Major depression is one of the leading causes of disease burden worldwide (Reference Berton and NestlerBerton & Nestler, 2006). Its impact on society with respect to human suffering and economic charge is enormous, and is even projected to increase in upcoming decades (Reference Lopez and MurrayLopez & Murray, 1998). Since the discovery of drugs with antidepressant properties in the 1950s, no essentially innovative treatment strategy has been established for routine clinical use. Resistance to the available treatment strategies is encountered in 15–30% of patients. New treatment approaches are therefore needed. Repetitive transcranial magnetic stimulation (rTMS) was introduced as a promising new treatment option for depression and showed beneficial effects in single-centre studies (Reference Burt, Lisanby and SackeimBurt et al, 2002; Reference Kozel and GeorgeKozel & George, 2002; Reference Loo and MitchellLoo & Mitchell, 2005). However, it remains difficult to draw general conclusions about the antidepressant efficacy of rTMS because of heterogeneous study designs, variable stimulation parameters and low sample sizes (Reference Martin, Barbanoj and SchlaepferMartin et al, 2003).
METHOD
Study design and participants
The aim of this multicentre trial was to evaluate whether the application of rTMS in a routine clinical setting as an additional strategy to standard antidepressant medication would enhance the clinical improvement of depression compared with sham treatment with regard to the number of responders and the decrease in depression rating scores. Psychiatric departments in seven university clinics – Munich (Ludwig-Maximilian University), Regensburg, Rostock, Tübingen, Ulm and Würzburg in Germany, and Vienna in Austria – with experience in transcranial magnetic stimulation studies participated in this randomised double-blind placebo-controlled, multicentre trial. Randomisation to the real and sham treatment conditions was performed centrally prior to the study by the Institute of Biometrics of the University of Ulm. Patients, raters and medical staff at the in-patient units were all masked to the treatment conditions. The principal investigator (U.H.) at the University of Zürich was responsible for study coordination and central data collection. The Institute of Biometrics of the University of Ulm performed the statistical analysis. All patients gave written informed consent. The study was conducted according to the latest version of the Declaration of Helsinki and was approved by the local ethics committees in each centre.
Inclusion criteria were age 18–75 years; a moderate or severe major depressive episode meeting ICD–10 and DSM–IV criteria (World Health Organization, 1992; American Psychiatric Association, 1994), including bipolar affective disorder, assessed with the Structured Clinical Interview for DSM–IV Axis I Disorders (SCID; Reference First, Spitzer and GibbonFirst et al, 1998); and a score of 18 points or more on at least two of three depression rating scales: the Beck Depression Inventory (BDI; Reference Beck, Ward and MendelsonBeck et al, 1961), the 21-item Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960) and the Montgomery–Åsberg Depression Rating Scale (MADRS; Reference Montgomery and AsbergMontgomery & Åsberg, 1979). The cut-off at 18 points was chosen because in all three scales it is within the range of the transition from mild to medium severity of depression. Exclusion criteria were neurological and severe medical disorders, psychiatric disorders other than depression, history of epileptic seizures, brain lesions or neurosurgery, cardiac pacemaker, inability to give informed consent, and involuntary hospitalisation. Included patients were given an identification number linked to a centralised computer-generated randomisation code determining real or sham stimulation condition. Randomisation was stratified for centre and for HRSD score >30 or ≤30 at enrolment. Raters underwent training at the beginning of the study to increase interrater reliability.
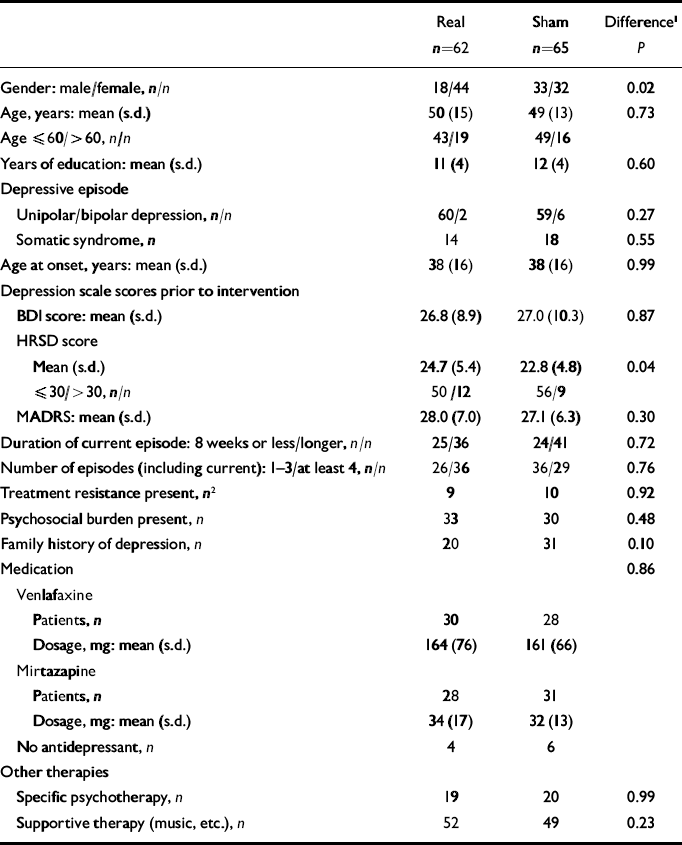
The following individual and clinical features at baseline were documented (see Table 1): duration of the current episode before rTMS, number of episodes in the history including the current episode (1–3 v. >3), treatment resistance (no response to two different antidepressant medications and one combination treatment with treatment periods of at least 4 weeks each in sufficient dosage for the current episode), polarity (depressive episode within unipolar or bipolar disorder), a medical record of family history for depression, and history of a severe psychosocial stressor in the year before manifestation of the current episode (such as death of a close relative, separation from a partner or loss of work).
