Hyperprolactinaemia, a commonly encountered adverse effect of antipsychotic medication, used to be regarded as an inevitable consequence of the treatment and ignored (Reference Bostwick, Guthrie and EllingrodBostwick 2009). However, it has now been established that hyperprolactinaemia can be avoided with certain newer antipsychotics, such as aripiprazole, olanzapine, quetiapine, asenapine and clozapine. In addition, increasing knowledge about the long-term adverse consequences of hyperprolactinaemia has led to greater efforts in improving the management of antipsychotic-induced hyperprolactinaemia.
Over the past decade, a growing evidence base has improved our clinical understanding of managing the disorder. It has been increasingly recognised in various guidelines (Reference BarnesBarnes 2011; NICE 2014a,b), but most of these do not give any specific guidance about managing its adverse effects. In this article, we will present an overview of the management of antipsychotic-related hyperprolactinaemia based on current evidence.
Physiology of prolactin
Prolactin is an anterior pituitary hormone, which is also secreted by extra-pituitary sources such as the hypothalamus, brain stem, spinal cord, mammary glands, some immune cells and circumventricular organs (Reference Peuskens, Pani and DetrauxPeuskens 2014). However, the physiological significance of extra-pituitary prolactin is yet to be determined.
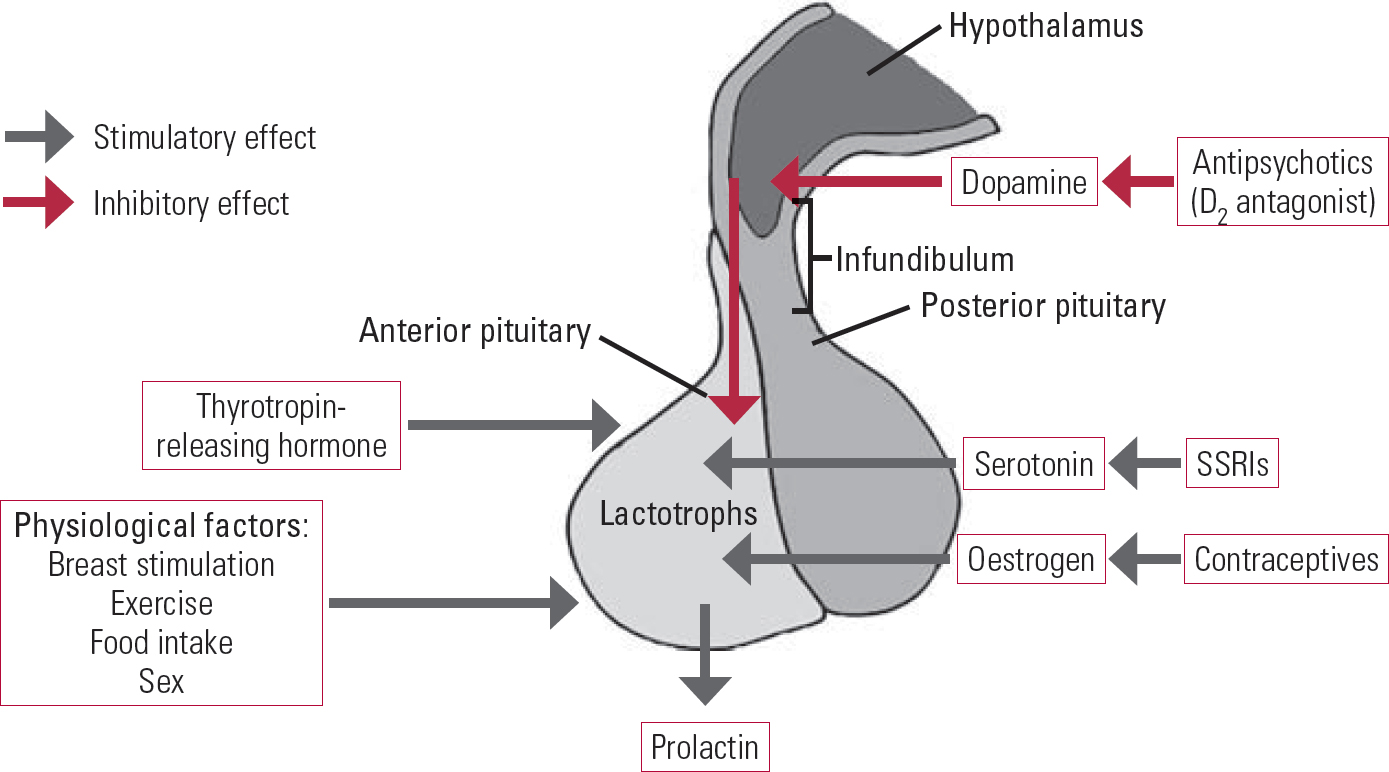
Prolactin plays an important role in breast development and lactation in women, but it has many other biological functions related to osmo-regulation, angiogenesis and immune regulation (Reference Freeman, Kanyicska and LerantFreeman 2000). The hormone is under the inhibitory control of dopamine, which is produced in the tuberoinfundibular neurons of the hypothalamus. Dopamine is transported by the hypophyseal portal circulatory system to the pituitary gland, where it binds to and stimulates D2 receptors on lactotroph cells. D2-receptor stimulation inhibits prolactin gene transcription, the synthesis and release of prolactin, and lactotroph proliferation. Other factors also influence prolactin secretion: serotonin, thyrotropin-releasing hormone (TRH) and oestrogen have stimulatory effects; and gamma-aminobutyric acid (GABA) has an inhibitory effect (Fig. 1).
