Introduction
Spermatozoa need low and controlled levels of reactive oxygen species (ROS) for successful fertilization. In addition, ROS molecules such as hydrogen peroxide (H2O2), superoxide (O2 ●–), and nitric oxide (NO) are important for sperm metabolic processes, capacitation, hyperactivation of motility, acrosome reaction and gamete fusion (Zini & Al-Hathal, 1993; Aitken et al., Reference Aitken, Paterson, Fisher, Buckingham and Van Duin1995; Baumber et al., Reference Baumber, Sabeur, Vo and Ball2003; O'Flaherty et al., Reference O'Flaherty, Beorlegui and Beconi2003; Lamirande et al., Reference Lamirande, Lamothe and Villemure2009).
Although controlled ROS generation facilitates physiological functions in sperm, excessive concentrations of these molecules can cause oxidation of macromolecules, thereby generating damage in sperm DNA, proteins, and lipids. Moreover, high levels of ROS and nitrogen species can contribute to male infertility by triggering metabolic dysfunction, and consequently decrease sperm viability and motility (Armstrong et al., Reference Armstrong, Rajasekaran, Chamulitrat, Gatti, Hellstrom and Sikka1999; Duru et al., Reference Duru, Morshedi, Schuffner and Oehninger2000; Krzyzosiak et al., Reference Krzyzosiak, Evenson, Pitt, Jost, Molan and Vishwanath2000; Baumber et al., Reference Baumber, Vo, Sabeur and Ball2002; Bilodeau et al., Reference Bilodeau, Blanchette, Cormier and Sirard2002; Buzadzic et al., Reference Buzadzic, Vucetic, Jankovic, Stancic, Korac, Korac and Otasevic2015).
Sperm possesses an endogenous antioxidant system to counteract the effects of the high ROS levels that are constantly being generated. For example, O2 ●– is a product of aerobic metabolism that yields adenosine triphosphate (ATP) in mitochondria and leukocytes in semen also generate large quantities of ROS molecules. Thus, there is also an antioxidant exogenous system present mainly in seminal fluid, which contains molecules such as glutathione, thiols, taurine, vitamin E, and ascorbic acid (Zini & Al-Hathal, Reference Zini and Al-Hathal2011).
Exogenous antioxidants are also relevant to assisted reproductive techniques such as spermatozoa cryopreservation, given that cryopreservation is mandatory for donated sperm due to the quarantine requirements needed to ensure that semen is infection free (Holt, Reference Holt2000). With increasing numbers of cancer survivors of a young age, improvement in freezing–thawing methods for sperm is also relevant to preserve the reproductive capacity of children and young adult cancer patients (Wallace, Reference Wallace2011).
Several cryoprotectants, such as glycerol, dimethyl sulfoxide, ethanediol, and propanediol are used to reduce ice formation, and have biologically acceptable properties with low toxicity. Besides preserving cell viability, the freezing process should also ensure maintenance of cell structure and function. Previous studies have demonstrated that cryopreservation of human semen increases ROS production, which causes serious damage to sperm (Ball et al., Reference Ball, Vo and Baumber2001; Woods et al., Reference Woods, Benson, Agca and Critser2004; Aitken & Roman, Reference Aitken and Roman2008; Zribi et al., 2012). Attempts have therefore been made to optimize sperm cryopreservation by adding antioxidant compounds when freezing (Branco et al., Reference Branco, Garcez, Pasqualotto, Erdtman and Salvador2010; Garcez et al., Reference Garcez, dos Santos, Lara, Pasqualotto and Salvador2010; Kalthur et al., Reference Kalthur, Raj, Thiyagarajan, Kumar, Kumar and Adiga2011; Li et al., Reference Li, Yang, Lu, Lu, Zhang, Yao, Meng and Lu2012) and analyzing the cryoprotective effects of various chemicals on sperm function and viability. Commonly used antioxidants do not always provide effective protection against oxidative stress generated by the freezing and defrosting processes (Zhu et al., Reference Zhu, Fan, Lv, Zhang, Fan, Zhang and Zeng2015; Bucak et al., Reference Bucak, Ataman, Başpınar, Uysal, Taşpınar, Bilgili, Öztürk, Güngör, İnanç and Akal2015).
To identify alternative, more effective antioxidants for sperm cryopreservation, chemical matrices from nutritional foods, such as guaraná (Paullinia cupana), which possesses antioxidant and cytoprotective properties, should also be considered. Previous studies on extracts of Amazon guaraná seeds powder depended on the modulation of higher NO levels by exposure to sodium nitroprusside (Bittencourt et al., Reference Bittencourt, Machado, Machado, Dos Santos, Algarve, Marinowic, Ribeiro, Soares, Barbisan, Athayde and Cruz2013). Guaraná also provided important cytoprotection for neural cell lines exposed to rotenone (Oliveira et al., Reference Oliveira, Barreto, Galeano, Romero, Holubiec, Badorrey, Capani and Alvarez2011) and showed anti-inflammatory effects in vivo and in vitro, as peripheral blood mononuclear cells (PBMCs) exposed to guaraná had reduced proinflammatory cytokines compared with cells treated only with ascorbic acid and resveratrol (Krewer et al., Reference Krewer, Ribeiro, Ribeiro, Moresco, da Rocha, Montagner, Machado, Viegas, Brito and da Cruz2011). Cell culture supplementation with guaraná was also able to revert some senescence in adipocyte stem cells from adult lipoaspirates (Machado et al., Reference Machado, Cadoná, Azzolin, Dornelles, Barbisan, Ribeiro, Mânica-Cattani, Duarte, Saldanha and da Cruz2015). The beneficial effects of guaraná are probably due to bioactive molecules in its composition such as caffeine, theobromine, and catechins (Cadoná et al., Reference Cadoná, Machado, Azzolin, Barbisan, Dornelles, Glanzner, Gonçalves, Assmann, Ribeiro and da Cruz2016) that be generally useful against cryodamage associated with freezing cell storage. In order to test this hypothesis, we evaluated the effects of guaraná supplementation on human spermatozoa during cryopreservation by first producing a new chemical compound based on methylxanthine and polyphenols molecules (CCMP) present in the guaraná chemical matrix and enriching CCMP with resveratrol, a well known antioxidant from plant sources. Thus, we tested whether resveratrol-enriched CCMP could improve the viability and modulate differentially ROS and NO levels of thawed sperm.
