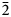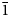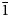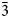Introduction
Eudialyte-group minerals (EGMs) are important components of some types of peralkaline rocks in which they are the main concentrators of zirconium. Currently, 32 mineral species belonging to the eudialyte group are known (https://www.mindat.org/). Their crystal structures are based on heteropolyhedral frameworks composed of nine- and three-membered rings of SiO4 tetrahedra, six-membered rings of edge-sharing M1O6 octahedra and isolated ZO6 octahedra. The simplified general formula of EGMs is [N1N2N3N4N5]3M16M23M3M4Z 3(Si9O27)2(Si3O9)2Ø4–6X1X2 where N1–5 are extra-framework cations (mainly, Na+ or H3O+, sometimes with species-defining H2O, K+, Ca2+, Sr2+, Mn2+ or REE 3+ as well as minor H+ and/or Ba2+); M1 = Ca2+, Na+, Fe2+, Mn2+ and REE 3+; M2 = Na+, Fe2+, Mn2+, Fe3+ or Zr, occurring between rings of M1O6 octahedra; M3 and M4 are [4]Si, [4]Nb, [4]W or [4]Ti atoms occurring at the centres of the Si9O27 rings; Z = Zr or Ti; Ø, X1 and X2 are additional extra-framework cations (OH–, Cl–, F–, O2–, CO32–, SO42– and S2–) and water molecules (Johnsen et al., Reference Johnsen, Ferraris, Gault, Grice, Kampf and Pekov2003; Rastsvetaeva et al., Reference Rastsvetaeva, Chukanov and Aksenov2012). All sites except those belonging to the rings of tetrahedral and octahedra can be partly vacant. Most EGMs are characterised by the space groups R $\bar{3}$![]() m or R3m, but in some species (members of the oneillite subgroup) different M1 cations are ordered in the M1(1) and M1(2) sites alternating in the six-membered rings of octahedra which results in lowering of symmetry from R $\bar{3}$
m or R3m, but in some species (members of the oneillite subgroup) different M1 cations are ordered in the M1(1) and M1(2) sites alternating in the six-membered rings of octahedra which results in lowering of symmetry from R $\bar{3}$![]() m or R3m to R3 (Johnsen et al., Reference Johnsen, Grice and Gault1999; Chukanov et al., Reference Chukanov, Pekov, Zadov, Korovushkin, Ekimenkova and Rastsvetaeva2003, Reference Chukanov, Aksenov, Pekov, Belakovskiy, Vozchikova and Britvin2020, Reference Chukanov, Vigasina, Rastsvetaeva, Aksenov, Mikhailova and Pekov2022, Reference Chukanov, Aksenov, Kazheva, Pekov, Varlamov, Vigasina, Belakovskiy, Vozchikova and Britvin2023; Khomyakov et al., Reference Khomyakov, Nechelyustov and Rastsvetaeva2007, Reference Khomyakov, Nechelyustov and Rastsvetaeva2009).
m or R3m to R3 (Johnsen et al., Reference Johnsen, Grice and Gault1999; Chukanov et al., Reference Chukanov, Pekov, Zadov, Korovushkin, Ekimenkova and Rastsvetaeva2003, Reference Chukanov, Aksenov, Pekov, Belakovskiy, Vozchikova and Britvin2020, Reference Chukanov, Vigasina, Rastsvetaeva, Aksenov, Mikhailova and Pekov2022, Reference Chukanov, Aksenov, Kazheva, Pekov, Varlamov, Vigasina, Belakovskiy, Vozchikova and Britvin2023; Khomyakov et al., Reference Khomyakov, Nechelyustov and Rastsvetaeva2007, Reference Khomyakov, Nechelyustov and Rastsvetaeva2009).
This paper describes a new oneillite-subgroup member amableite-(Ce), with Na+ + REE 3+ and Mn2+ ordered in the M1(1) and M1(2) sites and Mn2+-dominant M2 site. Amableite-(Ce) is named after its discovery locality, Saint-Amable Sill. The mineral and its name (with symbol Ambl-Ce) have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2023–075, Chukanov et al., Reference Chukanov, Zolotarev, Schäfer, Varlamov, Pekov, Vigasina, Belakovskiy, Aksenov, Vozchikova and Britvin2024). The holotype specimen is deposited in the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, Russia with the registration number 6921/1.
Experimental methods and data processing