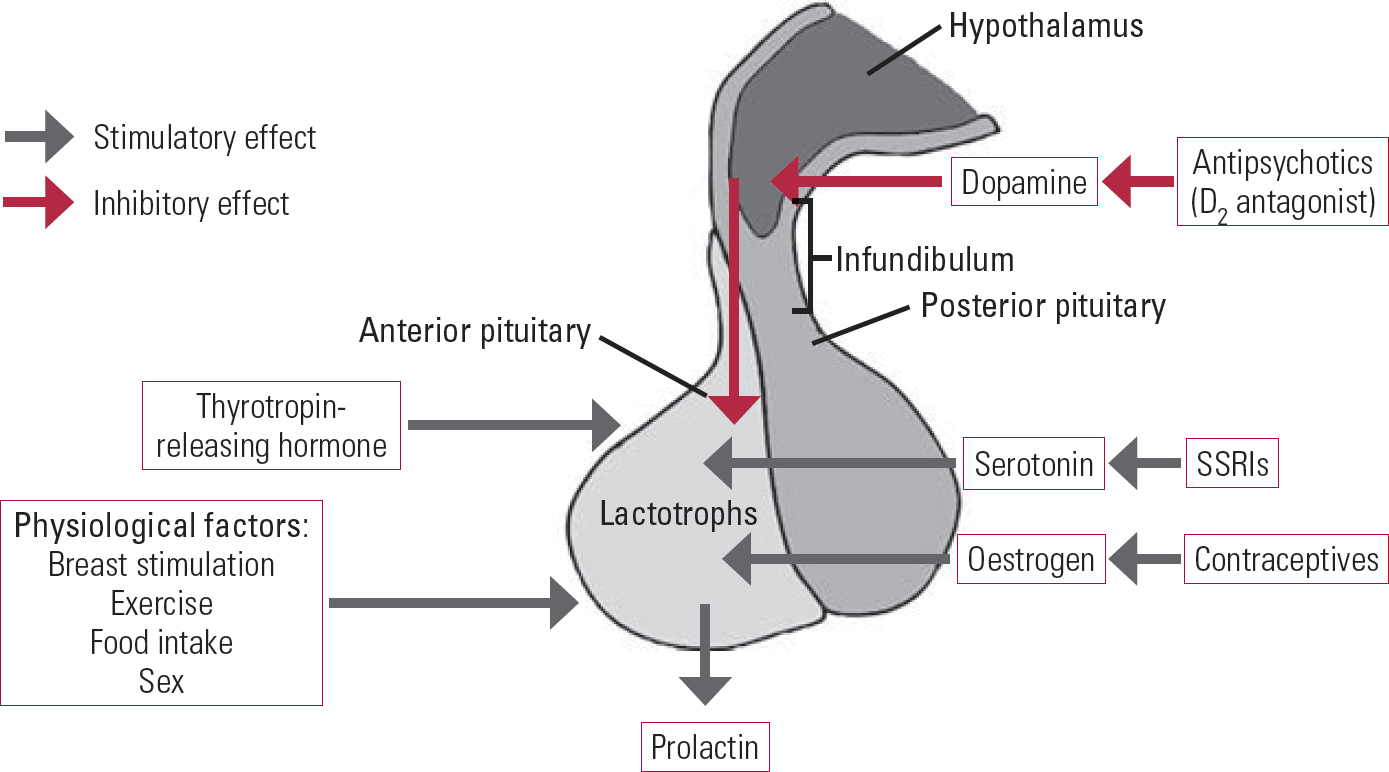
FIG 1 Factors that affect prolactin hormones.
Hypothyroidism can increase the levels of prolactin by raising TRH levels. Drugs that enhance serotonin levels, such as selective serotonin reuptake inhibitors (SSRIs), can also increase prolactin levels. Conversely, drugs that stimulate GABA, such as benzodiazepine and sodium valproate, can reduce serum prolactin levels. Oestrogen also increases prolactin release and is reported to sensitise women to the prolactinelevating properties of antipsychotic medications (Reference Peuskens, Pani and DetrauxPeuskens 2014). High prolactin levels inhibit the hypothalamic secretion of gonadotropin-releasing hormone (GnRH), thereby decreasing the secretion of both luteinising hormone and follicle-stimulating hormone by the pituitary and resulting in low gonadal hormone levels (oestrogen in women and testosterone in men). Reduced gonadal hormone levels lead to infertility, sexual problems and osteoporosis.