Materials and methods
Reagents
All reagents were obtained from Sigma Chemical Co. (St Louis, MO, USA), Invitrogen Co. (USA) and Cultilab Co. (São Paulo, Brazil).
Study design
In vitro protocols using human spermatozoa cells were performed. Cells were treated with different concentrations of guaraná extract (0.1, 1 5 or 10 mg/ml) and incubated for 30 min at 37°C in a 5% CO2 in air incubator. Samples were transferred to screw-top plastic vials and submitted to a slow cooling rate process. In the first step, samples were cooled at 0°C and maintained at this temperature for 15 min. Samples were then frozen at −80°C for 24 h. Finally, samples were slowly thawed and viability, as well as the oxidative biomarkers, were evaluated. An additional protocol was performed to evaluate the guaraná protective effect on spermatozoa without freezing using sperm aliquots exposed to a pro-oxidant compound (H2O2 at 200 µM) over a 2 h incubation period at 37°C in 5% CO2 in air.
Another in vitro experiment was performed using sperm cells treated with three different concentrations of CCMP (composed of caffeine, theobromine, catechin molecules found in guaraná chemical matrix) and resveratrol,. Caffeine, catechin and theobromine concentrations were based on the best guaraná cryoprotective concentration used in the previous experiment. Resveratrol concentrations were used according to Garcez et al. (Reference Garcez, dos Santos, Lara, Pasqualotto and Salvador2010).
Sperm cells were exposed to high concentrations of CCMP (H-CCMP), intermediate concentrations (I-CCMP) and low concentrations (L-CCMP). The percentages of bioactive molecules used for H-CCMP, I-CCMP and L-CCMP were for caffeine (12–1.2%), theobromine (6–0.6%), catechin (4–0.4%) and resveratrol (1–0.1%).
Cells were incubated with CCMPs at 37°C in a 5% CO2 in air incubator for 1 h (Garcez et al., Reference Garcez, dos Santos, Lara, Pasqualotto and Salvador2010). After this step, sample volumes were divided into three sterile vials. One part of the sample was used to conduct pre-freezing analyses of viability and oxidative metabolism and the other two parts were frozen either in liquid nitrogen (−196°C) or in an ultrafreezer (−80°C) for 24 h. Samples were submitted to a slow cooling rate: frozen at 20°C for 10 min, suspended in the liquid nitrogen vapour phase for 2 h and stored in liquid nitrogen at −196°C. To perform post-thawing viability and oxidative metabolism analyses, samples were removed from liquid nitrogen after 24 h storage and then thawed at room temperature for 5 min, and incubated at 37°C for 5–10 min (Garcez et al., Reference Garcez, dos Santos, Lara, Pasqualotto and Salvador2010).
Samples collection
All the donors were healthy young adults of similar age (20–25 years old), diet and habits. Donors did not ingest antioxidant foods 1 day before collection to avoid experimental interferences. The study was approved by the Institutional Review Board of the Universidade Federal de Santa Maria (UFSM RS, Brazil, process number: 23081.015838/2011-10). All individuals gave informed, written consent for the study procedures and the use of their biological samples for research purposes. The study group included seven young fertile male donors.
Semen samples were collected at the Laboratório de Biogenômica, Universidade Federal de Santa Maria. An individual room was used specifically for this purpose, by masturbating into a sterile, non-toxic disposable container. All men were asked to abstain from ejaculation for at least 72 h before their semen was collected. Samples were placed in 5% CO2 incubation at 37°C for 30 min for seminal liquefaction.
All semen analyses were performed manually within 1 h after the sample had been collected. Semen analyses were performed by the same investigator, according to the World Health Organization (WHO) standards. Sperm concentration (number of spermatozoa/ml), percentage of normal morphology [(number of spermatozoa with normal head, mid-piece and tail/total number of spermatozoa) × 100] and per cent motility [(number of motile spermatozoa/ total number of spermatozoa) × 100] were evaluated on fresh samples to ensure sperm quality (Stanic et al., Reference Stanic, Tandara, Sonicki, Simunic, Radakovic and Suchanek2000). Sperm samples were initially counted to estimate the sperm concentration using formal saline (2.9%). All experiments were performed with a 1 × 106/ml sperm cell concentration in a Tris modified medium.
Cryopreservation medium
A cryopreservation medium was prepared using a modified Tris buffer containing 2.42 g Tris(hydroxymethyl)aminomethane, 1.50 g citric acid monohydrate, 1.25 g glucose, 20 ml egg yolk, 6.0 ml glycerol, and 0.02 g streptomycin/ampicillin, in a 100 ml final volume. Samples were diluted 1:1 in cryoprotective Tris modified medium.
