Choline is an essential nutrient that is a precursor for the cell membrane components phosphatidylcholine (PC) and sphingomyelin, required for synthesis of the neurotransmitter acetylcholine, and is a source of methyl groups for synthetic reactions( Reference Collier and Lang 1 , Reference Voelker and Kennedy 2 ). The adverse effects of choline deficiency on liver lipid accumulation and muscle dystrophy are well established across species( Reference Hove and Copeland 3 , Reference Zeisel, Da Costa and Franklin 4 ). Previous studies have demonstrated that the demand for choline increases significantly during lactation, and that it is required for optimal growth and development of the infant( Reference Shaw, Carmichael and Yang 5 , Reference Zeisel, Mar and Zhou 6 ). However, the diet of women during lactation may not be meeting this increase. Our recent cohort study has found that the majority of lactating women were consuming well below the present recommended ‘adequate intake’ level( Reference Lewis, Subhan and Bell 7 ).
Dietary nutrients can modify and optimise immune function, and this may be particularly important during lactation since the high nutrient requirements of breast milk might limit the availability of nutrients to the mother. It has been demonstrated that choline is essential during pregnancy for the offspring, and an early study has reported that dams fed diets devoid or marginally deficient in dietary lipotropes (including choline and folate) had pups more susceptible to infection in postnatal life, and this could not be corrected by postnatal supplementation( Reference Gebhardt and Newberne 8 ). Furthermore, choline is a structural component of acetyl-choline, which, in addition to its role as a neurotransmitter, has been demonstrated to be involved in mediating the anti-inflammatory response of the vagus nerve( Reference Rosas-Ballina and Tracey 9 ). Optimising the immune system in the dam is not only important for the composition of breast milk( Reference Hosea Blewett, Cicalo and Holland 10 ) but also for the health of the mother( Reference Palmer 11 ).
PC and sphingomyelin have critical structural and functional roles in cell membranes, and alterations in these molecules due to choline deficiency would be predicted to impair immune function. However, due to the high demands of the placenta, fetus and infant (breast milk) and the reported depletion of maternal choline status during pregnancy and lactation, one would predict that a less than adequate intake of choline would also have adverse effects on immune cell function. Only one previous feeding study has examined the effects of choline deficiency on immune function in adult female rats. Courrèges et al. ( Reference Courrèges, Benencia and Uceda 12 ) reported that, relative to a diet containing 2·6 g choline/kg (fed as 3·5 g choline chloride/kg), 60-d-old rats consuming a diet devoid of choline for 3 weeks had lower delayed-type hypersensitivity responses and ex vivo their splenocytes had a significantly reduced response to concanavalin A (ConA) stimulation. As there was no effect of the devoid diet on the proliferative response to lipopolysaccharide (LPS), the authors concluded that primarily T-cell function was affected. The optimal level of dietary choline required to support maternal immune function has not been established. Although an exogenous source of choline is required in rats during lactation( Reference Zeisel, Mar and Zhou 6 ), the implications of differing levels of intake of choline during lactation on maternal immunity have not been studied. Therefore, the connection between choline intake during lactation and maternal and infant immunity merits further research.
The purpose of the present study was to compare the parameters of maternal immune function and pup growth during suckling when mothers are fed a diet containing the recommended minimal concentration (AIN-93G/M( Reference Reeves, Nielsen and Fahey 13 )) found in most commercial rodent chows (1 g choline/kg diet) to (1) a choline-devoid diet, (2) a diet containing the amount of choline that can be found in many high-fat diets (2·5 g choline/kg diet) and (3) a diet containing a higher amount of choline (6 g choline/kg diet) representing 2·5–6 times the concentration usually found in commercial rodent diets. We hypothesise that not providing choline in the diet during lactation will adversely affect maternal immune function and that intake above the current minimum recommendation of 1 g choline/kg in rodent diets( Reference Reeves, Nielsen and Fahey 13 ) will improve immune cell function.