Prolactin is released in a pulsatile manner: its level reaches a peak at night and a nadir in the afternoon. The prolactin level temporarily increases soon after food intake, exercise and sex, and after stressful events, including venepuncture. Prolactin levels also increase after epileptic seizures and after electroconvulsive therapy. Furthermore, the rate of prolactin clearance by the kidneys influences its concentration in the blood.
During pregnancy, the prolactin level starts increasing after 2 months of gestation and it can increase to 20–30 times the normal value. It remains high during the lactation period and normalises 3 weeks after discontinuation of breastfeeding.
The normal serum prolactin concentration varies between men and women, and there are significant inter-individual differences. In pre-menopausal women, prolactin levels also vary over the menstrual cycle, with higher levels during the second half of the cycle. Prolactin levels are higher in premenopausal women than in postmenopausal women. Otherwise, there are no clinically significant changes in baseline prolactin with ageing.
Prolactin exists in various forms: the monomeric biologically active form (23 kDa in mass) and a higher molecular weight form (>100 kDa) known as macroprolactin, which is usually bound to immunoglobulin G. Macroprolactin is largely inactive. Macroprolactinaemia should be suspected in asymptomatic patients with high serum prolactin levels, because their hyperprolactinaemia is probably due to a delayed clearance of macroprolactin. This condition is also called ‘pseudo-hyperprolactinaemia’. About 10–25% of patients with hyperprolactinaemia also have positive findings for macroprolactin (Reference Shimatsu and HattoriShimatsu 2012). Most laboratories report on macroprolactinaemia along with serum prolactin levels.
Hyperprolactinaemia
Hyperprolactinaemia is defined as a sustained increase in the prolactin level, above the normal range. To circumvent the problems associated with physiological variations in the prolactin level, hyperprolactinaemia is usually defined as a fasting level (at least 2 h after waking) of >20 ng/mL (>424 mIU/L) in men and >25 ng/mL (>530 mIU/L) in women (Reference Peveler, Branford and CitromePeveler 2008), but values for the normal range vary between laboratories owing to the different assays used.
Short-term effects of hyperprolactinaemia
Women are more likely to suffer the symptoms of hyperprolactinaemia and commonly present with oligomenorrhoea, amenorrhoea, loss of libido, infertility, gynaecomastia and galactorrhoea. In men, the usual symptoms are decreased libido, impotence, infertility (hypospermatogenesis) and, less commonly, gynaecomastia.
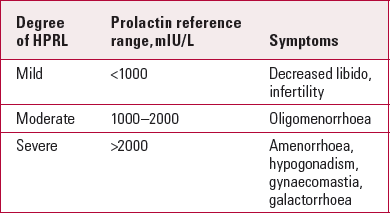
Table 1 illustrates the symptoms associated with hyperprolactinaemia, although there are significant inter-individual variations with regard to its signs and symptoms. Even a mild increase in serum prolactin levels can be associated with severe symptoms, and vice versa.
TABLE 1 Symptoms associated with hyperprolactinaemia (HPRL)
Long-term effects of hyperprolactinaemia
Hyperprolactinaemia has many long-term consequences, the most clinically significant of which are osteoporosis and, possibly, breast cancer. Therefore, special consideration should be given to the possibility of the development of these morbidities in clinically asymptomatic patients.
Osteoporosis
Osteoporosis is defined as a loss of bone mass and strength that leads to fragility fractures. Schizophrenia and other chronic mental illnesses are known to be associated with low bone mineral density (Reference De Hert, Correll and BobesDe Hert 2011). Schizophrenia and bipolar disorder are associated with higher prevalence of osteoporosis and, in fact, schizophrenia has been recognised as a risk factor for osteoporosis (Reference De Hert, Correll and BobesDe Hert 2011; Reference Correll, Detraux and De LepeleireCorrell 2015). However, many other factors can cause osteoporosis, including ageing, a poor diet, decreased mobility and weight-bearing activities, smoking, excessive alcohol intake, and reduced exposure to sunlight. Owing to these confounding factors, there have been contradictory reports of an association between raised prolactin levels and osteoporosis (Reference Peuskens, Pani and DetrauxPeuskens 2014).