Guaraná extract characterization
Guaraná powder used in sperm treatments was kindly supplied by EMBRAPA, Amazonia Ocidental (Agropecuary Research Brazilian Enterprise). Guaraná powder was protected from the light and stored in dry conditions at ±4°C until the extract preparations to perform the chemical analyses of main bioactive compounds were made. Guaraná powder was produced from a hydro-alcoholic extract based on the small solubility of guaraná powder from the standard ratio of alcohol and water (70:30) to 100 ml of extraction fluid prepared at a concentration of 300 mg/ml. After 21 days of extraction, the preparation was centrifuged at 3000 rpm for 10 min, and the supernatant was isolated. The resulting solution was lyophilised to determine xanthine and catechin compositions for the experimental procedures (Bittencourt et al., Reference Bittencourt, Machado, Machado, Dos Santos, Algarve, Marinowic, Ribeiro, Soares, Barbisan, Athayde and Cruz2013). The main bioactive compounds presented in guaraná extract were determined by chromatographic analysis performed on an HPLC system based on UV absorbance at 272 nm. A 150 mm × 4.6 mm ODS-3 column (5-μm particle size) was used for the separation as described in Carlson and Thompson. From these procedures, it was possible to determine that the extract used in this study had 12.240 mg/g of caffeine, 6.733 mg/g of theobromine, and 4.336 mg/g total catechins.
Cell viability assays
MTT assay
Sperm mitochondrial functional was evaluated by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The supernatants from the treatments were discarded and cells were washed and resuspended in phosphate-buffered saline (PBS; 0.01 M, pH 7.4) to avoid interference from polyphenols present in guaraná and CCMPs treatments. Samples were placed in a 96-well plate, MTT reagent (Sigma-Aldrich, St. Louis, MO, USA) was added (dissolved in 5 mg/ml PBS), and incubated for 1 h at 37°C. The formazan crystals generated were released from the cells through dimethyl sulphoxide (DMSO) addition and measured at 560 nm wavelength (Fukui et al. Reference Fukui, Yamabe and Zhu2010).
Flow cytometry
The viability of the samples was measured by flow cytometry using propidium iodide (PI). PI allows the identification of dead cells because this reagent only passes through damaged cell membranes. Analyses were performed according to manufacturer's instructions. Samples were washed twice with cold PBS and then resuspended in 1× binding buffer. Next, a 100 μl solution (1 × 105 cells) containing the cells was transferred to a 5 ml culture tube. Resuspended cells underwent smooth vortexing and staining with 5 μl of PI. Following a short incubation for 15 min in the dark at room temperature, 400 μl of 1× binding buffer was added to each tube, and cell florescence was analyzed by flow cytometry according to the manufacturer's specifications (Vermes et al., Reference Vermes, Haanen, Steffens-Nakken and Reutelingsperger1995).
Cell-free double-stranded DNA PicoGreen assay
We evaluated spermatozoa viability by quantifying dead cells from the concentration of cell-free double-stranded DNA (cfDNA) in the supernatant. When a cell dies, the membrane is disrupted and cfDNA fractions are released into the extracellular medium. As DNA PicoGreen® dye has a high affinity with cfDNA it is able to quantify the cfDNA released. The Quant-IT™ PicoGreen® double-stranded DNA kit (Invitrogen, Life Technologies) was used to perform cfDNA determination. Following the manufacturer's instructions, cfDNA was measured using 50 μl of the sample and 50 μl of DNA PicoGreen® dissolved in TE buffer, 1× (1:1; v/v), following incubation for 5 min in a dark room. Fluorescence was measured at an excitation wavelength of 480 nm and at an emission wavelength of 520 nm (Costa et al., Reference Costa, Barbisan, Assmann, Araújo, de Oliveira, Signori, Rogalski, Bonadiman, Fernandes and da Cruz2017).
Crystal violet assay
Spermatozoa viability was also measured by crystal violet assay. Cells were dyed and fixed with crystal violet 0.2% (in ethanol 2%) for 30 min at 37°C. The dye was dissolved using 1% sodium dodecyl sulfate (SDS) and absorbance was measured at 550 nm wavelength (Cubillos-Rojas et al., Reference Cubillos-Rojas, Amair-Pinedo, Peiró-Jordán, Bartrons, Ventura and Rosa2014).
ROS production measure
ROS production was measured using 2′-7′-dichlorofluorescein diacetate (DCFH-DA) oxidation assay. Intracellular ROS production was detected in sample cells using the non-fluorescent cell permeating compound DCFH-DA. DCFH-DA is hydrolyzed by intracellular esterases to DCFH, which is trapped within the cell. This non-fluorescent molecule is then oxidized to fluorescent dichlorofluorescein (DCF) by cellular oxidants. Sample cells from different treatments were treated with DCFH-DA (10 μM) for 60 min at 37°C. Fluorescence was measured at an excitation wavelength of 488 nm and an emission wavelength of 525 nm. The calibration curve was performed the DCF standard (0–1 mM), and the level of ROS production was calculated as nmol DCF formed/mg protein (Esposti, Reference Esposti2002).
Nitrite determination
The NO produced in the spermatozoa was measured using Griess reagent by reacting 100 µl Griess reagent with the same volume of sample supernatant for 10 min at room temperature in the dark. Absorbance was measured at 540 nm wavelength using a microplate reader (Choi et al., Reference Choi, Shin, Lee and Kim2012).