Experimental methods
Animals and diets
Primiparous Sprague–Dawley rats (n 42) were obtained from Charles River Laboratories on day 14 of gestation. Dams were fed standard rat chow (Lab diet 5001; PMI Nutrition International, containing 1 g choline/kg; Harland Teklad) throughout gestation, then randomised to one of four experimental diets (Table 1) 24–48 h before parturition. This timing was selected to ensure that the dam had the experimental diet at the initiation of suckling. The initial body weight of the dams at the time point of birth did not differ significantly between the experimental groups (271 (sem 5) g, n 40). The content of choline in the salt was verified by analysis( Reference Xiong, Zhao and Goruk 14 ), before adding it to the diets. Diets were fed ad libitum throughout lactation to the end of the study at 21 d postnatal. Animals were provided free access to food throughout each 24 h period, and the feed cups were dumped and refilled every 2–3 d. The four experimental diets were isoenergetic, isonitrogenous and differed only in the content of choline provided in the form of choline bitartrate (Table 1): devoid choline (0 g choline/kg diet; D, n 8) or sufficient choline, containing 1·0 g choline/kg diet (C1, n 11), 2·5 g/kg (C2·5, n 10), or 6·2 g/kg (C6, n 11). At birth, litters were standardised to ten pups (five males and five females when possible) per dam. Dietary intake and body weight were recorded regularly throughout lactation. Two of the original ten dams in the D group had to be killed before the end of the experiment due to significant weight loss. The protocol was reviewed and approved by the Committee of Animal Policy and Welfare of the Faculty of Agricultural, Life and Environmental Sciences at the University of Alberta, Edmonton, Alberta, Canada.
Table 1 Composition of experimental diets
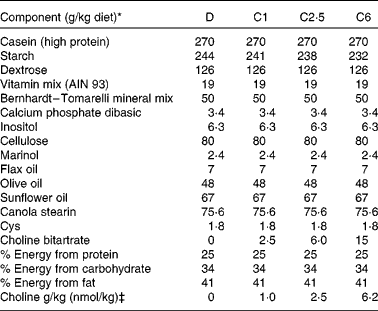
D, choline-deficient diet; C1, 1·0 g choline/kg diet; C2·5, 2·5 g choline/kg diet; C6, 6·2 g choline/kg diet.
* All ingredients were purchased from Harland Teklad with the exception of the dietary oils that were purchased from Safeway, Marinol was donated by Lipid Nutrition, and the stearin was purchased from CanAmera.
‡ Choline was added to the diets in the form of choline bitartrate (Harland Teklad) reach the desired choline concentration for the four diets.
Tissue collection
After 21 days of parturition, dams and two pups per dam were weighed and killed by CO2 asphyxiation and subsequent cervical dislocation in the morning hours. The dams were killed in the AM at the end of the dark cycle, under the assumption that the dams were in the postprandial state. Spleens and livers were collected, weighed and immune cells isolated from the spleen. Pups' stomach contents were collected aseptically, weighed, snap-frozen in liquid N2 and stored at − 80°C until analysis.
Liver total fat content
Total fat was isolated from dam and offspring livers using a modification of the Folch method( Reference Folch, Lees and Sloane-Stanley 15 ). After defrosting, 300 mg of liver tissue were homogenised in 1 ml of 0·025 % calcium chloride solution for 30 s at 6·5 m/s using a FastPrep-24 homogeniser (MP Biomedicals). In glass tubes, 12 ml of 2:1 chloroform–methanol and 1 ml of 0·025 % calcium chloride solution were added to the liver homogenate. These tubes were vortexed thoroughly and left overnight at 4°C. This treatment results in two layers and the lower, chloroform phase was transferred into a new, pre-weighed, glass tube and the remaining phase washed once more with 2:1 chloroform–methanol. After 1 h, the chloroform phase of the second wash was added to the first aliquot. The chloroform was evaporated under N2 until only the fat remained in the tube that was reweighed, with the difference between the two weights being the weight of the fat extracted. Total liver fat was expressed as mass of fat adjusted for original sample weight (mg/g).