In order to obtain infrared (IR) absorption spectra of amableite-(Ce) and the related eudialyte-group mineral voronkovite (Khomyakov et al., Reference Khomyakov, Nechelyustov and Rastsvetaeva2009), powdered samples were mixed with anhydrous KBr, pelletised, and analysed using an ALPHA FTIR spectrometer (Bruker Optics) at a resolution of 4 cm–1. A total of 16 scans were collected. The IR spectrum of an analogous pellet of pure KBr was used as a reference. The assignment of IR bands was made based on the analysis of IR spectra of several tens of structurally investigated eudialyte-group minerals, in accordance with Rastsvetaeva et al. (Reference Rastsvetaeva, Chukanov and Aksenov2012).
Raman spectra of randomly oriented grains of amableite-(Ce) and other eudialyte-group minerals used for comparison were obtained using an EnSpectr R532 spectrometer based on an OLYMPUS CX 41 microscope coupled with a diode laser (λ = 532 nm) at room temperature (Moscow State University, Faculty of Geology). The spectra were recorded in the range from 100 to 4000 cm–1 with a diffraction grating (1800 gr mm–1) and spectral resolution of ~6 cm–1. The output power of the laser beam was in the range from 5 to 13 mW. The diameter of the focal spot on the sample was 5–10 μm. The back-scattered Raman signal was collected with a 40× objective; signal acquisition time for a single scan of the spectral range was 1 s, and the signal was averaged over 50 scans. Crystalline silicon was used as a standard.
Compositional data (5 spot analyses) were obtained using a digital scanning electron microscope Tescan VEGA-II XMU equipped with an energy-dispersive spectrometer (EDS) INCA Energy 450 with semiconducting Si (Li) detector Link INCA Energy at an accelerating voltage of 20 kV, electron current of 190 pA and electron beam diameter of 160–180 nm. Attempts to use WDS mode, with a higher beam current, were unsuccessful because of the instability of the mineral under the electron beam due to partial dehydration and migration of Na. This phenomenon is typical for high-hydrous sodium minerals with microporous structures. A good agreement was observed between compositional data obtained under these standard conditions and those obtained under more ‘mild’ conditions (with a current lowered to 90–100 pA and electron beam defocused to an area of 30 × 30 μm). The L-lines of Ta are not observed in the spectrum, which indicates the absence of detectable amounts of tantalum in amableite-(Ce).
The H2O content was determined by means of a modified Penfield method. The CO2 content was not determined because characteristic bands of carbonate groups (in the range of 1350–1550 cm–1) are not observed in the IR spectrum of amableite-(Ce).
Powder X-ray diffraction (XRD) data were collected using a Rigaku R-AXIS Rapid II diffractometer (image plate), CoKα, 40 kV, 15 mA, rotating anode with the microfocus optics, Debye-Scherrer geometry, d = 127.4 mm and exposure time of 15 min. The raw powder XRD data were collected using the program suite designed by Britvin et al. (Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017). Calculated intensities were obtained by means of STOE WinXPOW v. 2.08 program suite based on the atomic coordinates and unit-cell parameters (Stoe, 2003).
Single-crystal X-ray diffraction studies were carried out using a Rigaku XtaLAB Synergy-S diffractometer (MoKα radiation) with high-stability sharp-focus X-ray source PhotonJet-S and a high-speed direct-action detector HyPix-6000HE. CrysAlisPro software was used for further processing (CrysAlisPro, 2015). An absorption correction was introduced using the SCALE3 ABSPACK algorithm. The obtained data were loaded into the Olex2 program software (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2009) and the crystal structure was solved and refined by the ShelX program package (Sheldrick, Reference Sheldrick2015). The crystal data, experimental details of the data collection and refinement results are shown in Table 1.
Table 1. Crystal data, data collection information and structure refinement details for amableite-(Ce).

