Wampold, Tilden, Hestad and Neupane2018). We also assessed the relationship between anti-depressants and GSI in the aforementioned strata in order to distinguish potential effects of different drugs. IL-1RA and MCP-1 were analyzed separately, thus constituting trivariate multilevel analyses with time as predictor variable, the cytokine as explanatory variable, and GSI as dependent variable. The material comprised three measurements of each patient, giving a structure of GSI score nested within patients. We disentangled the between- and within-subjects effects from the total effects. The within-subjects effects represent the expected change in GSI on average for a patient over the 12 weeks of treatment. The between-subjects effects indicate that, on average, higher cytokine levels increase the GSI by the given coefficient (Curran & Bauer, Reference Curran and Bauer2011). The process of disentangling the within- and between-subjects effects involved group-mean centering of the cytokine variables. The multilevel modelling was performed in a stepwise approach with fixed effects of cytokines and time, with subject ID as random intercept. A random slope of time was added to allow the slopes to vary across time. A likelihood ratio test was performed to assess model fit. The best model fit was chosen based on the −2 Log Likelihood and Bayes Information Criterion (BIC), and formally confirmed using likelihood ratio test. A model with fixed effects and random intercept gave a better fit than a model which included random slope (likelihood ratio test χ 2(2) = 1.93, p = 0.381). The predicted fixed and random effects of time on GSI are shown in Supplementary Table S3. The assumption of linearity in the dependent GSI variable across time was visually inspected with spaghetti plot and considered met. The assumption of homoscedastic residuals was assessed with a likelihood ratio test (χ 2(2) = 1.01, p = 0.603). The non-significant test confirmed that the assumption of homoscedastic residuals was met. Normality of residual distribution was assessed with QQ-plot and a histogram with a Gauss curve which showed normally distributed residuals and a nearly perfect normal curve. Restricted maximum likelihood (REML) was used in all multilevel model estimations due to small sample size, and maximum likelihood (ML) was used for model comparison. Those who did not submit all three GSI scores were defined as missing completely at random (MCAR), which indicated no specific pattern of missing data. Consequently, there was no increased variability which could have biased the regression coefficients. MCAR was confirmed by Little’s MCAR test (Little, Reference Little1988) with χ 2 = 0.576 (p = 0.902). All tests were two-sided, and p-values below 0.05 were considered statistically significant. No correction for multiple hypothesis testing was implemented as we considered the study to be exploratory. The HSCL-90R subscales anxiety, somatisation, and depression were analysed, but did not provide different results (included in Supplementary Table S4). Also, we explored all longitudinal analyses with adjustment for age and sex, but this did not significantly change any results (adjusted analyses are shown in Supplementary Table S5). Therefore, we chose to present unadjusted results. The statistical package STATA (StataCorp. 2015. Stata Statistical Software: Release 15. College Station, TX: StataCorp LP) was used for all statistical analyses.
Results
Table 1 shows demographic variables, cytokine levels, use of anti-depressive medications, and PTSD diagnosis in those not using or using anti-inflammatory drugs. There were significantly higher levels of MCP-1 and IL-1RA in those using anti-inflammatory drugs (p < 0.001 and p = 0.026, respectively). There were also significantly more patients without PTSD and without anti-depressive drugs who also were not using anti-inflammatory drugs (p < 0.001 and p = 0.008, respectively).
Table 1. Clinical information of study participants across the treatment period categorised on the use of anti-inflammatory drugs

GSI, Global Severity Index; IL-1RA, interleukin-1 receptor antagonist; MCP-1, monocyte chemoattractant protein-1; PTSD, post-traumatic stress disorder.
* Pearson chi square was used for categorical variables.
† The Mann–Whitney U-test was used for continuous variables.
‡ Cytokine levels in picograms per millilitre (pg/ml).