Choline metabolite analyses of pup stomach content
Rat pup stomach contents were used to determine the effect of maternal diet on the content of choline in the dam's milk. Frozen stomach contents were ground on liquid N2, and lipids were then extracted by a modified method following Bligh & Dyer( Reference Bligh and Dyer 16 ) as described in detail previously( Reference Xiong, Zhao and Goruk 14 ). All of the significant classes of choline-containing compounds in the extracts were quantified by hydrophilic interaction chromatography (HILIC) liquid chromatography–tandem MS (LC–MS/MS) using an Agilent 1200 series HPLC system coupled to a 3200 QTRAP mass spectrometer (AB SCIEX) as previously described in detail( Reference Xiong, Zhao and Goruk 14 ). Data were acquired and analysed with the use of Analyst 1.4.2 software. The total choline content and proportion of choline per molecule were calculated using the molecular weights of choline and the choline-containing molecules.
Immune cell isolation
Immune cells were isolated from spleens as described previously( Reference Field, Wu and Metroz-Dayer 17 ). Briefly, single-cell suspensions were obtained by disrupting tissue through a nylon mesh screen in sterile Krebs–Ringer HEPES buffer with bovine serum albumin (5 g/l; Sigma-Aldrich Canada Limited). Erythrocytes were removed from the mixture by treatment with ammonium chloride lysis buffer (155 mm-NH4Cl, 0·1 mm-EDTA, 10 mm-KHCO3; Fisher Scientific). Cells were washed and re-suspended in complete culture medium (Roswell Park Memorial Institute (RPMI) 1640 media (Life Technologies)) supplemented with 5 % (v/v) heat-inactivated fetal calf serum, 25 mm-HEPES, 2·5 mm-2-mercaptoethanol and 1 % antibiotic/antimycotic (pH 7·4; Fisher Scientific, as earlier). Cells were counted on a haemocytometer using trypan blue dye exclusion (Sigma-Aldrich, as above) and diluted to 1·25 × 106 cells/ml.
Immune cell phenotype analysis
Immune cell subsets in freshly isolated splenocytes were identified by direct immunofluorescence assay as described previously( Reference Field, Wu and Metroz-Dayer 17 , Reference Field, Thomson and Van Aerde 18 ). In brief, 200 000 immune cells were incubated for 30 min at 4°C with pre-labelled monoclonal antibodies applied in combination to quantify various immune cell phenotypes. Four-colour flow cytometry allowed determination of the following surface molecule combinations: CD28/CD3/CD8/CD4, CD4/CD8/CD152, CD25/CD8/CD4, CD3/CD71/CD8/CD4, CD3/CD45RA/CD27, CD68/CD284/CD11b/c, CD3/CD161, CD8/OX62/OX6, IgG/IgM, CD3/CD25/FOXP3/CD4 and OX12/CD27. All mAb with the exception of IgG, IgM and OX6 (BD Biosciences) were purchased from Cedarlane Laboratories. After incubation, cells were washed and fixed in paraformaldehyde (10 g/l; ThermoFisher) in PBS. To identify the intracellular protein forkhead box P3 (FOXP3), isolated cells were permeabilised before addition of the antibody, according to the manufacturer's directions (Cedarlane Laboratories).
All samples were acquired within 72 h by flow cytometry (FACSCalibur; Becton Dickinson) and analysed according to the relative fluorescence intensity using Kaluza Software (Beckman Coulter).
