Introduction
Wolff–Parkinson–White syndrome is a common cause of arrhythmias in the young. Reference Lu, Wu, Chen, Kao and Huang1 Radiofrequency transcatheter ablation has proven its efficacy, with a success rate reaching 95%, Reference Rodriguez, de Chillou and Schläpfer2 and currently represents the first-choice treatment for supraventricular tachycardia. Reference Brugada, Katritsis and Arbelo3 However, in some circumstances the anatomic features of the accessory pathway can cause technical difficulties, with risk of procedural failure. A common cause for unsuccessful ablation attempts with the standard endocardial approach is epicardial localisation of the accessory pathway.
The most common site of epicardial accessory pathways is posterior-septal, followed by left posterior. Coronary sinus-associated diverticulum can be found in 21% of patients and anomalies of the coronary sinus and its tributaries in other 9%. Ablation from the coronary sinus or its main tributaries is needed in these cases. Reference Sun, Arruda and Otomo4 This approach can be challenging and with a considerable risk of complications in children, Reference Alazard, Lacotte and Horvilleur5 due to the small size of the venous branches and their proximity to the coronary arteries. Reference Stavrakis, Jackman and Nakagawa6
We describe our most recent consecutive paediatric case series who underwent radiofrequency transcatheter ablation of epicardial posterior-septal accessory pathways using a transvenous approach through the coronary sinus in our Institution.
Methods
Study population
This case series included all children with epicardial posterior-septal accessory pathways who underwent radiofrequency transcatheter ablation using a transvenous approach through the coronary sinus in our Institution from September 2017 to September 2020.
All procedures were indicated according to current guidelines. Reference Brugada, Blom and Sarquella-Brugada7,Reference Philip Saul, Kanter and Abrams8
Written informed consent was obtained from the parents of all patients prior to the procedure. The study was approved by the institutional review board and fully complies with the Declaration of Helsinki.
Electrophysiological study and 3D mapping
All antiarrhythmic drugs were withdrawn at least five half-lives before catheter ablation to allow for complete pharmacological wash-out.
The procedure was performed under general anaesthesia, induced with sevoflurane or propofol and maintained with sevoflurane. A thermal mattress was used to maintain normal body temperature.
Surface electrocardiogram leads and endocardial potentials were recorded and stored on a multichannel recorder (Bard Electrophysiology, Billerica, MA, United States of America).
In all patients, an oesophageal electro-catheter (EsoflexTM, FIAB, Florence, Italy) and/or an endocavitary catheter (quadripolar or decapolar) were used to perform the electrophysiological study and the pacing manoeuvres. Additional diagnostic catheters were employed, if needed.
In all procedures, CARTO-3® with the CARTO-Univu® Module (Biosense Webster Inc., Diamond Bar, CA, United States of America) was used. CARTO-Univu® is an advanced imaging integration module that allows to combine fluoroscopy images with 3D electroanatomical maps.
At the beginning of the procedure, three fluoro-views (anterior–posterior, left–right anterior oblique) of the heart were acquired with the 3D system. The navigation catheter, inserted into the right femoral vein, was advanced into the right atrium and coronary sinus. In case of any doubts about venous access, catheter position, discordance between the electrogram recorded and catheter location showed by CARTO-Univu®, fluoroscopy was used. Reference Drago, Grifoni and Remoli9
Mapping was performed in the bipolar recording mode filtered at 0.005–800 Hz. The peak of the R wave on the surface ECG (commonly lead II) was used as the reference fiducial point in each patient to determine the local activation time. All mapping data acquired with the system were manually checked fixing the annotation of mapping points at the onset of the ventricular electrogram for precise system determination of the local activation time.
The map was created during sinus rhythm, and the site of earliest ventricular activation was considered as possible site of the accessory pathway ablation, but additional parameters were also used to evaluate the best ablation target, such as presence of an accessory pathway potential, the largest accessory pathway potential, accessory pathway potential-delta wave interval, and morphology of the unipolar recording.
In the last eight procedures, electroanatomical mapping was integrated with pre-acquired angio-CT scans using CARTO-Merge® Module in attempt to better understand coronary sinus anatomy and guide ablation.