Table 2A and B presents the multilevel models of levels and changes in GSI over time. The table is divided in three, where Table 2A presents the results of all patients (n = 128), Table 2B users of anti-inflammatory medication (n = 28) and Table 2C non-users (n = 100 in total, with 99 of these patients submitted blood samples). In Table 2A, each variable was run as a separate explanatory variable on GSI, first as main effect only and then in interaction with time. There was a significant main effect of IL-1RA (p = 0.005), MCP-1 (p = 0.020) and having PTSD disorder (p = 0.002) on GSI. The only variable related to the slope of GSI over time was age (p = 0.025), indicating that older patients improve more in mental distress over time.
Table 2A. Trivariate linear mixed effects models of characteristics and mean cytokine levels across treatment on GSI for all patients

GSI, Global Severity Index; β (SE), regression coefficient (standard error); IL-1RA, interleukin-1 receptor antagonist; MCP-1, monocyte chemoattractant protein-1; PTSD, post-traumatic stress disorder.
Bold values represent significance value at p = 0.01, p = 0.05 and p = 0.01.
* Anti-inflammatory drugs. All regression models run with time as predictor variable.
Table 2B. Trivariate linear mixed effects models of characteristics and mean cytokine levels across treatment on GSI for patients using anti-inflammatory drugs
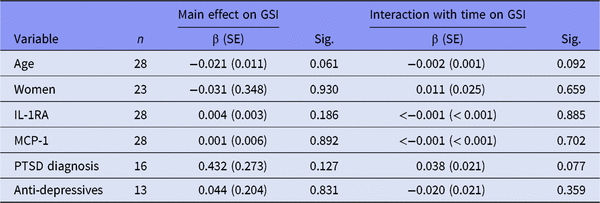
GSI, Global Severity Index; β (SE), regression coefficient (standard error); IL-1RA, interleukin-1 receptor antagonist; MCP-1, monocyte chemoattractant protein-1; PTSD, post-traumatic stress disorder.
All regression models run with time as predictor variable.
Table 2B shows the main effect and interaction with time on GSI in those patients using anti-inflammatory drugs. We found no associations in this stratum. In those not using anti-inflammatory drugs (Table 2C), there were significant main effects of IL-1RA (p = 0.023), MCP-1 (p = 0.018) and having PTSD disorder (p = 0.014) on GSI. Together, Tables 2B and C show that among those not using anti-inflammatory drugs, GSI levels were significantly higher in the PTSD sample compared to non-PTSD patients. This difference was not present in those using anti-inflammatory drugs. Time interaction did not show any significant results in either of the two strata.
Table 2C. Trivariate linear mixed effects models of characteristics and mean cytokine levels across treatment on GSI for patients not using anti-inflammatory drugs

GSI, Global Severity Index; β (SE), regression coefficient (standard error); IL-1RA, interleukin-1 receptor antagonist; MCP-1, monocyte chemoattractant protein-1; PTSD, post-traumatic stress disorder. IL-1RA, p = 0.023.
Bold values represent significance value at p = 0.05.
All regression models run with time as predictor variable.
Fig. 1 shows the intercept and slope of GSI over time in the patients according to their use of anti-inflammatory drugs. There was a tendency that patients who used anti-inflammatory drugs had higher level of GSI, also reflected in Table 2A (p = 0.093). The slope of GSI in those who did not use anti-inflammatory drugs was significantly different from zero (β = −0.03, SE = 0.005, p < 0.001), indicating that there was a reduction in symptoms over time. The slope of GSI in the users of anti-inflammatory drugs did not reach significance in decline over time (β=−0.01, SE = 0.01, p = 0.149), and the difference between the two groups was not significant, as shown in Table 2A (p = 0.108).