Results
Occurrence, general appearance and physical properties
The material with amableite-(Ce) was collected in the Demix-Varennes quarry, Saint-Amable Sill (45° 40′1″N, 73°20′35″W), Lajemmerais RCM, Montérégie, Québec, Canada (see: Horváth et al., Reference Horváth, Pfenninger Horváthm, Gault and Tarasoff1998). Amableite-(Ce) crystallised from a peralkaline post-magmatic fluid. It forms yellow thick-tabular, slightly flattened on (0001) crystals up to 2 mm across in cavities of peralkaline pegmatite (Fig. 1). The dominant crystal form is {0001}; the subordinate forms are {11$\bar{2}$![]() 0}, {10$\bar{1}$
0}, {10$\bar{1}$![]() 1} and {10$\bar{1}$
1} and {10$\bar{1}$![]() 0}. The associated minerals are albite, microcline, aegirine, serandite, natrolite, yofortierite, and an unidentified titanosilicate forming minute grains.
0}. The associated minerals are albite, microcline, aegirine, serandite, natrolite, yofortierite, and an unidentified titanosilicate forming minute grains.

Figure 1. Amableite-(Ce) crystals (yellow) in association with albite, aegirine and natrolite. Field of view width is 3.5 mm. Sample 6921/1 from the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, Russia. Photographer: V. Heck.
Amableite-(Ce) is brittle, with a Mohs hardness of 5. No cleavage is observed. The fracture is uneven. Density measured by flotation in heavy liquids (mixtures of methylene iodide and heptane) is equal to 2.89(1) g⋅cm–3. Density calculated using the empirical formula and unit-cell volume refined from single-crystal XRD data is 2.90 g⋅cm–3.
The new mineral is optically anomalously biaxial (+) with α ≈ β = 1.603(2) and γ = 1.608(2) (λ = 589 nm). Different causes of optical anomalies (microstrains, compositional inhomogeneity, including different degrees of dehydration in different zones or sectors) are discussed in detail by Shtukenberg and Punin (Reference Shtukenberg and Punin2007). A possible cause of optical anomalies may be deformation of the crystals during their growth or when they were being crushed. In particular, anomalous biaxiality is very typical for eudialyte-group minerals despite that all studied members of this group are trigonal (Rastsvetaeva et al., Reference Rastsvetaeva, Chukanov and Aksenov2012). Under the microscope, amableite-(Ce) is colourless and nonpleochroic. Dispersion is distinct with r > v.
Infrared spectroscopy
Absorption bands in the IR spectrum of amableite-(Ce) (curve a in Fig. 2) and their assignments are (cm–1, s – strong band, w – weak band, sh – shoulder): 3520, 3335, 2880w (O–H stretching vibrations); 1635w (H–O–H bending vibrations); 1170sh, 1010s, 991s (Si–O stretching vibrations of silicate rings); 935s (Si–O stretching vibrations of SiO4 tetrahedra at the M3 and M4 sites); 739 (mixed vibrations of rings of SiO4 tetrahedra – ‘ring band’); 700sh, 657 (mixed vibrations of rings of SiO4 tetrahedra combined with Nb–O stretching vibrations); 519sh (IVMn2+–O and/or VFe3+–O stretching vibrations); 481s, 446s (lattice mode involving predominantly bending vibrations of rings of SiO4 tetrahedra); and 390sh, 373 (lattice modes involving VI(Ca;Mn2+)–O stretching vibrations).