Ex vivo cytokine secretion by mitogen-stimulated splenocytes
Cytokine production by mitogen-stimulated splenocytes was measured as described previously( Reference Blewett, Gerdung and Ruth 19 ). In brief, immune cells (1·25 × 106 cells/ml) were cultured in 1 ml (RMPI-1640 medium as above) for 48 h at 37°C and 5 % CO2 without mitogen (unstimulated cells) or with ConA (2·5 μg/ml; MP Biomedicals), LPS (100 μg/ml; Sigma-Aldrich, as above) or both CD3 (1 μg/ml) and CD28 (5 μg/ml; both from e-Bioscience, Inc.). Cells were then centrifuged for 10 min at 1000 rpm and the supernatants kept at − 80°C until analyses. Commercial ELISA kits were used to measure the concentrations of IL-1β, IL-2, IL-6, IL-10, TNF-α and interferon-γ (IFN-γ) according to the manufacturer's instructions and as described previously( Reference Blewett, Gerdung and Ruth 19 ). All detection limits were 15·63–4000 pg/ml except IFN-γ, 9·76–2500 pg/ml (R&D systems). Concentrations were determined on a microplate reader (SpectraMax 190; Molecular Devices), and all measurements were conducted in duplicate with CV < 10 %.
Statistical analysis
Statistical analyses were conducted using SAS statistical software (version 9.3; SAS Institute, Inc.). Data were analysed for normal distribution in each dietary group with the use of a Kolmogorov–Smirnov test. Parametric data were subsequently analysed for differences by ANOVA and Tukey post hoc testing was used. Regression analysis was used to test the relationship between dietary choline intake and the concentration of free and total choline in the stomach content of the offspring. Non-parametric data were log-transformed before analysis by ANOVA as above. In some instances, log-transformation did not lead to normal distribution of the data, and groups were compared similarly using Mann–Whitney U-tests. For all statistical tests, significance was accepted with a CI of 95 % (P< 0·05). All results are presented as means with their standard errors and the actual number of values available for each measure is indicated with the results.
Results
Anthropometric characteristics
Dams fed the choline-deficient diet, D, had lower final body weights, spleen weights and average pup weight per dam compared with the C1 diet (P< 0·05; Table 2). Dams fed the diet with a high dose of choline, C6, had lower final body weight compared with the C1 and C2·5 dams, and had lower spleen weight and lower average pup weight at 21 d postnatal than C1 dams (P< 0·05; Table 2). Food intake in D dams was 29 % lower compared with C1 (P< 0·05, Table 2). Food intake in C6 dams was 24 % lower than C1 (P< 0·05, Table 2). Neither splenocyte numbers nor liver fat weight differed between any of the groups.
Table 2 Anthropometric data of lactating dams fed choline-deficient (D) or choline-sufficient diets (C1, C2·5 or C6) at the end of the study, 21 d postnatal* (Mean values with their standard errors)
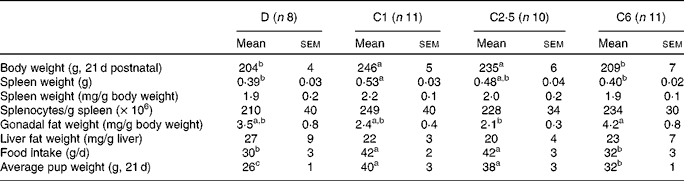
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* There was no significant difference between males and females, so the entire litter was pooled to represent the average pup weight. Discrepancies between total number of mothers per group and the number here depicted are due to technical difficulties.
Choline metabolites in pup stomach contents
The total choline content in the pups' stomachs was significantly lower in D compared with C1, C2·5 and C6 pups (Fig. 1), and there was a linear relationship between dietary choline content and total choline concentration in the offspring's stomach (R 2 0·28, P< 0·001). The difference in choline content between groups in the stomach content was primarily due to differences in the free choline content (Table 3), which also had a linear relationship with maternal dietary choline content (R 2 0·42, P< 0·001). Glycerophosphocholine (GPC) concentration was lower and lysophosphocholine concentration was higher in D stomach contents compared with pups of choline-sufficient mothers. These differences in total concentrations resulted in differences in the relative contribution of choline-containing molecules to the total choline content (Fig. 1). In D pups, the relative contributions of sphingomyelin, lysophosphocholine and phosphocholine (Pcho) to total choline content were significantly higher than in the other groups, due to the low content of free choline (Fig. 1). In C6 pups, due to the high concentration of free choline, the relative contribution of Pcho and GPC was lower than in C1 pups (Fig. 1). There were no significant differences in the absolute or relative concentrations of choline metabolites, between C1 and C2·5 pups' stomach contents (Table 3 and Fig. 1).