Statistical analysis
Data were expressed as mean ± standard deviation (SD). The results were expressed as a per cent (%) of the control group. The data of cells treated with CCMP were submitted to one-way analysis of variance (ANOVA) followed by Dunnett's post hoc test and spermatozoa were submitted to one-way ANOVA followed by Tukey's post hoc test. The statistical tests were performed using GraphPad Prism Software and Statistical Package for Social Sciences (SPSS), version 18.0 (SPSS Inc., Chicago, IL, USA). The alpha value was set to <0.05 to determine statistical significance. All experiments were repeated at least three times.
Results
Semen analyses
The baseline characteristics showed that sperm samples used in the present study were within WHO fertility criteria (Table 1).
Table 1 Sperm samples baseline characteristics

Data are mean ± standard deviation (SD).
Sperm viability treated with guaraná supplementation medium
Sperm viability of cells treated with guaraná supplementation medium was measured and the results are presented in Fig. 1. The MTT assay showed that guaraná medium supplementation at 5 and 10 mg/ml significantly increased spermatozoa viability after the freezing process when compared with the control group (F4,9 = 9.496, P = 0.003) (Fig. 1 a). Moreover, sperm samples exposed to the H2O2 prooxidant and supplemented at 10 mg/ml guaraná presented significantly higher viability than the H2O2 control and other guaraná concentrations (F4,9 = 5.436, P = 0.012) (Fig. 1 b).
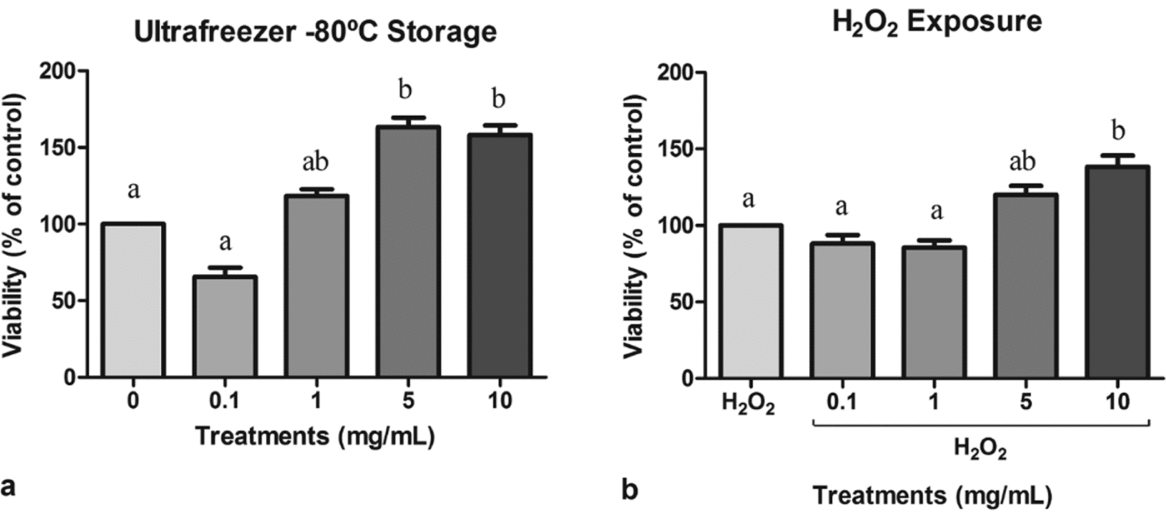
Figure 1 (a) Viability of sperm cells exposed to different concentrations of guaraná (0.1, 1, 5 and 10 mg/ml) and ultrafreezing storage (−80°C) for 24 h. The results were compared with the negative control (only cells and medium). (b) Viability of sperm cells exposed to different concentrations of guaraná (0.1, 1, 5 and 10 mg/ml) in association with hydrogen peroxide (H2O2 at 200 µM) and incubated at 37°C in 5% CO2 in air for 2 h. The results were compared with the positive control (cells and H2O2). N = 3. a,bDifferent letters indicate statistically significant differences at P < 0.05.
Pre-freezing analyses of sperm viability and oxidative metabolism treated with CCMP supplementation medium
Firstly, the effect of the three CCMP concentrations on sperm viability (Fig. 2) and oxidative metabolism (Fig. 3) at 37°C was analyzed. Addition of I-CCMP increased sperm cells viability by 10% in comparison with untreated control cells (P < 0.05). However, this effect was not observed in H-CCMP and L-CCMP (Fig. 2 a). The improvement in viability of I-CCMP was confirmed by crystal violet assay. In this assay, a significant increase in viability was also observed when sperm cells were supplemented with H-CCMP (Fig. 2 b). In contrast, the cfDNA assay did not show significant differences in viability among all treatments (Fig. 2 c). In summary, in all results, I-CCMP improved the sperm cell viability in comparison with control cells.