Figure 2. Powder infrared absorption spectra of (a) amableite-(Ce) and (b) holotype sample of voronkovite (Khomyakov et al., Reference Khomyakov, Nechelyustov and Rastsvetaeva2009), an oneillite-type EGM related to amableite-(Ce). The spectra are offset for comparison.
The band of IVFe2+–O stretching vibrations (in the range 539–545 cm–1) was not observed. The IR spectrum of amableite-(Ce) differs from that of the related eudialyte-group mineral voronkovite, ideally Na15[(Na,Са)3Mn3]Fe2+3Zr3Si2(Si24O72)(OH,O)4Cl⋅H2O (curve b in Fig. 2), with a lower intensity of the band at 933–935 cm–1, which reflects significant amounts of vacancies and Nb at the M3 and M4 sites, in accordance with the structural data (see below).
According to the correlation by Libowitzky (Reference Libowitzky1999), the band of O–H stretching vibrations with the maximum at 3335 cm–1 corresponds to a hydrogen bond with the O⋅⋅⋅O distance of 2.72 Å. Thus, this band should be assigned to the hydrogen bond O24⋅⋅⋅H–O26 with the O⋅⋅⋅O distance of 2.72(3) Å. The band at 3520 cm–1 may correspond to silanol groups formed as a result of protonation of pending vertices of the SiO4 tetrahedra, but this assignment is ambiguous because H atoms were not localised.
Raman spectroscopy
A specific feature of Raman spectra of the EGMs selsurtite, ideally (H3O)12Na3(Ca3Mn3)(Na2Fe)Zr3□Si[Si24O69(OH)3](OH)Cl⋅H2O (Chukanov et al., Reference Chukanov, Aksenov, Kazheva, Pekov, Varlamov, Vigasina, Belakovskiy, Vozchikova and Britvin2023), aqualite, ideally (H3O)9(K,Ba,Sr)2Ca6Zr3Na2Si2[Si24O66(OH)6](OH)3Cl⋅H2O (Khomyakov et al., Reference Khomyakov, Nechelyustov and Rastsvetaeva2007) and other hydronium-bearing EGMs is a series of bands in the range of 1200–2900 cm–1 corresponding to strong hydrogen bonds formed by hydronium cations in different local situations including Zundel- and Eigen-like bonds with short O⋅⋅⋅O distances of ~2.4 and ~2.6 Å (Chukanov et al., Reference Chukanov, Vigasina, Rastsvetaeva, Aksenov, Mikhailova and Pekov2022, Reference Chukanov, Aksenov, Kazheva, Pekov, Varlamov, Vigasina, Belakovskiy, Vozchikova and Britvin2023; see Discussion section for details). Similar but much weaker bands are observed in the Raman spectrum of amableite-(Ce), but such bands are absent in the Raman spectrum of eudialyte, which does not contain hydrated proton complexes including (H3O)+ cation groups (Fig. 3). Most probably, hydrated protons could form as a result of partial dissociation of silanol (SiOH) groups.

Figure 3. Raman spectra of (a) selsurtite, (b) amableite-(Ce) and (c) eudialyte.
The assignment of other bands in the Raman spectrum of amableite-(Ce) is as follows: 3430 cm–1 to O–H stretching vibrations of H2O molecules and/or OH groups; the range 900–1300 cm–1 to Si–O stretching modes; 780 cm–1 to mixed vibrations of rings of SiO4 tetrahedra; 653 and 689 cm–1 to mixed vibrations of rings of SiO4 tetrahedra combined with Nb–O stretching vibrations; 573 cm–1 to Zr–O stretching vibrations and bands ≤ 400 cm–1 are assigned to lattice modes.
Chemical data
Analytical data are given in Table 2. Contents of other elements with atomic numbers >8 are below detection limits. Based on IR spectroscopy data, all iron is considered as Fe3+. Note that Fe3+ and Fe2+ cations at the M2 site of eudialyte-group minerals are yellow and red (to brownish-red in the case of five-fold coordination) chromophores, respectively (Pol'shin et al., Reference Pol'shin, Platonov, Borutsky, Taran and Rastsvetaeva1991). Thus, the yellow colour of amableite-(Ce) indicates the absence of Fe2+ at the M2 site. The trivalent state of iron is also in agreement with a superior Gladstone–Dale compatibility index CI = 1–(K p/K c) = –0.014 (Mandarino, Reference Mandarino1981). In comparison, with Fe2+, the CI would be –0.026.
Table 2. Chemical composition of amableite-(Ce).