Prolactin inhibition of GnRH suppresses production of the gonadal hormones oestrogen and testosterone, which play important roles in preserving bone mass. Prolactin elevation is more detrimental to men and women who have not reached their peak bone mass (i.e. are under 25 years of age). Decreased bone marrow density occurs in women with hyperprolactinaemia and amenorrhoea, but not in women with hyperprolactinaemia who have normal gonadal function (Reference Klibanski, Biller and RosenthalKlibanski 1999). There are some suggestions that prolactin might also directly affect bone turnover by stimulating bone resorption relative to bone formation (Reference Wu, Deng and ZhaoWu 2013). A reduction in bone mass (associated with osteopenia and osteoporosis) results in reduced bone strength and increased bone fragility, thus increasing the risk of fracture, particularly of the spine, hip and wrist. Low bone mineral density and an increased risk of fractures, possibly mediated by hyperprolactinaemia, have been reported in patients taking antipsychotic drugs (Reference Crews and HowesCrews 2012; Reference Wu, Deng and ZhaoWu 2013). The degree of bone loss is related to the degree and duration of hypogonadism; moreover, restoring prolactin levels can partially reverse the loss of bone mass (Reference O'KeaneO'Keane 2008; Reference Lodhi, Masand and MalikLodhi 2016).
High-risk patients are those aged over 65 and female patients with amenorrhoea of more than 6 months’ duration, an early menopause (i.e. at <35 years), a previous fragility fracture or a family history of maternal hip fracture. In these patients, assessment of bone mineral density by dual energy X-ray absorptiometry should be considered. Restoration of prolactin levels by changing the antipsychotic medication or treating with a gonadal steroid hormone has been shown to improve bone density; however, the consequent risk of thromboembolism and breast and prostate cancer must be evaluated. Treatment alternatives are vitamin D supplementation, calcium supplementation and bisphosphonates. In established cases of osteoporosis, it is also advisable to avoid the use of any antipsychotic that is likely to be associated with hyperprolactinaemia.
Breast cancer
No causal link has been established between the use of antipsychotic drugs and the risk of breast cancer. In 1990 an animal study linked prolactin with breast cancer (Reference Wennbo, Gebre-Medhin and Gritli-LindeWennbo 1997). Since then many human studies have suggested a link between hyperprolactinaemia and the pathobiology and risk of progression of established breast cancer. However, thus far the relationship remains unclear (Reference Froes Brandao, Strasser-Weippl and GossFroes Brandao 2016). A retrospective cohort study in the Medicines and Healthcare products Regulatory Agency's General Practice Research Database did not find any difference between typical and atypical antipsychotic drugs in increasing the risk of breast cancer. In addition, the risks from other antipsychotics were similar to that of risperidone (Reference Azoulay, Yin and RenouxAzoulay 2011).
The magnitude of the breast cancer risk is unclear, but the absolute risk is probably very low. However, in clinical practice it would be prudent to preferentially treat breast cancer patients or those at a high risk of developing breast cancer with an antipsychotic that is less likely to raise prolactin levels (Reference Rahman, Clevenger and KaklamaniRahman 2014; Reference De Hert, Peuskens and SabbeDe Hert 2016).
Other long-term effects
Hyperprolactinaemia is also reported to be associated with many other physical and mental health consequences, such as an increased risk of prostate cancer, venous thromboembolism, reduced glucose tolerance and hyperinsulinaemia, as well as behavioural and mood alterations (Reference Landgraf, Landraf-Leurs and WeissmannLandgraf 1977; Reference Ben-Jonathan, Hugo and BrandebourgBen-Jonathan 2006; Reference Harvey, Everett and SpringallHarvey 2008; Reference Milano, D'Acunto and De RosaMilano 2011; Reference Ishioka, Yasui-Furukori and SugawaraIshioka 2015; Reference Wong-AnuchitWong-Anuchit 2016). However, data supporting these consequences are very limited and more research is needed to confirm their clinical significance.
Management of hyperprolactinaemia
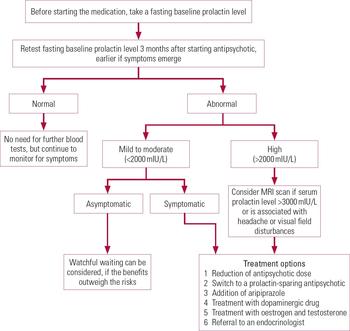
A procedure for monitoring and managing antipsychotic-induced hyperprolactinaemia is outlined in Fig. 2.
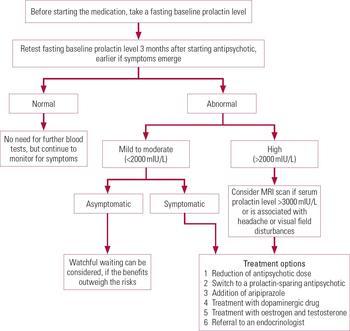
FIG 2 Procedure for the monitoring and management of antipsychotic-induced hyperprolactinaemia, created on the basis of our review of the literature.
Monitoring prolactin levels
There is no consensus on the need for regular monitoring of prolactin levels in patients receiving antipsychotic medication. Most guidelines do not recommend routine monitoring of prolactin levels in asymptomatic patients. NICE guidelines on schizophrenia and bipolar disorder (NICE 2014a,b) have suggested measuring the baseline prolactin level, but have not specified follow-up monitoring. Reference Peveler, Branford and CitromePeveler et al (2008) recommended measuring serum prolactin levels after 3 months of treatment to ascertain the effect of antipsychotic drugs.