Radiofrequency transcatheter ablation
Different types of mapping and ablation catheters were used in chronological order in attempt to increase success and reduce recurrences: standard radiofrequency catheter (Navistar®; Biosense Webster Inc.), standard open irrigated tip catheter (Navistar ThermoCool®; Biosense Webster Inc.), and contact force sensing irrigated tip catheter (ThermoCool® SmartTouch®; Biosense Webster).
Radiofrequency current was delivered through a Stockert generator (EP Shuttle® and Smartablate®, Stockert GmbH, Freiburg, Germany – distributed by Biosense Webster) from the electrode tip to the cutaneous patch, positioned on the left scapula, under closed loop temperature control. Radiofrequency power was titrated from 15 up to 25 W without or with saline irrigation of the ablation electrode at a constant flush rate of 17 cc/minute. During radiofrequency delivery, temperature was constantly monitored to avoid an increase over 60 °C with standard catheter and 43 °C with irrigated catheters. In case of an increase of impedance > 5 Ω, radiofrequency delivery was stopped.
When contact force sensing irrigated tip catheter was used, radiofrequency delivery was started on the target site with a minimal contact force of 5 g. Radiofrequency was stopped when conduction along the accessory pathway did not disappear within 10 seconds after the start of the pulse; when accessory pathway conduction disappeared during the ablation, radiofrequency delivery was continued for additional 60 seconds. The initial successful attempt was usually followed by an additional radiofrequency energy application at the same site to minimise possible recurrence.
Acute procedural success was defined as disappearance of ventricular pre-excitation and non-inducibility of atrioventricular re-entrant tachycardia by programmed stimulation, either at rest or under isoproterenol infusion (0.01–0.04 µg/kg/minute) for at least 30 minutes from the last radiofrequency delivery. In addition, an intravenous bolus of adenosine (0.2 mg/kg) was administered to evaluate if there was residual antegrade accessory pathway conduction during inhibition of antegrade atrioventricular nodal conduction.
Follow-up
After the procedure, continuous ECG monitoring was maintained for 24 hours and both standard ECG and transthoracic echocardiogram were recorded every 8 hours to exclude signs of myocardial ischaemia.
Clinical evaluation, ECG, 24-hour ECG Holter monitoring, and exercise test were performed 1 and 6 months after the procedure and then once a year.
Data analysis
Continuous variables were first tested for Gaussian distribution with the one-sample Kolmogorov–Smirnov test. They were expressed as frequencies, median ± interquartile range, and mean ± standard deviation as appropriate.
Results
During the study period, 20 children underwent 22 radiofrequency transcatheter ablation procedures due to a second ablation procedure in 2 patients. Demographic and clinical characteristics of this case series are summarised in Table 1. Four patients underwent unsuccessful cryoablation before radiofrequency transcatheter ablation.
Table 1. Characteristics of the case series

LVNC = left ventricular non-compaction
Most patients (Table 2) presented a positive delta wave in lead I (100%), a negative delta wave in lead II (60%), III (90%) and aVF (80%), and a positive delta wave followed by a deep S wave in precordial lead V1 (55%).
Table 2. Delta wave polarity. The delta wave was measured within the first 40 ms of the earliest delta wave onset (positive, negative, or isoelectric)

In most procedures (12 out of 22, 55%), two catheters were used (a quadripolar/decapolar deflectable diagnostic catheter and ablation catheter). In six patients, three catheters were used, whereas one catheter only in two procedures.
Navistar® was used in 6 out of 22 procedures (27%), Navistar ThermoCool® in 12 (55%), and Navistar SmartTouch® in 4 (18%).
In 14 patients, the ablation site was detected within the coronary sinus, while in the other 6 patients in the middle cardiac vein.
Mean procedural time was 174 ± 70 minutes. Median fluoroscopy time was 9 seconds (interquartile range 1–25 seconds). Median dose area product (DAP) 157mGy/cm2 (interquartile range 81–522).
Total acute success rate was 73% (16 out of 22 procedures).
No patient was lost to follow-up (mean time 11.4 ± 9 months).
There were no transient or permanent complications. In particular, coronary angiogram was not necessary in any patients due to the absence of electrocardiographic and echocardiographic anomalies.
The total recurrence rate was 19% (3 out of 16 successful procedures). In one patient, a recurrence was detected after 12 hours, while in the other 2 after 1 month.