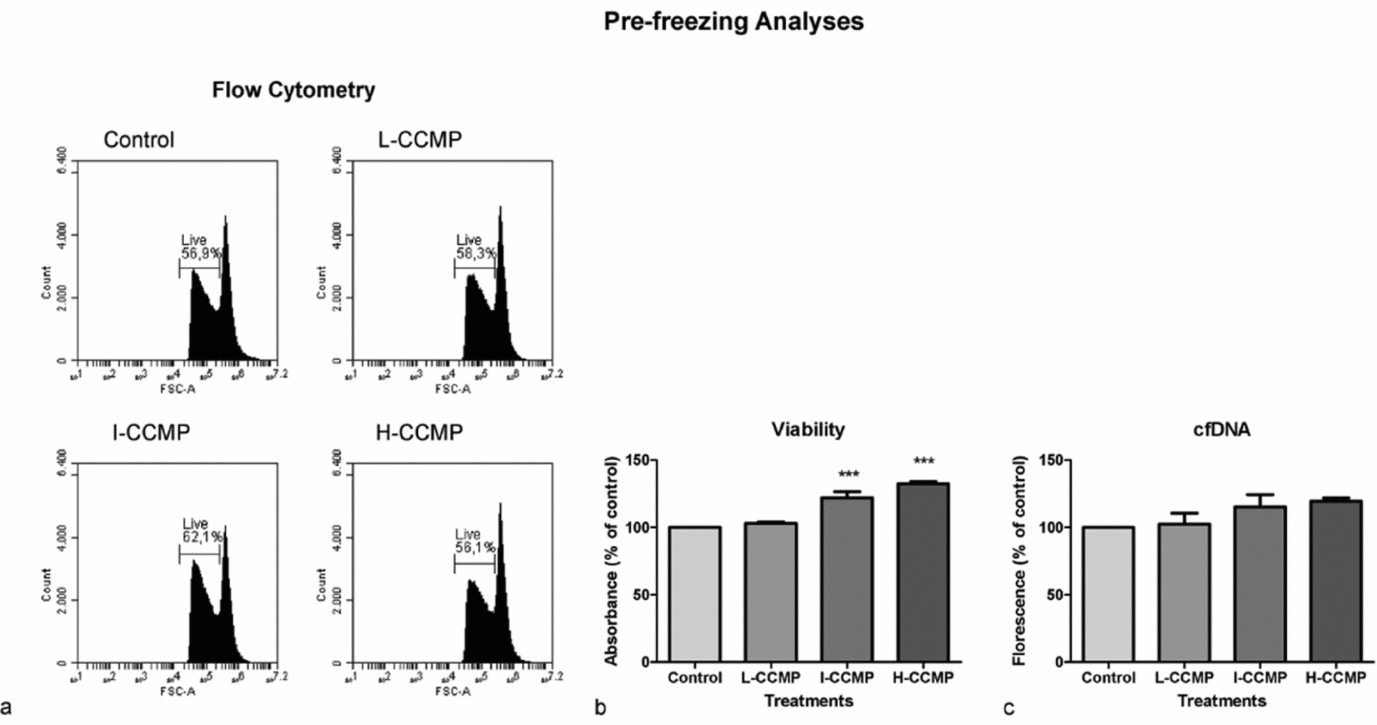
Figure 2 Pre-freezing analyses of sperm exposed to L-CCMP, I-CCMP or H-CCMP. (a) Cell viability was evaluated by flow cytometry using propidium iodide (PI) staining. (b) Cell viability was evaluated using crystal violet assay. (c) cfDNA was measured by PicoGreen® assay. The results were compared with the control (cells and medium only). N = 3, ***P < 0.0001.

Figure 3 Pre-freezing analyses of sperm exposed to L-CCMP, I-CCMP or H-CCMP. (a) ROS levels were evaluated by DCFH-DA assay. (b) NO levels were evaluated by Griess reaction. (c) Mitochondrial function was evaluated by MTT assay. The results were compared with the control (cells and medium only). N = 3, ***P < 0.0001, **P < 0.001 and *P < 0.005.
All CCMP concentrations reduced ROS levels significantly when compared with the control group (Fig. 3 a). However, lower NO levels were only observed in sperm cells supplemented with L-CCMP or I-CCMP (Fig. 3 b). H-CCMP increased mitochondrial function (Fig. 3 c). Similar metabolic activity was observed in all sperm samples independent of CCMP exposure.
Post-thaw analyses of sperm viability and oxidative metabolism treated with CCMP supplementation medium
The same variables were analyzed in post-thawing sperm samples stored at −196°C with cryopreservation medium supplemented with CCMP (Figs 4 and 5). Flow cytometry analyses showed that I-CCMP and H-CCMP improved post-thaw sperm cell viability. These concentrations increased number of viable sperm cells in relation to the untreated control group by about 12% (Fig. 4 a). The crystal violet assay showed an increase in sperm cell viability at all CCMP concentrations (Fig. 4 b). The cfDNA assay showed a low increased effect on sperm cell viability of L-CCMP supplementation (Fig. 4 c).

Figure 4 Post-thaw analyses of sperm exposed to L-CCMP, I-CCMP or H-CCMP and storage at liquid nitrogen. (a) Cell viability was measured by flow cytometry using PI. (b) Levels of viable cells were analyzed by crystal violet assay. (c) cfDNA levels were evaluated by PicoGreen assay. The results were compared with the control (cells and medium only). N = 3, *P < 0.005.