Fig. 1 Total choline content and relative contribution of the different forms of choline in the pups' stomach contents at 21 d postnatal from mothers who were fed one of four diets during lactation: (A) choline-devoid diet (D diet; n 6); (B) 1 g/kg choline diet (C1 diet; n 11); (C) 2·5 g/kg choline diet (C2·5 diet; n 8); (D) 6·2 g/kg choline diet (C6 diet; n 10). Discrepancies between the total number of mothers per group and the numbers shown here are due to technical difficulties. Total choline content in the D diet was significantly different from that in all the other diets (P< 0·05). Total choline content did not differ between the C1, C2.5 and C6 diets. Data are means with their standard errors for the total content. Values are means for the percentage composition of the total choline content within each chart. a,b,cDifferences in the relative content of choline originating from the different forms are indicated by letters next to the pie charts, indicating that between charts, wedges (clockwise from the top, sphyingomyelin (![]() ), lipopolysaccharide (
), lipopolysaccharide (![]() ), phosphocholine (
), phosphocholine (![]() ), phosphatidylcholine (
), phosphatidylcholine (![]() ), glycerophosphocholine (
), glycerophosphocholine (![]() ) and free choline (
) and free choline (![]() )) with unlike letters were significantly different (P< 0·05).
)) with unlike letters were significantly different (P< 0·05).
Table 3 Choline content from the choline-containing molecules (mg/100 g) of rat pup stomach contents from dams fed choline-deficient (D) or choline-sufficient diets (C1, C2·5 or C6)* (Mean values with their standard errors)
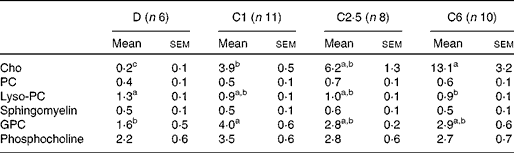
Cho, free choline; PC, phosphatidylcholine; Lyso-PC, lysophosphatidylcholine; GPC, glycerophosphocholine.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Concentrations calculated per 100 g total stomach contents. Discrepancies between total number of mothers per group and the number here depicted are due to technical difficulties.
Immune cell phenotypes
While splenocyte numbers were similar between groups, spleen-derived immune cell distributions differed between groups. Compared with C1, D dam spleens had a lower proportion of CD4+T cells (% CD3+CD4+, T helper cells; P< 0·05; Table 4). D also had a lower proportion of CD4+ (CD3+CD4+) and CD8+ cells (CD3+CD8+) expressing the co-stimulatory molecule CD28 (P< 0·05; Table 4). Although we found no difference in the proportion of total CD8+ cells, the proportion of CD8+ cells that expressed either the IL-2 receptor (CD25), the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, CD152), or the transferrin receptor (CD71) were higher (P< 0·05) in spleens from D compared with C1 dams (Table 4). There was a lower proportion of CD3+CD4+ cells; however, the percentage of the CD4+ population that was positive for CD152 was higher in D than in C1 and C2·5 dams (Table 4). There was no difference in the relative proportion of T regulator cells (CD4+CD25+FOXP3+). D spleens had a higher (P< 0·05) proportion of cells expressing MHC class II (OX6+); however, the proportion of macrophages (CD68+ and CD11b/c+) did not differ between groups (Table 4). Total B (Ig+ cells, OX12+) and IgG+ cells comprised a higher percentage of splenocytes in D mothers (Table 4). However, the proportion of B cells (OX12+) that expressed the TNF receptor (CD27) was lower in the D group (P< 0·05). Although only comprising a small proportion of total splenocytes, dendritic cells (OX62+OX6+) were 2-fold higher (P< 0·05) in the spleen of dams fed D (Table 4). Compared with the groups fed a diet with a normal choline content, feeding a diet with a higher choline content, C6, made no difference in the types of immune cells in the spleen, except for a lower proportion of CD3+CD4+ cells (P< 0·05; Table 4).