Hyperprolactinaemia has also been reported in drug-naive patients with schizophrenia and patients with prodromal psychosis, possibly as a result of stress (Reference Riecher-Rössler, Rybakowski and PfluegerRiecher-Rössler 2013; Reference Peuskens, Pani and DetrauxPeuskens 2014). Furthermore, even a single dose of antipsychotic medication can increase prolactin levels. Therefore, it is important to measure the baseline prolactin level.
Ideally, baseline testing should be performed on a morning fasting sample or at least an hour after a meal, as food intake can increase prolactin levels (Reference CitromeCitrome 2008). The morning dose of medication should not be taken before testing. Excessive stress due to venepuncture should be avoided by ensuring that phlebotomy is done by an experienced and competent person. In certain cases, one can also consider taking two or three samples separated by intervals of at least 20–30 minutes by using indwelling cannula to avoid the stress response (Reference Holt and PevelerHolt 2011). Once the correct antipsychotic dose has been established, prolactin levels should be retested after 3 months unless the patient becomes symptomatic, in which case the test should be repeated earlier.
In the absence of a baseline prolactin value, it is difficult to attribute a raised prolactin level to the antipsychotic drug. In a patient with suspected antipsychotic-induced hyperprolactinaemia and for whom the baseline prolactin level is not available, stopping the drug for 3 days and repeating the prolactin test should be considered. A significant decrease in the prolactin level or normalisation would confirm the clinical suspicion (Reference Melmed, Casanueva and HoffmanMelmed 2011).
In the case of a mild to moderate increase in prolactin level, or if there is any doubt about the cause, then the blood test should be repeated. However, usually a single blood test showing a very high level of prolactin (>2000 mIU/L) or a high prolactin level in a symptomatic patient is usually sufficient to confirm hyperprolactinaemia. (Reference Melmed, Casanueva and HoffmanMelmed 2011).
Assessment of patients presenting with hyperprolactinaemia
Health professionals often fail to detect the signs and symptoms of hyperprolactinaemia for several reasons. First, patients are less likely to volunteer sexual symptoms unless specifically asked. Second, unlike weight gain or extrapyramidal symptoms, the symptoms are not entirely apparent. Patients taking antipsychotic medications should be specifically asked about the signs and symptoms of hyperprolactinaemia. The presence of symptoms should trigger a request to test the serum prolactin level. The use of structured questionnaires for sexual problems (such as the Arizona Sexual Experiences Scale or sexual dysfunction checklist in the UKU Side Effect Rating Scale) or scales for antipsychotic adverse effects such as the Glasgow Antipsychotic Side-effect Scale is recommended to improve their detection.
Raised prolactin levels could be due to many reasons: Box 1 list some common causes. In psychiatric patients raised prolactin should not always be assumed to be caused by antipsychotic drugs unless there is clear evidence of a temporal relationship and the baseline level was normal. In the absence of baseline prolactin testing, other factors, such as the association of hyperprolactinaemia with the particular antipsychotic, the temporal association of symptom emergence with starting antipsychotic drug treatment and the presence of hyperprolactinaemia of mild to moderate severity (<2000 mIU/L), might suggest psychotropic-induced hyperprolactinaemia. Other common causes of hyperprolactinaemia, such as hypothyroidism, pregnancy, chronic kidney disease and pituitary adenoma, should be considered and excluded.
BOX 1 Common causes of hyperprolactinaemia
Physiological
-
Stress
-
Physical exercise
-
Sex
-
Food intake
-
Pregnancy
-
Lactation
Pharmacological
-
Antipsychotic drugs
-
Antidepressant drugs
-
Metoclopramide/domperidone
-
Oestrogen
-
Verapamil
-
Cimetidine, omeprazole, opiates
Pathological
-
Pituitary adenoma
-
Hypothyroidism
-
Chronic kidney disease
-
Polycystic ovary disease
-
Cirrhosis
-
Epileptic seizure
Patients should also be assessed for the long-term consequences of hyperprolactinaemia, such as osteoporosis (history of fragile fracture) and a personal or family history of breast cancer (Reference Milano, D'Acunto and De RosaMilano 2011; Reference Wong-AnuchitWong-Anuchit 2016). They should also undergo a physical examination, blood tests (for renal, hepatic and thyroid function) and visual field charting, if indicated.
Magnetic resonance imaging (MRI) is recommended for patients with high serum prolactin levels (>3000 mIU/L), those presenting with symptoms suggestive of raised intracranial pressure effects (headaches, visual field disturbances) and those for whom a high prolactin level cannot be easily attributed to antipsychotic medication.
Common differential diagnoses for hyperprolactinaemia in a psychiatric setting are a reaction to stress, a pituitary adenoma and an adverse effect of psychotropic medications.