*Bivalent state of Mn and trivalent state of Fe are in agreement with the IR spectrum (see above) and structural data. In addition, Mn3+ is a very strong purple chromophore whereas amableite-(Ce) is yellow.
**The content of H2O was determined by means of a modified Penfield method.
The bulk empirical formula of amableite-(Ce) is H5.76Na14.00K0.27Ca1.03Ce0.78La0.58Nd0.11Pr0.03Mn3.55Fe0.92Ti0.26Zr2.77Hf0.035Nb0.35Si25.12Cl0.22O75.36. Based on the structural data (see below), it can be written as: (Na12.93K0.27Ce0.06)Σ13.26[(Mn2.49Ce0.30Ca0.21)Σ3.00(Ce1.14Na1.04Ca0.82)Σ3.00](Mn1.05Fe0.90□1.05)Σ3.00(Zr2.85Ti0.12Hf0.03)Σ3.00(□0.40Nb0.36Si0.24)Σ1.00(Si0.88□0.12)Σ1.00[Si24(O70.44(OH)1.56)Σ72.00][(OH)2.20(H2O)1.27]Σ3.47Cl0.22.
The simplified formula is (Na,H,K,Ce)15[(Ce,Na,Ca)3(Mn,Ce,Ca)3](Mn,Fe,□)3(Zr,Ti)3(□,Nb,Si)(Si,□)Si24(O,OH)72](OH,H2O,O)3, and the ideal formula is Na15[(Ce1.5Na1.5)Mn3]Mn2Zr3□Si[Si24O69(OH)3](OH)2⋅H2O.
X-ray diffraction and crystal structure
Powder X-ray diffraction data for amableite-(Ce) are given in Table 3. The unit-cell parameters refined from the powder data are: a = 14.140(3) Å, c = 30.37(1) Å and V = 5258(4) Å3. The crystal structure of amableite-(Ce) (Figs 4 and 5) was refined on the basis of 6462 independent reflections with I > 2σ(I). The final refinement cycles converged to R 1 = 4.23%. The atomic coordinates occupancies, site-scattering values and equivalent isotropic parameters are given in Table 4. The anisotropic displacement parameters and selected bond lengths are given in Tables 5 and 6, respectively. See Discussion section for further details. The crystallographic information file (cif) has been deposited via the joint Cambridge Crystallographic Data Centre CCDC/FIZ Karlsruhe deposition service https://www.ccdc.cam.ac.uk/structures/ (the deposition number is CSD 2325823) and are also available as Supplementary Material (see below).
Table 3. Powder X-ray diffraction data (d in Å) of amableite-(Ce).

*For the calculated pattern, only reflections with intensities ≥1 are given. **For the unit-cell parameters calculated from single-crystal data. The strongest lines are given in bold.

Figure 4. The crystal structure of amableite-(Ce): general view. Drawn with the DIAMOND program (Brandenburg and Putz, Reference Brandenburg and Putz2005).

Figure 5. A local arrangement involving 6-membered rings of octahedra in the amableite-(Ce) structure. Drawn with the DIAMOND program (Brandenburg and Putz, Reference Brandenburg and Putz2005).
Table 4. Atomic coordinates, occupancies, site-scattering values and equivalent isotropic displacement parameters (Å2) for amableite-(Ce).

*s.s. – site scattering; calc. s.s. – calculated site scattering (electrons per formula unit). ** Fixed parameters.
Table 5. Anisotropic displacement parameters (Å2) for amableite-(Ce).

Table 6. Selected bond lengths (d in Å) in amableite-(Ce).