Table 4 Splenocyte phenotypes of lactating rat dams fed choline-deficient (D) or choline-sufficient diets (C1, C2·5 or C6)* (Mean values with their standard errors)

CD, cluster of differentiation; FOXP3, forkhead box P3.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* The following phenotypes were not significantly different among groups (n 30–39); %CD3+CD27+: 87 (sem 1) %; CD284+: 24 (sem 1) %; %CD68+CD284+: 79 (sem 2) %; %CD11+CD284+: 42 (sem 2) %; CD3 − CD161+: 7·5 (sem 0·4) %. Discrepancies between total number of mothers per group and the number here depicted are due to technical difficulties.
Cytokine production
There was a significantly lower (P< 0·05) production of cytokines produced after stimulation with LPS (IL-10 and IFN-γ), ConA (IL-2, IL-6, TNF-α and IFN-γ) and CD3/CD28 (IL-2, IL-6, IL-10 and IFN-γ) in D compared with cells from animals that received choline (C1, C2·5 and C6; Table 5). Production of cytokines did not differ in ConA-stimulated splenocytes from C1, C2·5 and C6 dams. There was also no difference in the cytokine response to CD3/CD28 between the C1 and C2·5 group; however, splenocytes from C6 dams produced more IL-2, IL-6 and TNF-α (P< 0·05; Table 5). Splenocytes from C2·5 and C6 dams produced more IL-6 after stimulation with LPS than those from C1 dams (P< 0·05; Table 5).
Table 5 Ex vivo mitogen-stimulated splenocyte cytokine production from rat dams fed choline-deficient (D) or choline-sufficient diets (C1, C2·5 or C6)* (Mean values with their standard errors)

LPS, lipopolysaccharide; IFN-γ, interferon γ.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* The amount IL-2 in the media after LPS stimulation was below the detection levels. IL-1β was only measured in the supernatant of LPS-stimulated cells. Discrepancies between total number of mothers per group and the number here depicted are due to technical difficulties.
Discussion
We have established that choline is an essential micronutrient during lactation, and that endogenous synthesis, contributing to the concentration of choline in mother's milk( Reference Rohlfs, Garner and Mar 20 ), is not sufficient to ensure optimal growth in the offspring. In the present study, we found that the amount of choline salt in the maternal diet influences the content of choline that is provided to the offspring. As the total choline concentration in milk has been reported to be directly associated with the infant's circulating choline concentration( Reference Ilcol, Ozbek and Hamurtekin 21 ), the present results suggest that we can alter the choline status of the offspring by feeding choline to suckling dams even at levels beyond the current requirement for growth. In D offspring's stomach content, the sample we used to represent mother's milk contents, we showed a reduction in GPC by 50 %. GPC content in the mammary gland is either synthesised from Pcho( Reference Rohlfs, Garner and Mar 20 ) or by phospholipase-mediated breakdown of PC( Reference Samborski, Ridgway and Vance 22 ). As the content of Pcho and PC was not lower in D stomach contents, this suggests that substrate availability may not have been the limiting factor in the synthesis of GPC. Alternatively, the lower content of GPC could suggest that choline is being partitioned away from GPC synthesis in the dam to meet other biological needs. The higher concentration of lysophosphocholine in D stomach contents suggests that the action of phospholipase A.2, the enzyme enabling GPC synthesis, may have been inhibited in the mammary glands of the D dams. In contrast to our findings, a rat study feeding a choline-devoid diet during lactation has found a lower concentration of Pcho( Reference Rohlfs, Garner and Mar 20 ). The relative composition of choline metabolites in the pups' stomach contents in the present study is consistent with that reported for human milk( Reference Holmes-McNary, Cheng and Mar 23 ), which may be due to the shared higher fat content. This difference from the previously published study may be due to the high-fat diet used in the present study, which provided considerably more fat as a percentage of energy than the 10 % provided in the standard AIN-76a diet. Choline metabolism, particularly that of PC in the liver, has been reported to be altered by feeding high-fat diets( Reference Noga and Vance 24 ).