Stress response
Acute stress, including venepuncture, can lead to transient hyperprolactinaemia. Although a stressful event may result in a two- to threefold rise in the prolactin level, it should revert to baseline within a few hours.
Pituitary adenoma
Pituitary adenomas are a common cause of hyperprolactinaemia. They are usually small, benign and endocrinologically silent. However, benign prolactin-secreting tumours account for 40% of all pituitary tumours. Over 90% of these are small, interstellar tumours that rarely increase in size. A meta-analysis reported the very high prevalence of asymptomatic pituitary tumours in the general public: 14.4% in autopsy studies and 22.5% in radiological studies (Reference Ezzat, Asa and CouldwellEzzat 2004). When endo crinologically active, these tumours can be associated with very high levels of prolactin and related symptoms. A prolactin level of >2000 mIU/L indicates a possible pituitary adenoma, whereas levels of >4000 mIU/L strongly indicate a pituitary adenoma. Prolactin levels correlate with tumour size. A large tumour can also lead to other symptoms, such as headache, visual field defects and diplopia, mainly because of pressure effects on the surrounding structures. Pituitary adenoma is detectable on an MRI scan, but small adenomas can often be missed. Treatment involves use of dopaminergic drugs (bromocriptine and cabergoline) and/or surgical resection.
Antidepressants associated with hyperprolactinaemia
Antidepressant drugs, especially SSRIs, can be associated with hyperprolactinaemia; however, the increase in prolactin level is minimal and of not much clinical significance (Reference Torre and FalorniTorre 2007). Some case reports suggest that hyperprolactinaemia can be caused by sertraline, paroxetine, citalopram, fluoxetine, tricyclic antidepressants (e.g. amitrip-tyline, clomipramine) and monoamine oxidase inhibitors. However, as this adverse effect is not very common, routine serum prolactin testing is not recommended.
Antipsychotics associated with hyperprolactinaemia
Hyperprolactinaemia is a common adverse effect of antipsychotic drug therapy. Cross-sectional studies have indicated that the prevalence of hyperprolactinaemia is 44–75% in women and 23–72% in men (Reference Bushe and ShawBushe 2007). One naturalistic study, sponsored by a pharmaceutical company, assessed the prevalence of hyperprolactinaemia in a community mental health trust in the UK (Reference Bushe and ShawBushe 2007). Prolactin levels were assessed in 194 patients with schizophrenia or bipolar disorder who were taking antipsychotic medication but had no signs or symptoms of hyperprolactinaemia. Hyperprolactinaemia was found to be more common in the women than in the men (52% v. 26%). More women also had significantly raised prolactin levels compared with men: levels of >1000 mIU/L and >2000 mIU/L respectively were reported in 25% and 13% of women and 5% and 2% of men. Most patients on risperidone or amisulpride had hyperprolactinaemia.
Almost all antipsychotic drugs can increase prolactin levels, although to varying degrees. The differential effects of antipsychotics are thought to be due to the interplay of different factors, namely their antagonistic/partial agonist effects on the D2 receptor, rate of dissociation from the D2 receptor and concentration in the brain (which depends on the relative permeability of the blood–brain barrier).
The adverse effects are generally dose dependent (Reference Peuskens, Pani and DetrauxPeuskens 2014), although certain antipsychotics (such as risperidone, paliperidone and amisulpride) can have profound effects on the serum prolactin level at relatively low doses (Table 2). More severe hyperprolactinaemia is associated with oral than with injectable risperidone, probably because reduced peak levels are associated with a depot antipsychotic preparation (Reference Holt and PevelerHolt 2011).
TABLE 2 Antipsychotics associated with hyperprolactinaemia
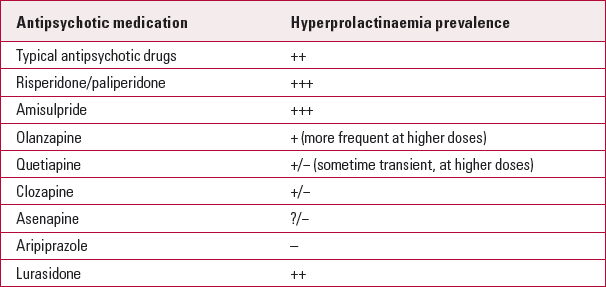
Aripiprazole, asenapine, clozapine, quetiapine and olanzapine, in comparison to other antipsychotic drugs, are less frequently associated with hyperprolactinaemia. Although these drugs can cause transient or sustained hyperprolactinaemia, the risk is much lower. Owing to its partial dopaminergic agonist effect, aripiprazole is likely to reduce prolactin levels and, in some patients, can cause hypoprolactinaemia. The clinical significance of aripiprazole-induced hypoprolactinaemia is yet to be determined, especially in men (Reference Peuskens, Pani and DetrauxPeuskens 2014). However, the drug is also associated with hyperprolactinaemia, especially at higher doses.