Table 1 Baseline characteristics of the real and sham intervention groups (n=127)
| Real n=62 | Sham n=65 | Difference1 P | |
|---|---|---|---|
| Gender: male/female, n/n | 18/44 | 33/32 | 0.02 |
| Age, years: mean (s.d.) | 50 (15) | 49 (13) | 0.73 |
| Age ≤60/>60, n/n | 43/19 | 49/16 | |
| Years of education: mean (s.d.) | 11 (4) | 12 (4) | 0.60 |
| Depressive episode | |||
| Unipolar/bipolar depression, n/n | 60/2 | 59/6 | 0.27 |
| Somatic syndrome, n | 14 | 18 | 0.55 |
| Age at onset, years: mean (s.d.) | 38 (16) | 38 (16) | 0.99 |
| Depression scale scores prior to intervention | |||
| BDI score: mean (s.d.) | 26.8 (8.9) | 27.0 (10.3) | 0.87 |
| HRSD score | |||
| Mean (s.d.) | 24.7 (5.4) | 22.8 (4.8) | 0.04 |
| ≤30/>30, n/n | 50/12 | 56/9 | |
| MADRS: mean (s.d.) | 28.0 (7.0) | 27.1 (6.3) | 0.30 |
| Duration of current episode: 8 weeks or less/longer, n/n | 25/36 | 24/41 | 0.72 |
| Number of episodes (including current): 1-3/at least 4, n/n | 26/36 | 36/29 | 0.76 |
| Treatment resistance present, n 2 | 9 | 10 | 0.92 |
| Psychosocial burden present, n | 33 | 30 | 0.48 |
| Family history of depression, n | 20 | 31 | 0.10 |
| Medication | 0.86 | ||
| Venlafaxine | |||
| Patients, n | 30 | 28 | |
| Dosage, mg: mean (s.d.) | 164 (76) | 161 (66) | |
| Mirtazapine | |||
| Patients, n | 28 | 31 | |
| Dosage, mg: mean (s.d.) | 34 (17) | 32 (13) | |
| No antidepressant, n | 4 | 6 | |
| Other therapies | |||
| Specific psychotherapy, n | 19 | 20 | 0.99 |
| Supportive therapy (music, etc.), n | 52 | 49 | 0.23 |
Transcranial magnetic stimulation
Each clinic used the locally available magnetic stimulator with figure-of-eight coils: the Magstim Rapid (Magstim Company Ltd, Whitland, UK; double 70 mm coil, P7N 9790) in Munich, Tübingen, Vienna and Regensburg; the Medtronic Magpro (Medtronic Inc., Minneapolis, USA; coil MC-B70) in Ulm and Würzburg; and the Medtronic Maglite r25 (Medtronic Inc., Minneapolis, USA; coil MC-B70) in Rostock. A biphasic pulse waveform was selected for all stimulations. The participant was seated in a comfortable chair during the procedure. The real stimulation was applied above the left dorsolateral prefrontal cortex, targeted by guiding the coil to the position F3 according to the international 10–20 system for electroencephalography electrode placement (Reference Herwig, Satrapi and Schonfeldt-LecuonaHerwig et al, 2003b ). The real stimulation intensity was determined as 110% of the individual resting motor threshold (Reference Rossini, Barker and BerardelliRossini et al, 1994). Inter-individual differences in cortical excitability and the use of different stimulators were thereby taken into account. Stimulations were performed with a frequency of 10 Hz, trains of 2 s, inter-train-intervals of 8 s, 100 trains per session, 2000 stimuli per day on 15 subsequent working days. Sham stimulation was applied 5 cm lateral to F3, perpendicular to the parasagittal plane, above the left temporal muscle; in this position the coil–cortex distance is essentially larger (more than 3 cm v. 1–1.5 cm) than at F3, and the electromagnetic field reaching the cortex was therefore substantially weaker. To further reduce the possible effectiveness of the sham stimulation the coil was angled at 45°, touching the skull not with the centre but with the rim opposite the handle, and the stimulation intensity was reduced to 90% of motor threshold. Although the angling of the coil might have been registered by the patients as being different from the coil handling involved in measuring the motor threshold, this was a compromise made in an attempt to make the sham condition as similar as possible concerning side-effects to the real one but with minimum efficacy. Owing to the substantially weaker electromagnetic field reaching the cortex in this condition compared with real rTMS, neuronal depolarisation (Reference Loo, Taylor and GandeviaLoo et al, 2000; Reference Lisanby, Gutman and LuberLisanby et al, 2001) was unlikely, as was any possible antidepressant effect. Nevertheless, this form of sham stimulation had the effect of inducing local sensations above the temporal muscle similar to the disturbances caused by the real stimulation (Reference Praeg, Herwig and LutzPraeg et al, 2005), helping to reduce bias from patient awareness of the difference between the two applications (Reference Abler, Walter and WunderlichAbler et al, 2005). Using a sham coil with no stimulation would have been even more different from real stimulation because of the absence of local sensations compared with the experience of motor threshold determination.