We show for the first time that feeding a diet devoid of choline significantly impairs maternal immune function. T-cell function may be reduced in D dams due to a lower proportion of T cells expressing the co-stimulatory molecule CD28, which is crucial for T-cell activation, their proliferation, cytokine secretion and exertion of effector functions( Reference Rudd, Taylor and Schneider 25 ). A reduction of CD28 expression has also been found in other conditions of depressed T-cell function, e.g. in childhood malnutrition( Reference Nájera, González and Cortés 26 ) and in Fe depletion( Reference Kuvibidila and Porretta 27 ), making this a feasible mechanism to reduce immune response in D splenocytes. At the same time, a higher proportion of D CD8+T cells carried markers of early and later activation (CD71 and CD25), and their activation in vivo may have contributed to the lower production of IL-2, a marker of proliferation. Proportions of both CD8+CD25+T cells, known to suppress IL-2 response( Reference McNally, Hill and Sparwasser 28 ), and of CD152+T cells, involved in the resolution of immune activation( Reference Rudd, Taylor and Schneider 25 ), are increased in D splenocytes, suggesting an immunosuppression in these dams. The production of IL-2, representing the ability of splenocytes to proliferate, after stimulation with a polyclonal T-cell stimulus (ConA) and a T-cell antigen (CD3/CD28) was 51 and 75 % lower, respectively. This is consistent with a study of feeding adult rats a diet devoid of choline for 60 d, resulting in a much reduced ex vivo proliferative response to ConA compared with animals fed 2·6 g/kg choline( Reference Courrèges, Benencia and Uceda 12 ). These animals also showed a reduced hypersensitivity response, providing evidence that T-cell-mediated functions are impaired in vitro ( Reference Courrèges, Benencia and Uceda 12 ). We further show that splenocytes of D animals had a reduced capacity to produce almost all cytokines involved in T-cell function (with the exception of IL-10 after ConA stimulation), which may be due, in part, to the reduced proportion of CD28+T cells. Importantly, production of IFN-γ was considerably reduced in D splenocytes after stimulation. This cytokine is crucial in the response to viral, as well as to some bacterial and protozoal infections( Reference Magombedze, Reddy and Eda 29 ), and is produced by both CD4+ and CD8+T cells and by Natural Killer cells (CD3-CD161+)( Reference Boehm, Klamp and Groot 30 ).
LPS activates antigen-presenting cells, including macrophages, dendritic cells and B cells, by first binding to their Toll-like receptor 4 (CD284). We found no difference in the proportion of splenocytes overall carrying CD284 nor of macrophages with this receptor between any of the dietary groups. Although there was a higher proportion of B cells (OX12+ and IgG+) in the spleen of D animals, there was a lower proportion of activated OX12+CD27+B cells, which are the cells that respond to LPS( Reference Rickert, Jellusova and Miletic 31 ). Not ruling out changes in the functional capacity of antigen-presenting cells, the present results are overall suggestive of a depressed T-cell function influencing the cytokine response to LPS. The cytokine most affected in the D splenocyte response to LPS was IFN-γ, which is also involved in the activation of macrophages and induces the MHC Class II, again affecting the response to LPS( Reference Schroder, Sweet and Hume 32 ). A reduced activation of antigen-presenting cells as represented by fewer OX12+CD27+B cells and reduced IFN-γ production probably exacerbates the reduced T-cell function in response to LPS. Cells do not proliferate to any great extent when stimulated with LPS, and this probably explains the failure of an earlier study to demonstrate a significantly lower proliferative response to LPS in splenocytes from choline-deficient adult rats( Reference Courrèges, Benencia and Uceda 12 ). It is possible that the effect on body weight contributed to the reduced immune function in the dams fed the devoid diet; however, this immunosuppression was not observed in the C6 choline group where body weight was similar. This suggests that the immune effects are more likely due to a limited supply of choline than a reduced food intake or lower body weight.