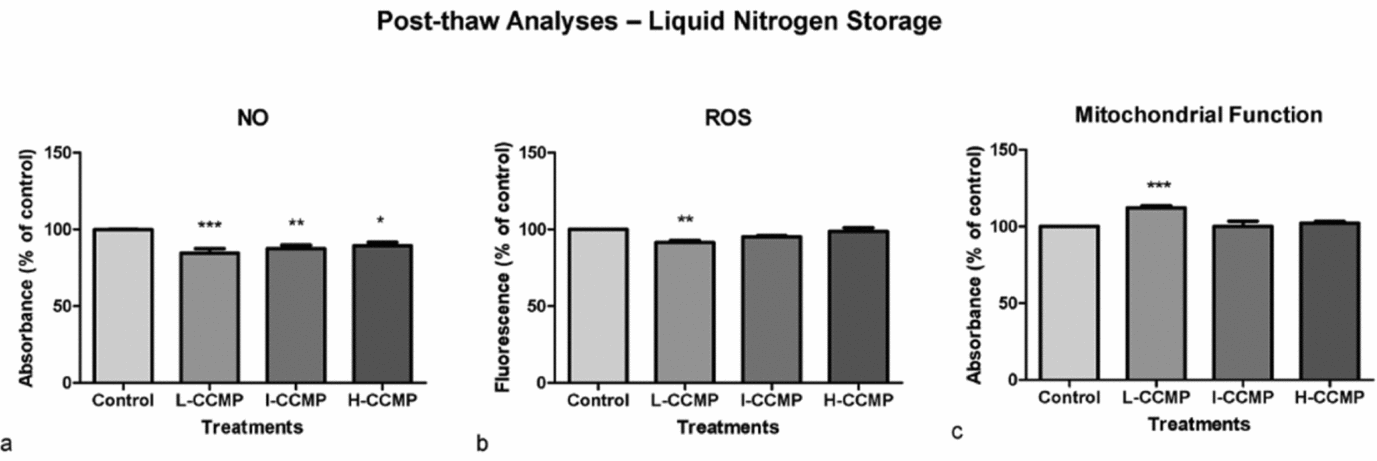
Figure 5 Post-thaw analyses of sperm exposed to L-CCMP, I-CCMP, or H-CCMP and storage at liquid nitrogen. The levels of NO (a), ROS (b) and mitochondrial function (c) were evaluated by Griess reaction, DCFH-DA, and MTT assay, respectively. The results were compared with the control (cells and medium only). N = 3, ***P < 0.0001, **P < 0.001 and *P < 0.005.
CCMP supplementation decreased NO levels of post-thawing sperm samples significantly, independently of concentration (Fig. 5 a). However, only L-CCMP supplementation presented a significant ROS lowering effect (Fig. 5 b). In this treatment, an increase in mitochondrial metabolism was also observed (Fig. 5 c).
A complementary analysis was performed in sperm samples thawed at −80°C with or without CCMP supplementation (Fig. 6). Flow cytometry analyses showed that L-CCMP improved sperm cell viability significantly. Following this treatment, numbers of viable cells were 15% higher than those of the control group (Fig. 6 a). Unfortunately, the other viability assays did not confirm these data (Fig. 6 b, c). ROS (Fig. 6 d) and NO (Fig. 6 e) levels also decreased when sperm cells were treated with L-CCMP, NO levels also decreased following I-CCMP treatment. Mitochondrial activity presented similar levels in all treatments (Fig. 6 f).
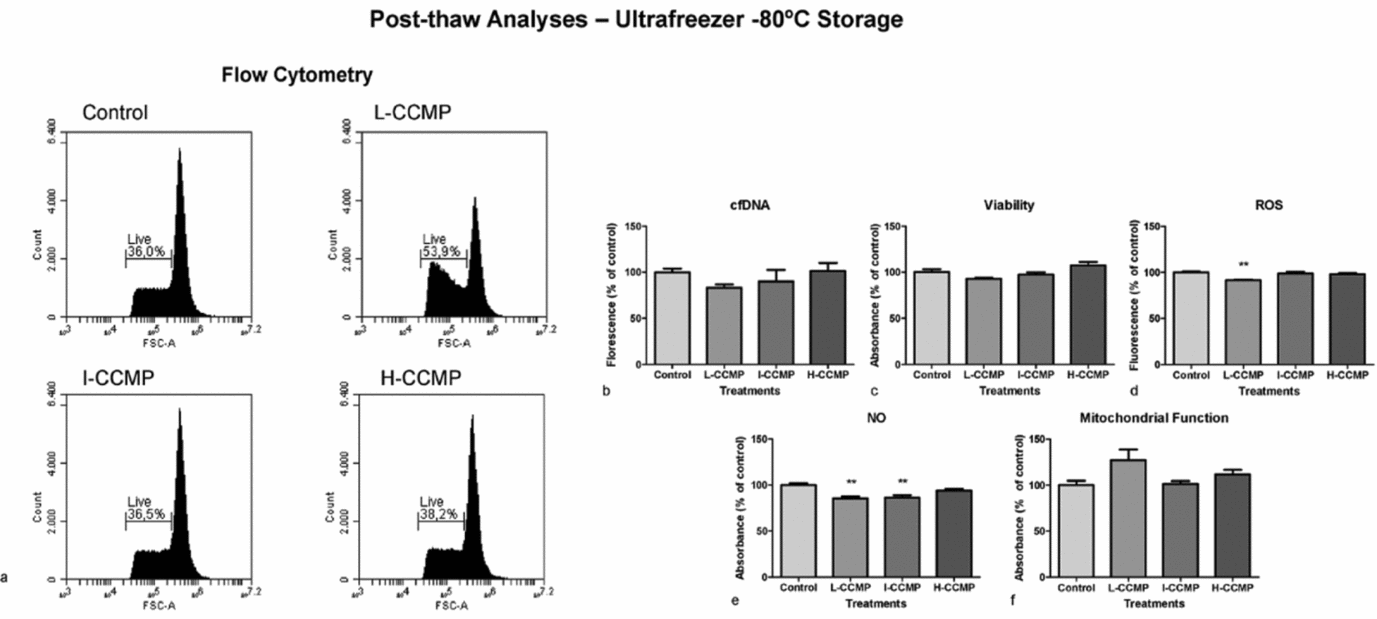
Figure 6 Post-thaw analyses of sperm exposed to L-CCMP, I-CCMP, or H-CCMP and storage in an ultrafreezer at −80°C. (a) Live cells were determined by flow cytometry using PI. Cell viability was measured by cfDNA using PicoGreen® assay (b), as well as by crystal violet assay (c). Levels of ROS (d), NO (e), and mitochondrial function (f) were measured by DCFC-DA, Griss reaction, and MTT assay, respectively. The results were compared with the control (cells and medium only). N = 3, **P < 0.001.