Concomitant treatments
In order to integrate rTMS in a naturalistic routine clinical setting, and for ethical and safety reasons, rTMS was applied in parallel with a standardised antidepressant medication or as monotherapy when no medication was possible. The stimulation sessions were started together with a venlafaxine or mirtazapine treatment, both selected because of their combined serotonergic and noradrenergic profile in order to rule out neurotransmitter-specific confounding effects. Prior antidepressant medication was washed out (4 t ½). Venlafaxine was started at a dosage of 75 mg per day in the first week, and mirtazapine at a dosage of 15 mg per day. Both treatments could be increased later according to clinical need as evaluated by the responsible psychiatrist. No other antidepressant or concomitant antipsychotic medication was allowed. A maximum of 1.5 mg lorazepam per day was permitted as crisis medication. Patients whose condition had been stable on lithium treatment for at least 3 months before starting rTMS were allowed to continue taking this medication. Anticonvulsants were not allowed. Non-psychiatric medication was continued as needed and documented. All other treatments, such as psychotherapy and supportive therapies (music, occupational therapy, etc.), were also continued and documented, and compared between the real and the sham stimulation group.
Efficacy variables and statistical procedure
Baseline values were analysed with descriptive statistics. Frequencies were calculated for categorical data and means and standard deviations for quantitative variables. Furthermore, the baseline values of the real and the sham groups were compared with chi-squared tests for categorical variables or t-tests for quantitative variables.
The primary objective was to demonstrate that rTMS adjunctive to standard antidepressant treatment results in a greater number of responders (defined as patients with an improvement in scores on at least two of the three rating scales by at least 50% after 3 weeks of rTMS) than sham treatment (primary hypothesis). The secondary objective was to show a greater decrease in the depression rating scores with real rTMS than by sham treatment (secondary hypothesis). Remission was defined descriptively as a score of 10 points or below in all three scales. The BDI, HRSD and MADRS rating scales were administered prior to the stimulation sessions (rating 1); after 1 week and 2 weeks (ratings 2 and 3); at the end of the stimulation series after 3 weeks (rating 4); and at a follow-up interview 3 weeks later (rating 5). The first rating was made on the day before the stimulation period commenced. If rTMS was started the day after recruitment, the recruitment ratings were considered instead.
On the basis of previous reports (e.g. Reference Pascual-Leone, Rubio and PallardoPascual-Leone et al, 1996; Reference George, Nahas and MolloyGeorge et al, 2000; Reference Padberg, Zwanzger and KeckPadberg et al, 2002; Reference Herwig, Lampe and JuenglingHerwig et al, 2003a ) and presuming a clinically meaningful response in the real treatment group, we assumed a response rate of 50% due to augmentative and accelerative effects of rTMS after 3 weeks of stimulation compared with a sham response rate of 20% with the response due to medication assumed to occur later. Accordingly, the calculation of the sample size indicated that 45 patients were needed in each group to detect a difference in response rates between groups with 80% power at a 5% significance level. Presuming an estimated withdrawal rate of 20%, we aimed to include 120 patients in the study.