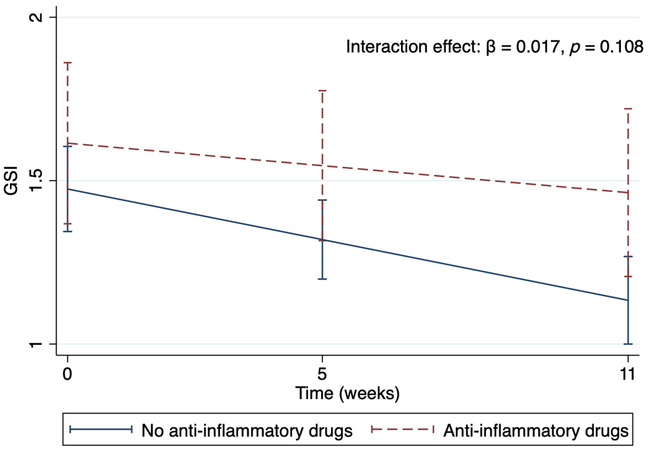
Fig. 1. Intercept and slopes in users and non-users of anti-inflammatory drugs depicting the decline levels of GSI (95% CI) during the treatment period.
Interaction effect of anti-inflammatory drugs with time on GSI. The graph visualises the trajectories of the GSI from the multilevel model found in Table 2A. GSI, GSI, Global Severity Index.
Fig. 2A and B shows the intercepts and slopes of GSI over time for the patients who did not use anti-inflammatory drugs, as shown in Table 2C. The three slopes categorise the patients by the mean level ± 1 SD of IL-1RA and MCP-1 according to their corresponding GSI level. The slopes of the GSI scores declined throughout treatment for all patients, and these slopes were significantly different from zero (p < 0.001).
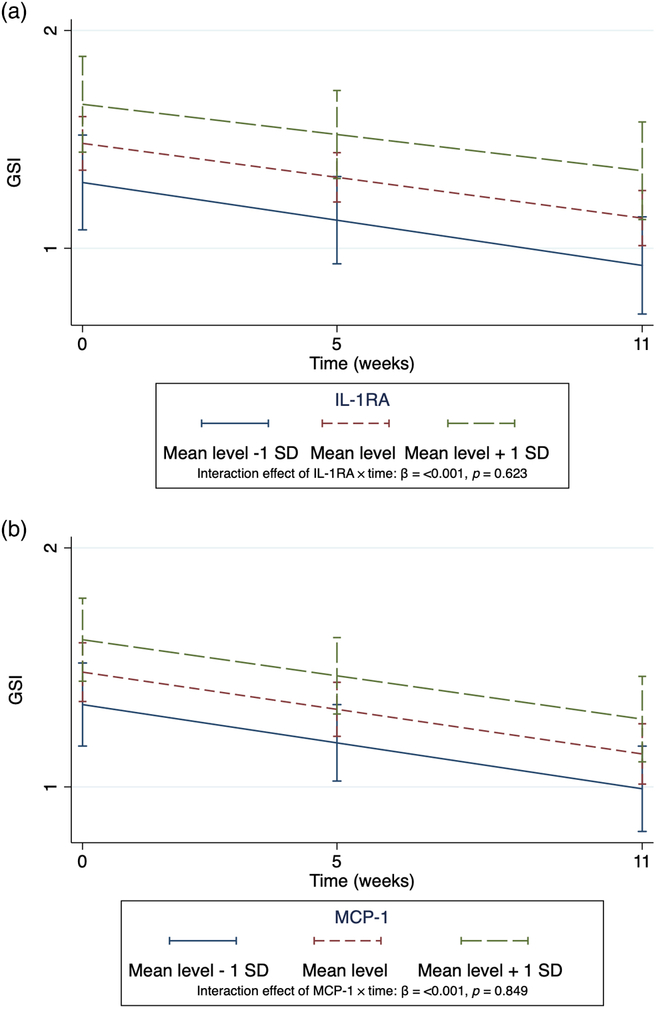
Fig. 2. (A and B) Intercepts and slopes of GSI in non-users (n = 99) of inflammatory drugs.
The declining trajectories of GSI are explained by the time variable (as shown in Supplementary Table S5). The GSI is categorised according to level of the inflammatory markers (mean level ± 1 SD). The graphs visualise the trajectories of the GSI from the multilevel models in Table 2C. GSI, Global Severity Index. IL-1RA, interleukin-1 receptor antagonist; MCP-1, monocyte chemoattractant protein-1.