Discussion
In an attempt to reduce and/or minimize detrimental effects of the spermatozoa freezing process, the effect of antioxidant supplementation with guaraná extract on human sperm post-thaw viability and oxidative stress biomarkers were studied. We found that guaraná supplementation at 5 or 10 mg/ml in freezing medium could significantly improve spermatozoa viability. Approximately 50% more viable spermatozoa cells were found at these concentrations compared with the control groups without addition of guaraná supplement. Data from sperm samples exposed to H2O2 confirmed that the best protective effect of guaraná on viability was found at a 10 mg/ml concentration.
The present study revealed that the different concentrations CCMP tested (L-CCMP, I-CCMP and H-CCMP) based on the best cryoprotective guaraná concentration (10 mg/ml), did not result in cytotoxicity in sperm samples when analysed pre-freezing or post-thaw. CCMP, at some concentrations, was able to improve viability and oxidative metabolism in sperm samples pre-freezing and showed cryoprotective activity by increasing viability and decreasing oxidative stress in post-thaw sperm samples.
As cryopreservation techniques increase oxidative stress and induce serious detrimental changes in sperm function (Gürler et al., Reference Gürler, Malama, Heppelmann, Calisici, Leiding, Kastelic and Bollwein2016), previous reports have suggested that some bioactive molecules that are present in CCMP exhibited cryoprotective effects in sperm cells (Amidi et al., Reference Amidi, Pazhohan, Shabani, Khodarahmian and Nekoonam2016; Liu et al., Reference Liu, Wang, Yu, Cheng, Li and Guo2016). A recent investigation reported that (−)-epigallocatechin gallate improved the motility and penetrability into oocytes of frozen–thawed boar spermatozoa (Kaedei et al., Reference Kaedei, Naito, Naoi, Sato, Taniguchi, Tanihara, Kikuchi, Nagai and Otoi2012).
The effect of natural antioxidants such as copherol and ascorbic acids in the maintenance of sperm activity during the freeze–thaw process was initially investigated by Askari et al. (Reference Askari, Check, Peymer and Bollendorf1994). These authors tested the hypothesis that excessive generations of ROS and increased peroxidation of phospholipids in the membrane reduced the activity of spermatozoa in cryopreserved semen. However, this study did not specifically evaluate the effect of antioxidants on spermatozoa viability.
Studies to test whether freezing medium supplementation with plant extracts rich in antioxidant compounds are currently being conducted. Malo et al. (Reference Malo, Gil, Cano, Martínez and Galé2011) recently explained that fennel extracts (Foeniculum vulgare) and rosemary (Rosmarinus officinalis) provide antioxidant protection for boar semen cryopreservation, increasing spermatozoa viability. While most studies were performed using animal spermatozoa, studies involving human sperm and antioxidant supplements added into a freezing medium were also limited to pure antioxidant compounds such as ascorbate, and vitamin E (Taylor et al., Reference Taylor, Roberts, Sanders and Burton2009; Li et al., Reference Li, Lin, Liu, Xiao and Liu2010).
Most studies showed that antioxidant supplementation can improve the post-thaw viability of the sperm. The increase in sperm viability is most probably related to positive modulation of antioxidant compounds on ROS produced during the thawing process. Investigations have demonstrated that cryopreservation of human semen produces ROS which causes significant sperm damage. The high content of unsaturated fatty acids in the plasma membrane makes boar spermatozoa particularly sensitive to the harmful effects of ROS (White, Reference White1993), therefore increase in ROS causes lipid peroxidation damage, which is initiated when ROS attack polyunsaturated fatty acids in the sperm cells. In addition to the effects on the membrane, several researchers have reported DNA damage in human spermatozoa associated with ROS production.
Due to the high susceptibility of sperm to ROS, the sperm cell presents a multitude of ROS scavengers including antioxidant enzymes, such as superoxide dismutase, catalase and glutathione peroxidase/reductase as well as non-enzymatic antioxidant compounds such as α-tocopherol, ascorbic acid, glutathione and pyruvate (Aitken & Baker, Reference Aitken and Baker2006). However, when ROS production increases, as occurs in the freezing process, the endogenous antioxidant molecules cannot efficiently degrade these molecules. For this reason, investigations into sperm cryopreservation involve additional studies about sperm oxidative stress.
We observed that guaraná treatment had an important effect on spermatozoa in post-thaw ROS production, which was greater on lipoperoxidation and protein carbonylation at 10 mg/ml guaraná. However, the cause of the lower guaraná protective effect on lipoperoxidation and protein carbonylation needs to be explored in future studies.
Several investigations have described the positive influence of antioxidants on oxidative stress modulation of post-thaw sperm. Data on sperm from humans and different animal species revealed that resveratrol, melatonin, vitamin E, curcumin and epigallocatechin-3-gallate (EGCG) are among the antioxidants that present positive effects on sperm oxidative stress.