The primary efficacy variable analysed in the intention-to-treat set was treatment response. The comparison between treatment groups was performed by means of a Wald chi-squared test in a logistic regression model for the primary efficacy variable, adjusting for the stratification variables ‘centre’ (the centres Munich, Regensburg and Vienna, which had a joint rTMS training, were pooled in order to avoid numerical problems due to too small sample sizes), and ‘HRSD’ (score ≤30 v. >30). Treatment × centre and treatment × HRSD interactions were tested in the model but were eliminated because P values exceeded 0.05. Results are described using odds ratios, 95% confidence intervals and P values. Secondary efficacy variables were the absolute and relative changes from rating 1 to 4 and 5 (before and after 3 weeks of stimulation, and at the follow-up) in the depression scores on HRSD, MADRS and BDI. They were compared between treatment groups using an F-test in a three-way analysis of variance (ANOVA) with treatment, centre and HRSD score as the main effects. Treatment × centre and treatment × HRSD interactions were again tested, and eliminated as P values were greater than 0.05. Least square means with 95% confidence intervals and P values for the comparisons between groups are reported.
Additional explorative analyses assessing the interaction effect of age (≤60 years v. >60 years), gender, device type and concomitant medication with treatment on the primary end-point were performed, by also including age or gender respectively in the models used for efficacy analyses. Owing to associations between device type and centre, device type was used instead of centre in the respective models.
All statistical analyses were performed with the Statistical Analysis System software package, version 8.02 for Windows.
RESULTS
Participants
The intention-to-treat (ITT) sample comprised 127 patients (Fig. 1, Table 1). The study commenced in 2003, and most of the patients were recruited between June 2004 and November 2005 after the researchers obtained a supporting grant from the German Research Foundation. The numbers of patients recruited by the different centres were Ulm n=37, Würzburg n=24, Rostock n=21, Tübingen n=16, Regensburg n=14, Munich n=11 and Vienna n=4. Of the 127 patients, 62 were randomised to the real stimulation group and 65 to the sham group. In the period between enrolment and start of the treatment, 5 patients showed an improvement in their depressive symptoms such that they no longer fulfilled the inclusion criteria. Two patients had to be excluded during or after stimulation (1 because of psychotic symptoms and 1 because a data review revealed an erroneous inclusion) and were not considered for the per protocol analysis. Fifteen participants withdrew during the stimulation series, 6 from the real intervention group and 9 from the sham group, for the reasons detailed in Fig. 1. Thus, 105 patients received stimulation according to protocol over the whole period of 3 weeks: 52 with real stimulation and 53 with the sham condition. Follow-up ratings 3 weeks after the end of the stimulation sessions were performed in 50 participants in the real group and 48 in the sham group; the remaining patients refused to participate or could not be contacted. Administration of concomitant medication was similar in both groups, including mean dosages (Table 1). Treatment groups were similar with respect to a continuation of supportive treatments such as occupational therapy, music therapy, relaxation techniques, supportive psychotherapy (real, n=52; sham, n=49) and, if established, a continuation of cognitive–behavioural or interpersonal therapy (real, n=19; sham, n=20). In the frame of the multiple comparisons of the baseline characteristics we found the real group to include more women than the sham group and to score marginally higher on the HRSD in the ITT set (no difference in an additional testing of the PP set: P=0.08), but not on the BDI or the MADRS. The other features and clinical baseline characteristics were similar in the two groups (Table 1).

Fig. 1 CONSORT flowchart (rTMS, repetitive transcranial magnetic stimulation).
Primary and secondary efficacy outcome
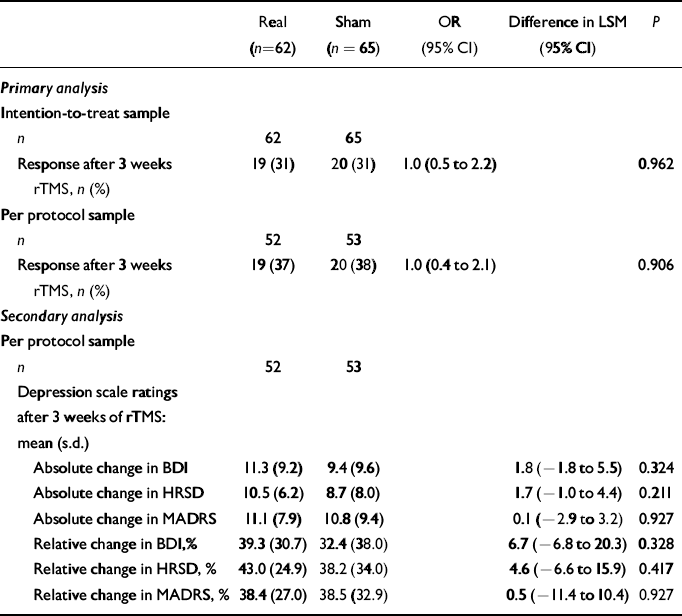
Within the ITT sample the analysis of treatment response revealed 19 responders (31%) in the real condition and 20 responders (31%) in the sham condition v. 33 non-responders (53%) and 33 non-responders (51%) respectively. The remaining patients withdrew from the trial or were excluded (real, n=10, 16%; sham, n=12, 18%; Fig. 1). For the ITT analysis of primary efficacy, missing values for the patients who withdrew were recorded as non-response. After adjusting for centre and HRSD score at the start of the study, there was no significant difference in responder rates between the different groups (OR=1.0, 95% CI 0.5–2.2, Wald χ2 test, P=0.962; Table 2). There was no meaningful difference in the response rates between the centres (P=0.339).