Discussion
In patients undergoing psychiatric treatment, those with higher levels of IL-1RA and MCP-1 had higher levels of mental distress. There was a decreasing slope of GSI over time for the older patients, indicating that older patients benefited more from treatment. When stratifying according to the use of anti-inflammatory drugs during treatment, we found associations between levels of cytokines and GSI only among those not using anti-inflammatory drugs. There seemed to be an interaction between the use of anti-inflammatory drugs and cytokine levels with the level of mental distress in psychiatric patients in treatment. We did not find a relationship between cytokine levels and the development of GSI scores over time. We still believe our finding underscores the need for taking anti-inflammatory drugs into account in immune-psychiatric investigations and in treatment effect evaluation.
The relationship between cytokine level and degree of mental distress is in line with numerous studies on many different groups of patients with psychiatric diagnosis (Maes et al., Reference Maes, Bosmans, Suy, Vandervorst, De and Raus1990; Smith, Reference Smith1991; Dowlati et al., Reference Dowlati, Herrmann, Swardfager, Liu, Sham, Reim and Lanctôt2010). The chemokine MCP-1 induces leukocyte infiltration in the CNS and is associated with neuronal damage, which is why it has been studied for its role in depression (Young et al., Reference Young, Bruno and Pomara2014). Our finding that MCP-1 was related to level of mental distress is in accordance with a series of studies. A review of studies on inflammatory processes in major depressive disorder showed that the synthesis of MCP-1 is involved in both the aetiology and progress of the disorder (Young et al., Reference Young, Bruno and Pomara2014). The association between elevated MCP-1 and depression and anxiety symptom severity has also been found by others (Vogelzangs et al., Reference Vogelzangs, De Jonge, Smit, Bahn and Penninx2016), but results are mixed, as serum MCP-1 has also been found to be lowered in patients with MDD (Young et al., Reference Young, Bruno and Pomara2014). The anti-inflammatory cytokine IL-1RA is also known to be elevated in depression and anxiety patients. The reason for this has been suggested to be due to an increased innate immune response, which involves both pro- and anti-inflammatory cytokine and chemokine production (Maes et al., Reference Maes, Bosmans, De, Kenis, Vandoolaeghe and Neels1997; Dahl et al., Reference Dahl, Ormstad, Aass, Malt, Bendz, Sandvik, Brundin and Andreassen2014).
We did not find that cytokine levels were related to the progress of mental distress. This discrepancy may have different explanations. An observation period of 12 weeks may not be sufficient to pick up on changes over time. Furthermore, patients enrolled at Modum Bad Psychiatric Center were patients with severe mental disease, often for many years, possibly carrying high cytokine levels as a trait feature. Also, the variety of diagnoses may explain a wide spread in cytokine levels in our study population. It has been suggested that anti-inflammatory drugs may play a role in outcome of PTSD by altering neuro-immune processes (Miller et al., Reference Miller, Lin, Wolf and Miller2018; Waheed et al., Reference Waheed, Dalton, Wesemann, Ibrahim and Himmerich2018) and thus contribute to mental recovery. Among those not using anti-inflammatory drugs, the PTSD patients had significantly higher GSI levels than non-PTSD patients. This difference was attenuated in those using anti-inflammatory drugs. This implies that the use of anti-inflammatory drugs affects GSI levels across PTSD diagnosis and suggests other researchers in the field should assess the use of such drugs in future studies of PTSD patients.