Two patients underwent a successful redo-procedure; thus, the overall long-term success rate was 65% (13 out of 20 patients).
A flow chart including the ablation site, the relative acute success, and recurrence rate obtained with the different type of ablation catheters used is represented in Figure 1.
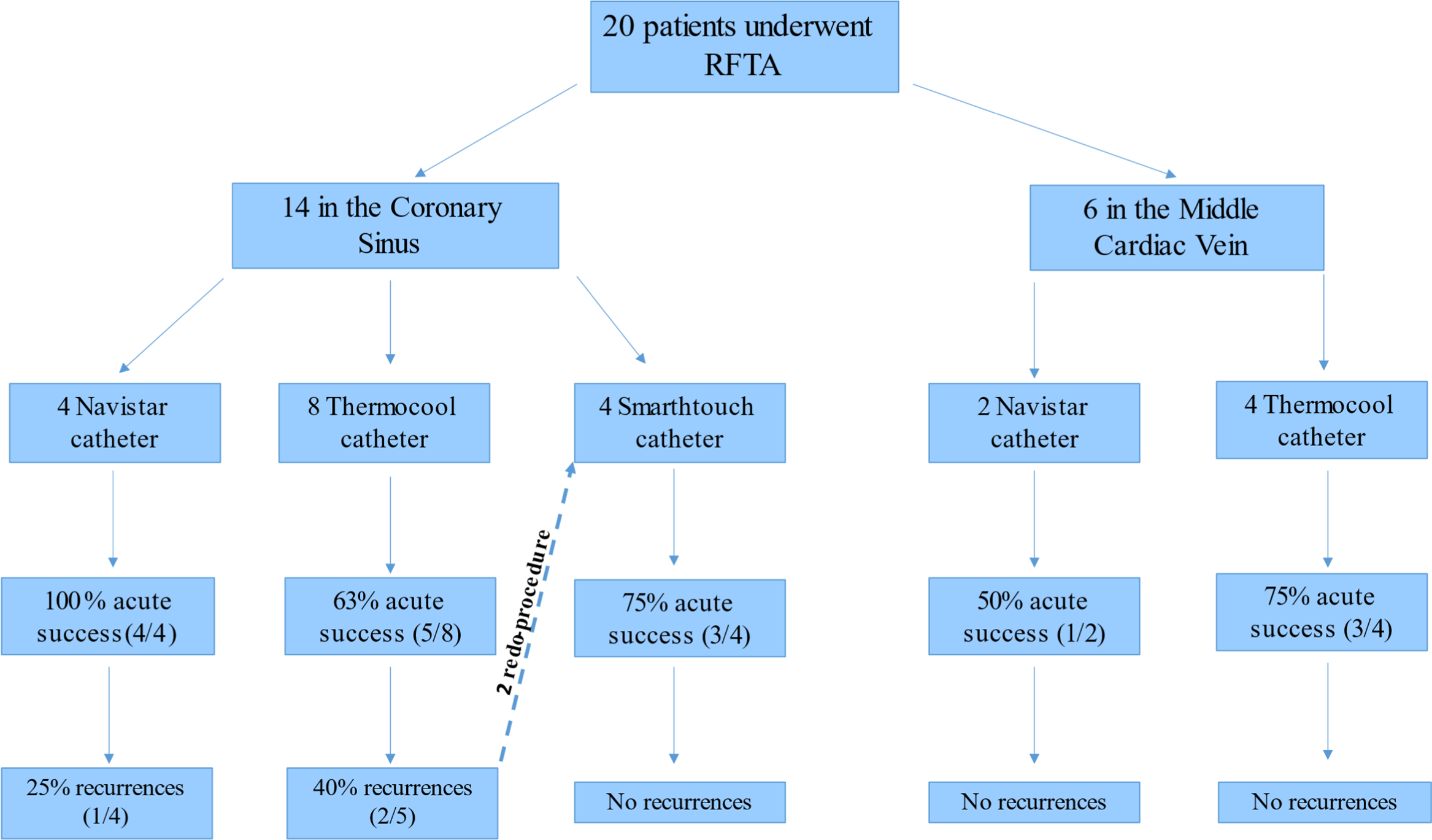
Figure 1. A flow chart including the ablation site, the catheter type, and the relative acute success and recurrence rate. RFTA = radiofrequency transcatheter ablation.