Table 2 Analysis of efficacy
| Real (n=62) | Sham (n=65) | OR (95% CI) | Difference in LSM (95% CI) | P | |
|---|---|---|---|---|---|
| Primary analysis | |||||
| Intention-to-treat sample | |||||
| n | 62 | 65 | |||
| Response after 3 weeks rTMS, n (%) | 19 (31) | 20 (31) | 1.0 (0.5 to 2.2) | 0.962 | |
| Per protocol sample | |||||
| n | 52 | 53 | |||
| Response after 3 weeks rTMS, n (%) | 19 (37) | 20 (38) | 1.0 (0.4 to 2.1) | 0.906 | |
| Secondary analysis | |||||
| Per protocol sample | |||||
| n | 52 | 53 | |||
| Depression scale ratings after 3 weeks of rTMS: mean (s.d.) | |||||
| Absolute change in BDI | 11.3 (9.2) | 9.4 (9.6) | 1.8 (-1.8 to 5.5) | 0.324 | |
| Absolute change in HRSD | 10.5 (6.2) | 8.7 (8.0) | 1.7 (-1.0 to 4.4) | 0.211 | |
| Absolute change in MADRS | 11.1 (7.9) | 10.8 (9.4) | 0.1 (-2.9 to 3.2) | 0.927 | |
| Relative change in BDI,% | 39.3 (30.7) | 32.4 (38.0) | 6.7 (-6.8 to 20.3) | 0.328 | |
| Relative change in HRSD, % | 43.0 (24.9) | 38.2 (34.0) | 4.6 (-6.6 to 15.9) | 0.417 | |
| Relative change in MADRS, % | 38.4 (27.0) | 38.5 (32.9) | 0.5 (-11.4 to 10.4) | 0.927 |
The ANOVA of the secondary efficacy variables, i.e. the absolute and relative changes from rating 1 to rating 4 (end of the rTMS period; Table 2, Fig. 2) and rating 5 (follow-up) of the depression scores on the HRSD, MADRS and BDI, revealed no difference between the real and sham groups at the end of the stimulation sessions. In the per protocol data-set, logistic regression showed no difference in the responder rates between the real and sham stimulation groups at any point during the course of stimulation, and thus no accelerated antidepressant effect (Fig. 3). Further, there was no meaningful difference in the responder rates between the treatment groups after the follow-up period (Wald χ2 test, P=0.34). With regard to the absolute and relative changes in the rating scores, no meaningful difference was observed between the real and sham stimulation groups in the ratings after 1 week, after 2 weeks and at follow-up (Fig. 4). Remission of depression was found in 6 people in the real group and 10 people in the sham group.
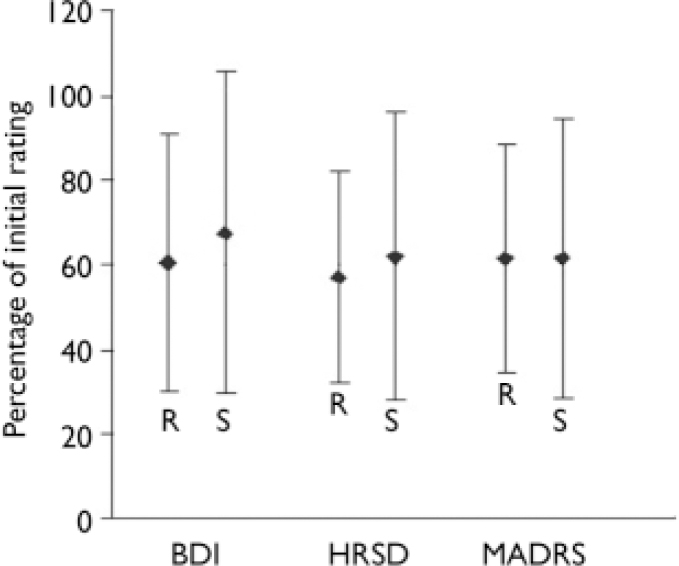
Fig. 2 Mean percentage and standard deviation of the rating scores after 3 weeks repetitive transcranial magnetic stimulation relative to the initial ratings (100%), secondary efficacy variable. No meaningful difference between the real group (R) and the sham group (S) was observed. (BDI, Beck Depression Inventory; HRSD, Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scale).