Discussion
Crystal structure
Amableite-(Ce) is isostructural with the other 12-layered members of the oneillite subgroup of the eudialyte group with the space group R3. Based on the refined site-scattering factors, the crystal chemical formula of amableite-(Ce) can be written as follows (Z = 3):
N 1–5(Na12.93H+1.74K0.27Ce0.06)Σ15 ZrZ(Zr2.85Ti0.12Hf0.03)Σ3
M 1(1)(Mn2.49Ce0.30Ca0.21)Σ3 M 1(2)(Ce1.14Na1.04Ca0.82)Σ3
M 2(Mn1.05Fe0.90□1.05)Σ3 M 3(□0.40Nb0.36Si0.24)Σ1 M 4(Si0.88□0.12)Σ1
[Si1,2,5–10Si24O70.44(OH)1.56] Ø,O25–30[(OH)1.78O1.08Cl0.22(H2O)0.61]
where [Si1,2,5–10Si24O70.44(OH)1.56] are rings of tetrahedra.
The following combination of structural features of amableite-(Ce) distinguishes it from other eudialyte-group minerals:
(1) Cation ordering within the six-membered ring of octahedra resulting in a lowering of symmetry (Fig. 5). The six-membered ring is formed by M1(1)O6- and M1(2)O6-octahedra with different occupancies. The M1(1)O6-octahedron is predominantly occupied by manganese (2.49 atoms per formula unit), whereas the M1(2)O6-octahedron is predominantly occupied by Na and REE, with subordinate Ca.
(2) The predominance of Mn2+ at the M2 site.
(3) The predominance of vacancies over NbO6 octahedra and SiO3(OH) tetrahedra at the M3 site.
(4) The predominance of Si at the M4 site.
Comparative data for amableite-(Ce) and related eudialyte-group minerals are given in Table 7.
Table 7. Comparative data for amableite-(Ce) and related eudialyte-group minerals with R3 symmetry.

Raman spectroscopy of hydronium and hydrated proton complexes
Numerous ab initio quantum-chemical calculations of hydronium and other hydrated proton complexes, including Zundel (H5O2+) and Eigen (H3O+⋅3H2O) cations have shown that these clusters are characterised by variable configurations and strong hydrogen bonds with the O⋅⋅⋅O distances in the range of 2.38–2.8 Å (Vyas, Reference Vyas, Sakore and Biswas1978; Komatsuzaki and Ohmine, Reference Komatsuzaki and Ohmine1994; Corongiu, Reference Corongiu, Kelterbaum and Kochanski1995; Kim et al., Reference Kim, Schmitt, Gruetzmacher, Voth and Scherer2002; Sobolewski and Domcke, Reference Sobolewski and Domcke2002a, Reference Sobolewski and Domcke2002b; Asmis et al., Reference Asmis, Pivonka, Santambrogio, Brümmer, Kaposta, Neumark and Wöste2003; Christie, Reference Christie2004; Headrick et al., Reference Headrick, Bopp and Johnson2004; Laria et al., Reference Laria, Martí and Guàrdia2004; Asthagiri et al., Reference Asthagiri, Pratt and Kress2005; Ortega et al, Reference Ortega, Escribano, Herrero, Maté and Moreno2005; Paddison and Elliott, Reference Paddison and Elliott2005; Vener and Librovich, Reference Vener and Librovich2009; Biswas et al., Reference Biswas, Carpenter, Fournier, Voth and Tokmakoff2017; Carpenter, Reference Carpenter2020). Calculated wavenumbers of vibrational modes corresponding to the hydrated proton complexes are in the range of 1070–3000 cm–1.
There is a negative correlation between the frequency of O–H stretching vibrations and O⋅⋅⋅O distance between the O atom of OH group and the O atom – acceptor of the hydrogen bond (McClellan and Pimentel, Reference McClellan and Pimentel1960; see Fig. 6). This correlation is nearly linear in the range of the O⋅⋅⋅O distances from 2.4 to 2.8 Å and significantly deviates from the linearity for weaker hydrogen bonds.