The mean contact force used with Navistar SmartTouch® catheter in the site of successful ablation was 15 ± 3 g (range 5–31). Interestingly, Navistar® catheter presented the highest acute success rate in the coronary sinus and the same long-term success rate of Navistar SmartTouch®. This latter, however, was the only catheter that did not present recurrences after the acute success. Moreover, Navistar SmartTouch® was successfully used in two patients previously unsuccessfully treated with a Navistar ThermoCool®.
Of note, no recurrences were observed in successful procedures performed in the middle cardiac vein.
At the end of the study, 13 out of 20 patients (65%) were successfully treated with radiofrequency transcatheter ablation.
All the remaining patients underwent antiarrhythmic therapy with flecainide that was combined with nadolol in 2. No tachyarrhythmias occurred in these patients during follow-up.
Image integration
Acute success rate was 79% (11 out of 14 procedures) in patients undergoing radiofrequency transcatheter ablation without image integration with angio-CT, while the overall long-term success rate was 64% (9 out of 14) due to 2 recurrences.
After the introduction of CARTO-Merge®, acute success rate was 63% (5 out of 8 procedures), while the overall long-term success rate was 50% (4 out of 8) due to 1 recurrence. However, among patients treated using CARTO-Merge®, there was a patient, with a recurrence after a previous radiofrequency transcatheter ablation without image integration, who underwent a successful procedure using CARTO-Merge® and Navistar ThermoCool® catheter. In this patient, the angio-CT showed the presence of a large coronary sinus diverticulum, located near the crux cordis in the inferior side of the coronary sinus (high-riding) (Fig. 2).

Figure 2. 3D transvenous radiofrequency ablation of a manifest epicardial posterior-septal accessory pathway in the neck of a very large coronary sinus diverticulum using a Navistar Smarttouch® catheter. ( a ) Activation map of the right atrium and the coronary sinus pre-ablation using Carto-UnivuTM together with Carto-Merge® module. ( b ) Ablation site (red dots) in the coronary sinus with disappearance of delta wave during radiofrequency delivery.
Discussion
Posterior-septal accessory pathways represent 20–25% of all accessory pathways detected in patients referred to an electrophysiological study. Reference Jackman, Wang and Friday10 Standard endocardial approach can result in ineffective treatment due to epicardial localisation of the accessory pathway (19% of the cases in a previous series). Reference Sun, Arruda and Otomo4
These epicardial pathways are a portion of the myocardial coat, normally surrounding coronary sinus, which can extend along its tributaries (more often the middle cardiac vein, but also the posterior cardiac vein or other branches) to the epicardial ventricular surface creating an electrical connection. Sometimes, this can occur in correspondence of a local venous dilatation or at the neck of a proper coronary sinus diverticulum.
Endocardial mapping performed in sinus rhythm identifies earliest ventricular signal about 1 cm below the A-V annulus with local activation not anticipating the surface delta wave, preceded by an earlier far field activation and with “r wave” at the unipolar mapping.
When the catheter is placed in the coronary sinus and its branches are mapped, it is possible to record the epicardial potential generated by the coronary sinus myocardial coat prolongation extending to the ventricle (corresponding to the far field seen endocardially), identifying the ideal site for ablation. However, ablation at these sites provides access to a limited portion of the epicardium and is not always associated with elimination of the accessory pathway often requiring an ablation at multiple sites (e.g. endo + epicardial) or using very high energy. Reference Derejko, Miszczak-Knecht, Sliwka, Dzwonkowska and Bieganowska11
Furthermore, the coronary sinus and its branches can be stenotic or occluded, as in already treated patients, or too small to be entered by ablation catheter, as in children. Furthermore, radiofrequency delivery in such a delicate site can be associated with risk of venous thrombosis/occlusion, wall damage with pericardial effusion, and coronary artery injury. These risks can be particularly high in very young patients, due to the small size of the cardiac veins and their greater proximity to the coronary artery branches.