Fig. 3 Proportion of participants achieving response at each rating during the repetitive transcranial magnetic stimulation sessions until follow-up (intention-to-treat sample). At no point was a meaningful difference between the real and the sham intervention groups observed.
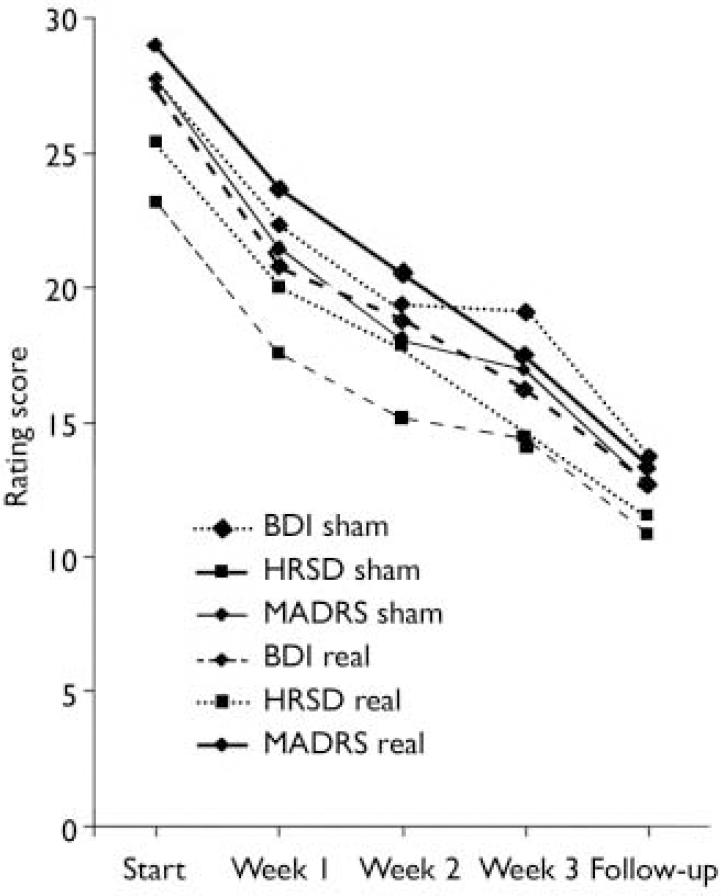
Fig. 4 Course of the mean rating scores of the per protocol set at each rating during the course of stimulations and at follow-up. No meaningful difference between the real and the sham intervention groups was observed. Standard deviations are not implemented for reasons of overview; those of the rating scores at the end of the stimulation session are provided in Table 2 (BDI, Beck Depression Inventory; HRSD, Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scale.
Explorative analyses did not show any meaningful interaction effect of age, gender, device type or concomitant medication with treatment on the primary efficacy outcome.
Side-effects
Patients complained of the following side-effects related to rTMS: headache (real, n=3; sham, n=1), dizziness (real, n=0; sham, n=1) painful local sensation (real, n=1; sham, n=2) and nausea (real, n=1; sham, n=0). Most patients reported that the stimulation generally caused an uncomfortable local sensation but they did not complain about this as a side-effect. We observed no epileptic seizure or other severe side-effect.
DISCUSSION
The aim of this multicentre trial was to investigate the antidepressant effect of rTMS as an augmentative and/or accelerative treatment to simultaneously initiated antidepressant medication in a routine clinical setting. We did not find beneficial effects of active rTMS compared with the sham condition with regard to responder rates or changes in the rating scores. Furthermore, no acceleration of a clinical improvement was observed. No severe side-effect such as epileptic seizure occurred, indicating that the method may be considered to be safe within the frame of our study design and as far as the limits of our sample size allow.
Transcranial magnetic stimulation depolarises neurons in targeted cortex areas focally and non-invasively through induction of a transient electromagnetic field that is generated by a pulsed electrical current running through a wound copper coil. The induction of local and trans-synaptically mediated metabolic and biochemical changes in pathophysiologically relevant brain areas was suggested as a rationale for an antidepressant effect (Reference Post and KeckPost & Keck, 2001). The left dorsolateral prefrontal cortex was selected as a main target area for stimulation in patients with depression on the basis of imaging studies that attributed depressive symptoms to a regional hypometabolism which might be upregulated by rTMS (Reference Pascual-Leone, Rubio and PallardoPascual-Leone et al, 1996). The antidepressant properties of rTMS have now been investigated for more than 10 years, and initial positive studies elicited hope in both the scientific community and the public. Presumably in routine clinical care rTMS would be mainly applied concomitantly with other antidepressant treatments; for this reason an additional benefit of rTMS should be demonstrated in controlled clinical trials.