The relationship between cytokine level and mental distress was only present among those not using anti-inflammatory drugs. The lack of a relationship between cytokines and mental distress among those using anti-inflammatory drugs may be that both the reason for using these drugs, often an inflammation, and the drugs themselves may influence the level of cytokines much more than mental distress. Furthermore, it has been suggested that anti-inflammatory drugs could be used in the treatment of depression, as it reduces inflammation (Young et al., Reference Young, Bruno and Pomara2014), thus reducing depressive symptoms, especially for the depression patients who are labelled as treatment-resistant due to treatment unresponsiveness (Kornstein & Schneider, Reference Kornstein and Schneider2001). The mechanism is believed to be the inhibition of enzyme cyclooxygenase-1 (COX-1). COX-1 is a major player in modulation of pro-inflammatory microglia activation, and aspirin in particular has been postulated as particularly promising, but still experimental and hypothetical (Baune, Reference Baune2017). However, the effect of anti-inflammatory medications has been found to exhibit efficacy in treatment-resistant depression patients with high-sensitivity CRP (hs-CRP) concentrations greater than 5 mg/l, but not for treatment-resistant depression with smaller hs-CRP concentrations (Raison et al., Reference Raison, Rutherford, Woolwine, Shuo, Schettler, Drake, Haroon and Miller2013). This suggests subgroups of depression patients respond differently to drugs according to inflammatory level. Together with our finding on different associations in PTSD patients according to stratification, a screening of inflammatory markers in patients seeking treatment could be warranted.
There are some limitations to mention. The patients were not fasting when blood samples were drawn. Eating has been found to be associated with increased MCP-1 (Dixit et al., Reference Dixit, Yang, Sayeed, Stote, Rumpler, Baer, Longo, Mattson and Taub2011). Body mass index (BMI) of the patients was not assessed in this study. Adipose tissue produces pro-inflammatory cytokines, meaning that body fat is a possible confounder (Calder et al., Reference Calder, Ahluwalia, Brouns, Buetler, Clement, Cunningham, Esposito, Jonsson, Kolb, Lansink, Marcos, Margioris, Matusheski, Nordmann, O’brien, Pugliese, Rizkalla, Schalkwijk, Tuomilehto, Warnberg, Watzl and Winklhofer-Roob2011). We did not assess smoking status. Smokers have been found to have a higher basal level of cytokines when compared to non-smokers (Belchamber et al., Reference Belchamber, Hall and Hourani2014). Physical exercise might affect the levels of circulating cytokines (Phillips & Fahimi, Reference Phillips and Fahimi2018). We did not record the level of exercise before blood samples were drawn. Conditions causing systemic inflammation, for instance hepatitis, could potentially bias the cytokine levels. The blood samples were collected in the afternoon for 16 patients. During clotting, monocytes may begin releasing MCP-1. However, increase in the protein levels of MCP has been found only after several hours (Campbell et al., Reference Campbell, Vieira-De-Abreu, Rowley, Franks, Manne, Rondina, Kraiss, Majersik, Zimmerman and Weyrich2017). Also, future studies will benefit from more sensitive assay techniques as well as inclusion of multiple cytokines of the pro-, anti-, and regulatory classes. The reader should bear in mind that the sample size is small and there is a risk of type 2 errors. The study showed that the levels of cytokine IL-1RA, chemokine MCP-1 and PTSD diagnosis were related to level of GSI. The use of anti-inflammatory drugs appeared to have an immunomodulating effect on this relationship.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/neu.2019.36
Acknowledgements
The study was funded by South-Eastern Norway Regional Health Authority through the Inland Hospital Trust. We want to thank the staff and the patients at Modum Bad Psychiatric Center for participating in the study. The authors have no conflicts of interest that might influence the current study.
Author contributions
HT was responsible for recruiting patients, collecting the blood samples, and drafting and revising the manuscript. HT also performed the statistical analyses. LL was responsible for the design and planning of the study and was involved in writing and revision of the manuscript. SPN was involved in designing and planning of the study and in writing and revision of the manuscript. DSA was involved in revision of the manuscript and supervision of the statistical analyses. TT was involved in planning of the study and in revision of the manuscript. BEW was involved in planning of the study and in revision of the manuscript. JGB was, together with LL, responsible for the design and planning of the study, and was involved in writing and revision of the manuscript. All authors revised the final version and approved for submission.
Financial support
The process of designing the study, collecting and analysing the data material, and writing the manuscript was funded by South-Eastern Norway Regional Health Authority through the Innlandet Hospital Trust.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008 (Williams, Reference Williams2008).