Overall, there is significant inter-individual variability regarding the severity of hyperprolactinaemia associated with any particular antipsychotic drug. Furthermore, the degree of prolactin elevation correlating with D2-receptor occupancy is suggested to be >50%, which is less than level of the D2 occupancy postulated to be required for an antipsychotic effect (Reference Tsuboi, Bies and SuzukiTsuboi 2013).
Prolactin levels increase within a few hours of initiating antipsychotic medication, reach a peak within 1–2 months and then gradually decrease. There is some evidence to suggest that tolerance develops, leading to a decreased prolactin level, but in most cases the level remains above the normal range (Reference Peuskens, Pani and DetrauxPeuskens 2014). The prolactin level significantly decreases soon after discontinuation of the antipsychotic and usually returns to normal within 3 weeks (Reference Wong-AnuchitWong-Anuchit 2016). However, for depot antipsychotics normalisation can take up to 6 months (Bergiota 2013).
Antipsychotic-induced hyperprolactinaemia is usually linked to serum prolactin levels of <2000 mIU/L, but antipsychotic medications such as risperidone and amisulpride can be associated with much higher levels. Studies have reported serum prolactin levels of up to 6000 mIU/L due to antipsychotic medications (Reference Peveler, Branford and CitromePeveler 2008). These drugs can also be associated with micro-adenomas, which can disappear after withdrawal of the offending agent or the addition of aripiprazole. Regarding gender and age, young female patients are at a greater risk of hyperprolactinaemia than elderly male patients.
Treatment of antipsychotic-induced hyperprolactinaemia
The management of hyperprolactinaemia should aim to normalise the prolactin level, restore gonadal dysfunction (menstrual and sexual problems) and, importantly, prevent osteopenia and osteoporosis.
Symptomatic patients should be treated. However, it is not possible to recommend a threshold or cut-off point for serum prolactin levels above which asymptomatic hyperprolactinaemia should be treated. The decision to intervene will vary, depending on the individual patient's risk of long-term consequences. Mild asymptomatic hyperprolactinaemia without significant medical comorbidity might not need active intervention, apart from careful monitoring. In these patients, it would be worth checking the gonadal hormone levels because these predict the future risk of osteoporosis and osteopenia. However, if a patient presents with moderate to severe hyperprolactinaemia, symptomatic hyperprolactinaemia (irrespective of the serum prolactin level) or low levels of gonadal hormones, serious consideration should be given to active intervention.
The management strategy will depend on the distress caused by the symptoms, the benefit from the antipsychotic drug and the risk of relapse should the dose of antipsychotic be substantially reduced or stopped. Management options are reducing the antipsychotic dose, switching to an antipsychotic that is less likely to be associated with hyperprolactinaemia, or adding aripiprazole or a dopamine agonist (bromocriptine or cabergoline) to the treatment regimen.
When treating a premenopausal female patient, it is important to remember that reducing the prolactin level will restore fertility; hence, the patient should be counselled about contraception.
Reducing the antipsychotic dose
There is evidence that a raised serum prolactin level might be a dose-related adverse effect of antipsychotic medication; hence, it makes clinical sense initially to try to reduce the antipsychotic dose. However, it is necessary to be aware of the risk of relapse, especially for patients with psychotic illnesses. A prospective study compared reducing the risperidone dose with switching to another antipsychotic in patients with risperidone-induced amenorrhoea: switching was reported to be the more effective strategy. In these patients, dose reduction was associated not with symptomatic improvement but instead with a higher relapse rate (Reference Lee, Han and KimLee 2005).
Switching to a prolactin-sparing antipsychotic
Antipsychotic-induced hyperprolactinaemia can also be managed by switching to a prolactin-sparing antipsychotic. Most studies report a significant improvement in symptoms combined with normalisation of prolactin levels within a few weeks (Reference Madhusoodanan, Parida and JimenezMadhusoodanan 2010; Reference Inder and CastleInder 2011). However, in an individual patient it would be difficult to predict the antipsychotic response to another antipsychotic. Therefore, this option should be chosen only after careful consideration of its risks and benefits. It might not be a suitable option for patients who are resistant to multiple antipsychotics and have done well on the current medication. If this option is taken, cross-tapering the antipsychotics is preferable to abruptly switching to the new antipsychotic (Reference Buckley and CorrellBuckley 2008).