Comparison with other rTMS treatment trials and limitations
Our multicentre results are in contrast to several positive reports from single-centre studies of rTMS for depression (reviewed by Reference Burt, Lisanby and SackeimBurt et al, 2002; Reference Martin, Barbanoj and SchlaepferMartin et al, 2003; Reference Loo and MitchellLoo & Mitchell, 2005), but they are in line with other negative reports (Reference Loo, Mitchell and CrokerLoo et al, 2003; Reference Nahas, Kozel and LiNahas et al, 2003; Reference Poulet, Brunelin and BoeuvePoulet et al, 2004; Reference Miniussi, Bonato and BignottiMiniussi et al, 2005). Our results are to be compared in particular with studies addressing the specific issue of rTMS as an add-on or augmentative treatment to antidepressant medication. Recent studies of this topic that reported positive results require discussion in more detail in relation to our results. In a trial investigating rTMS (5 Hz, 120% of motor threshold, 1200 stimuli per day) above the left dorsolateral prefrontal cortex, given in parallel with amitriptyline titrated up to a therapeutic dosage during the week before starting rTMS, beneficial effects were found already after the first week of stimulation and were sustained for the stimulation period of 4 weeks (Reference Rumi, Gattaz and RigonattiRumi et al, 2005). Another study, combining rTMS (15 Hz, 100% of motor threshold, 900 stimuli per day) above the left dorsolateral prefrontal cortex with venlafaxine, citalopram or sertraline started simultaneously and titrated up quickly, found beneficial effects after 2 weeks of stimulation, but these benefits had disappeared at the follow-up assessment 3 weeks later (Reference Rossini, Magri and LuccaRossini et al, 2005). Concerning stimulation parameters, the values used in our study (10 Hz, 110% motor threshold) were between those of the two studies mentioned above but our daily amount of stimuli was higher, so that these differences can hardly account for our negative results. A further study reporting beneficial effects (Reference Anderson, Delvai and AshimAnderson et al, 2007) applied rTMS at 10 Hz, 110% of motor threshold, 1000 stimuli per day, three times per week for 4–6 weeks while the patients were maintained on established medication. Here, the difference from our results might be due to unchanged medication in largely treatment-resistant patients, with thus no further medication effect as indicated by a low response in the sham group (7%), and to the longer stimulation period. Generally, different regimens of co-medication in these studies are to be considered when comparing the results. Other add-on rTMS studies with negative results might have suffered from insufficient stimulation parameters such as sub-threshold intensity and low number of stimuli (Reference Poulet, Brunelin and BoeuvePoulet et al, 2004).
The stimulation parameters for our study were chosen as those most likely to have a possible antidepressant effect, based on the evidence available at the time of study conception: higher intensities (≥100% of motor threshold), frequencies (≥5 Hz) and total amounts of stimuli (≥10 000); treatment periods of at least 10 days; and targeting the left dorsolateral prefrontal cortex (e.g. Reference Pascual-Leone, Rubio and PallardoPascual-Leone et al, 1996; Reference Padberg, Zwanzger and KeckPadberg et al, 2002; Reference Grunhaus, Schreiber and DolbergGrunhaus et al, 2003; Reference Herwig, Satrapi and Schonfeldt-LecuonaHerwig et al, 2003b ; Reference Loo, Mitchell and CrokerLoo et al, 2003; Reference Martin, Barbanoj and SchlaepferMartin et al, 2003). One might argue that our chosen stimulation period of 3 weeks was too short. However, the above-mentioned papers and the majority of other relevant studies reported positive effects even earlier, i.e. after 1–2 weeks of stimulation. A single-centre study that used the same parameters concerning intensity, frequency, location and duration as we did, albeit with fewer stimuli per day (1600) in 5 s trains and with a different study design, recently reported beneficial rTMS effects in treatment-resistant depression (Reference Avery, Holtzheimer and FawazAvery et al, 2006). Thus, on the basis of the literature, we could have expected to detect an anti-depressant effect from the stimulation parameters used in our trial. The improvement observed in both groups of our study may be explained as an effect of medication, a general placebo effect or the spontaneous course of the disease. Further, clinical factors such as short episode duration and lack of treatment resistance, whenever we had a strict definition, in some of our patients might have accounted for the generally good antidepressant response. Accordingly, one may argue that a possible antidepressant effect of rTMS might have been hidden by the medication effect and by these clinical factors; but one can at least state that no beneficial effect of rTMS in addition to newly initiated medication with mirtazapine or venlafaxine at the standard lower dose range was observed. In this context, it may also be argued that our study might have been underpowered and that more patients should have been included in order to reveal a significant difference. However, we observed the same rates of responders (31%) in both groups, implying that even if many more patients had been treated the outcome in the primary efficacy variable would not have been any different. As concerns the number of included patients, it should be noted that this study is one of the largest of rTMS in depression reported to date. The antidepressant response found in our study for both stimulation conditions is comparable with the results reported for a 3-week period of