Figure 6. The correlations between wavenumbers of O–H stretching vibrations and O⋅⋅⋅O distances for hydrogen bonds in crystals drawn using data by McClellan and Pimentel (Reference McClellan and Pimentel1960) (curve a), Libowitzky (Reference Libowitzky1999) (curve b), and Novak (Reference Novak1974) (squares).
The following empirical correlations between O–H stretching frequencies in IR spectra of minerals and O⋅⋅⋅O and H⋅⋅⋅O distances (obtained from structural data) were established by E. Libowitzky (Reference Libowitzky1999):
A similar correlation was obtained by Novak (Reference Novak1974).
In reality, equations 1 and 2 are a very rough approximation and have restricted applicability. In particular, above 3500 cm–1 substantial deviations from correlations 1 and 2 are common because O–H stretching frequencies depend not only on O⋅⋅⋅O and H⋅⋅⋅O distances, but also on the nature of cations coordinating O–H groups and H2O molecules, as well as on the O–H⋅⋅⋅O angle, and the influence of these factors becomes most evident in the case of weak hydrogen bonds. The equations 1 and 2 predict that maximum possible values of O–H stretching frequencies for minerals are 3592 and 3632 cm–1, respectively, but in many minerals including for magnesium serpentines, brucite, kaolinite, amphiboles etc. observed frequencies are much higher and can exceed 3700 cm–1. However, these correlations can be used for semiquantitative estimations, at least for relatively strong hydrogen bonds.
According to the equation (1), the Raman bands of amableite-(Ce) observed at 1815, 2433 and 2807 cm–1 correspond to the O⋅⋅⋅O distances of 2.51, 2.56 and 2.61 Å, respectively which is quite close to the distances of 2.50, 2.58 and 2.59 Å for O7⋅⋅⋅O30, O7⋅⋅⋅O27 and O24⋅⋅⋅O29 in strong hydrogen bonds O–H⋅⋅⋅O in amableite-(Ce).
Implications
Minerals belonging to the eudialyte group are considered as potential sources of REE, Zr, Hf, Nb and Ta for industrial use (Lebedev, Reference Lebedev2003; Lebedev et al., Reference Lebedev, Shchur, Maiorov, Popova and Serkova2003; Zakharov et al., Reference Zakharov, Maiorov, Alishkin and Matveev2011; Friedrich et al., Reference Friedrich, Hanebuth, Kruse, Tremel and Vossenkaul2016; Davis et al., Reference Davis, Stopic, Balomenos, Panias, Paspaliaris and Friedrich2017; Ma et al., Reference Ma, Stopic, Huang and Freidrich2019). Almost all the samples of EGMs studied contain detectable amounts of rare-earth elements (Rastsvetaeva et al., Reference Rastsvetaeva, Chukanov and Aksenov2012). Samples of EGMs from peralkaline pegmatites are characterised by the highest contents of these elements (typically, from 2 to 8 wt.% of REE 2O3). Amableite-(Ce) is the third EGM after zirsilite-(Ce), (Na,□)12(Ce,Na)3Ca6Mn3Zr3Nb(Si25O73)(OH)3(CO3)⋅H2O (Khomyakov et al., Reference Khomyakov, Dusmatov, Ferraris, Gula, Ivaldi and Nechelyustov2003) and johnsenite-(Ce), Na12(Ce,La,Sr,Ca)3Ca6Mn3Zr3W(Si25O73)(OH,Cl)2(CO3) (Grice and Gault, Reference Grice and Gault2006) containing REE as a species-defining component.
As a rule, REE-rich EGMs are enriched in Mn whereas REE-poor EGMs are enriched in Fe. This regularity may have a crystal-chemical origin and correspond to the situations where relatively small (Fe- and Ca-centred) or larger (Mn2+ and REE-centred) M2- and M1-octahedra share common edges. However, geochemical factors may also play a significant role.
Acknowledgements
The authors are grateful to Dr. Henrik Friis and anonymous reviewers for useful comments. A part of this work, including chemical analyses, infrared spectroscopy, interpretation of the Raman spectra and identification of associated minerals was carried-out in accordance with the state task of Russian Federation, registration number 124013100858-3. The authors thank the X-ray Diffraction Centre of Saint-Petersburg State University for instrumental and computational resources. Structure determination was done at the Center for X-Ray Diffraction Studies of the Research Park of St. Petersburg State University within the project AAAA-A19-119091190094-6.
Conflict of interest
The authors declare no conflict of interest.
Supplementary material
Crystallographic information files have been deposited at https://www.ccdc.cam.ac.uk/structures/ (the deposition number is CSD 2325823). In addition they can be found with this article at https://doi.org/10.1180/mgm.2024.26