Taking into account all these difficulties and risks, in our case series, we attempted the ablation in the coronary sinus and its branches after adequate growth of the patient, ideally when a weight of 40–45 kg was reached for patients identified at younger age. Moreover, more diagnostic catheters were used compared to our standard 3D non-fluoroscopic approach for left accessory pathways using CARTO-Univu®. Reference Drago, Grifoni and Remoli9
Regarding ECG characteristics of such epicardial accessory pathways, Arruda reported a negative delta wave in lead II as the typical ECG landmark of overt posteroseptal epicardial accessory pathways, Reference Arruda, Mcclelland and Wang12 although, for other authors, this finding is absent in more than 30% of cases. Reference Pascale, Hunziker and Denis13 Differently from these studies, we found most of our patients with negative delta wave in III and aVF and all with positive delta wave in lead I. Interestingly, about 70% of the patients presented in V1 a “rS” morphology of the QRS.
In this study, the overall acute success with radiofrequency was 73% and only two patients underwent a redo-procedure. Of note, all procedures were performed with an irrelevant use of fluoroscopy.
Comparing our acute success rate with those obtained in adult population, it is similar to that one not so recently reported by Payami et al. in a very small cohort of patients (75%) Reference Payami, Shafiee, Shahrzad, Kazemisaeed, Davoodi and Yaminisharif14 and lower than that one more recently reported by Stavrakis et al. (99% with non-irrigated and irrigated catheters). Reference Stavrakis, Jackman and Nakagawa6 In terms of chronic success rate, even though Leitz et al. seem to have a better result (87.5% with a mean of 2.1 procedures per patient) it was obtained with a higher number of procedures per patient (2.1 versus 1.3). Reference Leitz, Wasmer and Köbe15
Moreover, our results seem comparable with the only paper published in literature about transvenous ablation of epicardial accessory pathways in children Reference Alazard, Lacotte and Horvilleur5 (acute success 75% versus 80%, long-term success rate 65% versus 71%), even though Alazard et al. reported a larger cohort of patients (48) that were enrolled in a longer period. In addition, in contrast to our experience, Alazard et al. did not use contact force irrigated catheters. These latter allowed us to succeed in two patients with recurrences after procedures where an irrigated catheter was used. In this regard, contact force catheter could be an alternative to or the last chance before a questionable epicardial approach in children. Reference Upadhyay, Walsh, Cecchin, Triedman, Villafane and Saul16
The absence of recurrences was also noted in all ablations performed in the middle cardiac vein, irrespectively of catheter used. These data confirm that when anomalous myocardial fibres extending from the coronary sinus to the ventricle run along the middle cardiac vein, the placement of the catheter in this vein allows us to be close to the accessory pathway.
Moreover, our results seem to be better also than those reported by Collins et al. in a multicentre retrospective study evaluating the outcomes of cryoablation of these particular accessory pathways (APs) in children and in patients with CHD (acute success rate 71% and overall success rate 42.8%). Reference Collins, Rhee and Kirsh17 However, even if recurrence risk appears very high, the safety profile of cryoenergy makes the final success rate reasonable in these delicate arrhythmogenic substrates.
Finally, although the accuracy of direct dye-injections into the coronary sinus to evaluate individual anatomy and topographical relationships is indisputable, this case series suggests that image integration with cardiac-CT could be an alternative considering the value of the 3D anatomical reconstruction of this particular venous tree and its branches.
Limitations
This is a series of cases with a limited number of patients due to the rarity of the epicardial accessory pathways.
In this case series report, side effects of radiofrequency transcatheter ablation on the coronary arteries have been studied without the aid of coronary angiography and, in relation to patient numbers and study periods, there is too much variation in the use and incorporation of additional technical features and tools. The latter is due to the rarity of the arrhythmogenic substrate studied and to the rapid improvement of technology.
Conclusions
Epicardial posterior-septal accessory pathways, in the area of coronary sinus or middle cardiac vein, can be definitively eliminated by transvenous radiofrequency transcatheter ablation in more than half of the cases in children. Acute success rate does not seem to depend on the type of radiofrequency catheters used, but contact force catheter seems to be useful in case of recurrence.
Image integration with cardiac-CT does not increase success rate, but it can be an alternative to coronary sinus angiography to guide ablation.
Acknowledgements
The authors thank Elisa Del Vecchio for her valuable collaboration in the editorial revision.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional committees of Bambino Gesù Children’s Hospital IRCCS.