Addition of aripiprazole
Owing to its partial dopamine agonist effect, aripiprazole does not usually increase prolactin levels. In fact, it reduces levels in patients with antipsychotic-induced hyperprolactinaemia. Over the past few years, many case reports, case series, randomised controlled trials and meta-analyses have shown the effectiveness of this drug (Reference Li, Tang and WangLi 2013; Reference De Berardis, Fornaro and SerroniDe Berardis 2014; Reference Ranjbar, Sadeghi-Bazargani and Niari KhamsRanjbar 2015). When used as adjunctive therapy, aripiprazole normalises prolactin levels in up to 79% of patients who are developing antipsychotic-induced hyperprolactinaemia (Reference Li, Tang and WangLi 2013). A low dose of aripiprazole is usually sufficient to normalise prolactin levels: in most cases, 5 mg/day aripiprazole is sufficient, although a small proportion of patients might require a higher dose. Moreover, normalisation of prolactin is usually rapid, occurring within 3 months in most patients (Reference Ranjbar, Sadeghi-Bazargani and Niari KhamsRanjbar 2015). Commonly reported adverse effects of aripiprazole are headache, hypersomnia and insomnia. Usually the addition of aripiprazole is not associated with worsening of psychotic illness (Reference Li, Tang and WangLi 2013; Reference Ranjbar, Sadeghi-Bazargani and Niari KhamsRanjbar 2015); however, there are case reports of worsening of psychotic symptoms (due to the drug's dopaminergic agonist effect) (Reference Takeuchi and RemingtonTakeuchi 2013). A good response has been observed when aripiprazole is used to treat risperidone-induced hyperprolactinaemia, but there is some suggestion that aripiprazole might be less effective in treating sulpiride- or amisulpride-induced hyperprolactinaemia (Reference Chen, Huang and ReeChen 2010). However, it should be remembered that the addition of aripiprazole might lead to high-dose antipsychotic treatment and polypharmacotherapy. There is a lack of evidence for the long-term effects of adjunctive aripiprazole.
Addition of a dopaminergic drug
Bromocriptine and cabergoline are the dopamin-ergic agents most commonly used to treat hyperprolactinaemia due to a pituitary adenoma. These drugs have also been used to treat antipsychotic-induced hyperprolactinaemia. Studies have not shown any worsening of psychosis, although psychotic symptoms have been reported when these drugs have been used to treat other indications (Reference Inder and CastleInder 2011). Therefore, they should be used if aripiprazole is ineffective or if there is a pituitary adenoma, and the psychiatrist should preferably work in close liaison with an endocrinologist. A meta-analysis comparing cabergoline and bromocriptine treatment of hyperprolactinaemia suggested that cabergoline is more effective and has fewer adverse effects (Reference Wang, Mullan and LaneWang 2012).
However, most psychiatrists are familiar with aripiprazole. It has relatively benign adverse effect profile and, more importantly, is unlikely to worsen the psychotic symptoms. Hence, it should be preferred over dopaminergic agents.
Treatment with oestrogen and testosterone
In patients with long-term hypogonadism and/or osteoporosis, the use of oestrogen in women and testosterone in men can be considered, but this should ideally be done by a specialist.
Referral to an endocrinologist
Most patients with antipsychotic-induced hyperprolactinaemia can be managed by psychiatrists if the aetiology is certain. However, referral for specialist advice and management should be made if the aetiology is unclear, prolactin levels continue to rise despite intervention, the hyperprolactinaemia is severe (>3000 mIU/L) or there is suspected/confirmed pituitary adenoma.
Conclusions
Over the past decade, the importance of the physical health of patients with severe mental illness, including the endocrinal and metabolic consequences of antipsychotic drugs, has been increasingly recognised. Hyperprolactinaemia is no longer regarded as a benign abnormality. It has both short- and long-term negative consequences that can significantly affect the physical and psychological well-being of patients. An increased awareness of hyperprolactinaemia among clinicians and patients is a welcome development. However, there are still many unanswered questions and additional research is needed to clarify the appropriate level of monitoring, the long-term consequences of hyperprolactinaemia and the optimal treatment of antipsychotic-induced hyperprolactinaemia.
MCQs
Select the single best option for each question stem
-
1 Hyperprolactinaemia is not induced by:
-
a antipsychotics
-
b dopamine agonists
-
c opiates
-
d antidepressants
-
e omeprazole.
-
-
2 Hyperprolactinaemia is not associated with:
-
a hyperthyroidism
-
b polycystic ovary disease
-
c chronic renal failure
-
d liver cirrhosis
-
e epileptic seizure.
-
-
3 Antipsychotic-induced hyperprolactinaemia can be treated with:
-
a aripiprazole
-
b bromocriptine
-
c amisulpride
-
d cabergoline
-
e (a), (b) and (d).
-
-
4 Of the following antipsychotics, hyperprolactinaemia is least likely to be caused by:
-
a risperidone
-
b amisulpride
-
c olanzapine
-
d trifluoperazine
-
e haloperidol.
-
-
5 Hyperprolactinaemia has not been found to be associated with:
-
a osteoporosis
-
b breast cancer
-
c amenorrhoea
-
d pituitary adenoma
-
e hypertension.
-
MCQ answers

| 1 | b | 2 | a | 3 | e | 4 | c | 5 | e |





eLetters
No eLetters have been published for this article.