Guaraná extract is rich in methylxanthines such as caffeine, bromine and ophylline and also contains catechins and other antioxidant compounds present at lower concentrations, based on studies that described the use of methylxanthines as additives to sperm suspensions in order to improve sperm characteristics. In immature epididymal or testicular spermatozoa, the addition of methylxanthine is crucial for acquisition or improvement of sperm motility and fertilizing ability (Minnelli & Bellezza, Reference Minnelli and Bellezza2011). The mechanism of action on spermatozoa is generally assumed to be inhibition of sperm phosphodiesterase activity, resulting in an elevation of complementary adenosine monophosphate levels in spermatozoa. Complementary studies also indicated other possible mechanisms of action by methylxanthines on sperm involving alkaline phosphatase activity inhibition (Glogowski et al., Reference Glogowski, Danforth and Ciereszko2002). Pentoxifylline (PF) is a methylxanthine used as a cryoprotectant. It has been reported that the treatment of sperm with PF before freezing decreases acrosome loss during the freeze–thaw process and increases post-thaw agonist-induced acrosome reaction rate in normal semen. The treatment of poor quality human sperm with PF may enhance post-thaw sperm fertilizing ability (Esteves et al., Reference Esteves, Spaine and Cedenho2007).
A randomized double-blinded placebo-controlled study in 254 infertile men was recently performed by Safarinejad (Reference Safarinejad2011) to determine the safety and efficacy of oral PF administration in improving semen parameters. The results showed a significant increase in sperm concentration, motility and sperm with normal morphology after PF administration when compared with baseline and placebo treatment. The acrosome reaction was observed to increase in subjects treated with PF methylxanthine, therefore the results suggested that PF significantly improved semen parameters.
Unfortunately, investigations involving the effect of catechins on sperm viability as well as oxidative metabolism balance are still in progress. A recent investigation performed by Vallorani et al. (Reference Vallorani, Spinaci, Bucci, Tamanini and Galeati2010) evaluated the effect of supplementation with different antioxidants including EGCG, a catechins molecule present at high concentration in green tea, on acrosome, plasma membrane integrity, and activation of caspases of boar spermatozoa stored for 24 h at 15°C. EGCG supplementation resulted in a significant increase in the percentage of viable spermatozoa, inhibited caspase activation and led to a decreased number of dead cells. Therefore, the results suggested that EGCG could exert some compensatory protection against the detrimental effects of semen storage.
However, a negative result linked to EGCG supplementation on mouse spermatozoa using intracytoplasmic injection (ICSI) was also reported by Kusakabe & Kamiguchi (Reference Kusakabe and Kamiguchi2004). In this study, spermatozoa were collected by the swim-up method and treated with EGCG at 1 µM or 10 µM, but the results found pronuclear arrest and degenerated sperm chromatin mass after treatment with EGCG at 10 µM. These results questioned if natural products like guaraná extract could be used safely or if they also led to some genotoxic effects.
We suggest that CCMP, which also contains catechin, increased mitochondrial function of spermatozoa either at pre-freezing or post-thaw in liquid nitrogen, especially in sperm treated with H-CCMP or L-CCMP. Moreover, the present findings showed an increase in viability in pre-freezing analyses in sperm treated with I-CCMP or H-CCMP.
Our findings revealed that CCMP decreased the oxidative stress in sperm samples by reducing ROS production in post-thaw analyses at all the concentrations tested either by liquid nitrogen or L-CCMP following ultrafreezing. Furthermore, L-CCMP and I-CCMP sperm sample treatment decreased NO levels in liquid nitrogen and ultrafreezer post-thaw analyses.
Resveratrol is another bioactive molecule that has been studied as a cryoprotective supplement. An investigation by Garcez et al. (Reference Garcez, dos Santos, Lara, Pasqualotto and Salvador2010) reported that addition of resveratrol can avoid oxidative damage and protect DNA from injury caused by freezing. However, the protective action of resveratrol was not able to prevent loss of sperm motility, indicating that decrease in sperm motility is not only related to oxidative stress.
Conversely, a recent investigation reported that post-thaw sperm samples incubated with caffeine exhibited a progressive increase in motility and mitochondrial activity by increasing energetic levels within the cell (Pariz & Hallak, Reference Pariz and Hallak2016). Therefore, the association of bioactive molecules that present antioxidant properties with compounds that are able to restore sperm motility could be important in ensuring sperm quality for cryopreservation techniques.
In conclusion, the results described here suggest that guaraná extract at 5 and 10 mg/ml has a cryoprotective effect on post-thaw human sperm, increasing cell viability and decreasing cellular ROS production. These protective effects were also found in human sperm samples exposed to a prooxidant compound (H2O2) without freezing procedures. This protective effect in all probability is the result of the integrated action of xanthines and catechins at a ratio of about 5:1, estimated from the concentration of these compounds in the guaraná extract used in this study.
Moreover, we developed here a novel chemical supplement based on methylxanthines and polyphenol molecules found in guaraná chemical matrix and enriched with resveratrol. Overall, the findings of this study indicated that use of CCMP could improve cryopreservation techniques. The synergy of its bioactive molecules, caffeine, catechin, theobromine and resveratrol, could increase cryoprotective effect by improving viability and modulate ROS and NO levels differentially in thawed sperm. Therefore, CCMP supplementation could ensure sperm quality and reproductive success.
Acknowledgements
We are grateful to the Biogenomics Laboratory research team for helping us with data collection.
Financial support
The study was supported by Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).