treatment (within longer courses) in pharmacological studies that investigated the antidepressant response on mirtazapine and venlafaxine in terms of changes in HRSD and/or MADRS rating scores and response rate (e.g. Reference Amini, Aghayan and JaliliAmini et al, 2005; Reference Shelton, Haman and RapaportShelton et al, 2006). Accordingly, we found no evidence that the response to rTMS and medication in our study was superior to that reported by studies that investigated solely medication effects. Concerning patient characteristics, we found no influence of age and gender on outcome. Although other studies suggested an age-dependent rTMS effect with less efficacy in the elderly (Reference Mosimann, Schmitt and GreenbergMosimann et al, 2004; however, that study used lower intense stimulation parameters), in our study neither the younger nor the older patients responded to rTMS. Further, considering our gender distribution, gender showed no effect on treatment outcome in the explorative analysis, which also would not have been supported by any evidence in the literature. The HRSD baseline scores were slightly higher in the real stimulation group, whereas MADRS and BDI scores did not show any difference between the groups. Within a set of multiple comparisons it was likely that differences would be observed in relation to distinct features. The mean absolute difference in HRSD scores, however, was less than 2 points and therefore clinically marginal. Further, the analyses had been adjusted for HRSD score (≤30 v. >30) at the start of the study, and no different outcome dependent on HRSD score was observed. Considering these facts and that the study outcome was negative, there was no meaningful bias in our view. We further found no influence of stimulator type or concomitant medication on treatment outcome, and no difference in the clinical baseline variables.
Meta-analyses addressing rTMS studies in depression draw critical conclusions concerning the applied methodology and the clinical significance of the results. Kozel & George (Reference Kozel and George2002) found a mean difference in improvement in studies using real v. sham rTMS of 3 points on the HRSD, the clinical impact of which appeared to be marginal. Furthermore, for methodological reasons they considered only a small number of the studies on this topic. Martin et al (Reference Martin, Barbanoj and Schlaepfer2003) also criticised methodological issues and concluded that there was no strong evidence of benefit from using rTMS to treat depression, although the small sample sizes of the studies did not allow the possibility of such an effect being excluded. A recent meta-analysis concluded that rTMS may not differ from sham treatment in major depression (Reference CouturierCouturier, 2005). However, that analysis also excluded several studies because of methodological issues and therefore based its outcome on only a few studies. Therefore, the current literature and our data dampen early expectations about positive effects of rTMS on depression and indicate that one should be careful about generally implementing rTMS in clinical practice.
Future directions
Despite this critical report showing no augmentative or accelerating antidepressant properties of rTMS, previous positive reports still provide strong arguments for the possibility of rTMS providing an anti-depressant effect under certain circumstances. In particular, the possibility of beneficial rTMS effects in selected sub-populations of patients with distinct clinical variables and aetiological or psychopathological aspects in the sense of certain endo-phenotypes should be addressed. Also, rTMS may be advantageous for patients with treatment-resistant depression (Reference Avery, Holtzheimer and FawazAvery et al, 2006; Reference Fitzgerald, Benitez and de CastellaFitzgerald et al, 2006). This issue has been addressed in another multicentre trial not published at the time of the final submission of this manuscript. Further, the identification of more specific and neurobiologically based stimulation parameters, including alternative stimulation sites, may offer new approaches to finding an antidepressant rTMS effect. Notably, in the light of the diverse neurobiological effects of rTMS (e.g. Reference Post and KeckPost & Keck, 2001; Reference Pogarell, Koch and PopperlPogarell et al, 2006), the specific neurobiological basis for a possible treatment effect and for distinct stimulation parameters remains unclear in transcranial magnetic stimulation research.
To conclude, this first multicentre trial investigating rTMS over the left dorsolateral prefrontal cortex in depression in a routine clinical setting does not support the hypothesis of an augmentative or accelerative antidepressant effect of rTMS in patients with concomitant antidepressant medication. Major tasks for future research in this field will be to investigate whether patients with distinct subtypes of depression would respond preferentially, to identify which stimulation parameters might be most effective and to further reveal the neurobiological background. Given the heterogeneous nature of reports of this technique to date, it is recommended that the application of rTMS should be restricted to the scientific context for further exploration of its possible benefits.
Acknowledgements
The study was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG Annual Report 2004, 206–09, Clinical Neurosciences II) as a public sponsor. We are grateful to Eva Peron, Dr Anastasios Konstantinidis and Dr Romana Wimmer for participating in the performance of the study, and to Jacqueline Klesing for revising the manuscript.









eLetters
No eLetters have been published for this article.