The optimal choline content in the diet of lactating dams is not known and may be higher when a high-fat diet is fed, such as during breeding. Most commercial diets contain 1·0–2·5 g choline/kg diet that is usually provided, as was in the present study, as a choline salt. The present study confirms that supplementing a high-fat diet with 1 g choline/kg (in the form of salt) is equal to supplementing with 2·5 g choline/kg with respect to maintaining maternal and infant weight. Similarly, based on the immune measures collected in the present study, we found very little difference in the immune response between the C1 and C2·5 dams. The only difference between the two diets was a substantially higher (150 %) production of IL-6 by C2·5 splenocytes after stimulation with LPS, suggestive of a more robust macrophage response. Although CD4+T cells might produce some IL-6, an effect on T-cell function can be ruled out as there was no difference in response to either of the T-cell mitogens between C1 and C2·5. A source of choline has been demonstrated to be required for the release of IL-6 by macrophages stimulated with LPS( Reference Tian, Pate and Andreolotti 33 ). Recently, it was also demonstrated that LPS stimulates the release of acetylcholine from macrophages, which is involved in the induction of their inflammatory response( Reference Lv, Hu and Lu 34 ). This suggests that the 2·5 g choline/kg diet may have some benefit over the 1 g/kg diet to support the dam's innate immune system.
Our data suggest for the first time that there may be an upper level of intake during lactation for the health of both dam and offspring. Feeding the highest level of choline (6·2 g/kg diet, approximately six times the concentration in most research diets) had negative effects on maternal and pup body weight. Whether the lower pup body weight was due to the higher total choline content, the higher amount of free choline or the different balance of choline containing metabolites (lower proportion of GPC, PC and lysophosphocholine in stomach contents) cannot be determined by the present study. A recent study has been designed specifically to address that choline toxicity did not observe any negative effects in adult mice given a relatively low daily oral gavage of 200 mg choline chloride/kg body weight (approximately 150 mg choline/kg body weight) for 28 d( Reference Mehta, Arora and Gaur 35 ). The present study found no effects on growth, food and water intake, total leucocyte concentration, neutrophils, lymphocytes, eosinophils, monocytes and number of spleen mononuclear cells( Reference Mehta, Arora and Gaur 35 ). We estimated that the dams in the present study consumed a much higher amount of choline (approximately 248 mg choline/d or 1240 mg choline/kg body weight based on 200 g body weight and 40 g/d food intake), which may furthermore differ during periods of high choline demand, such as early life, pregnancy and lactation. There is a growing literature on the possible toxicity of trimethylamine-N-oxide to humans, a metabolic product of PC( Reference Miller, Corbin and da Costa 36 ), but this has, to our knowledge, not been associated with the intake of choline in the form of a salt.
Despite the effect on body weight and breast milk composition (stomach content of the pups), there were minimal effects of feeding the C6 diet on maternal immune function. The weight and, with that, total amount of cells in the spleen was lower in C6 than in C1; however, there was no substantial difference in the proportion of different cells present compared with the other groups fed choline, nor did the cytokine response to LPS and ConA differ among the choline-containing diet groups. Nevertheless, T cells were more robustly activated when stimulated through the T-cell receptor (CD3/CD28), so that there was a higher production of pro-inflammatory IL-2, IL-6 and TNF-α in cells from C6 dams. There are numerous studies in adult rodents demonstrating an increase in choline and its metabolites in brain after feeding diets high in choline (reviewed in Babb et al. ( Reference Babb, Ke and Lange 37 )). It is likely that choline concentration is also increased in lymphocytes in those animals, which may result in higher production of choline-derived metabolites as T cells, including their cholinergic system and, when stimulated via the T-cell receptor complex, cells may produce more acetylcholine that induces their activation( Reference Fujii, Takada-Takatori and Kawashima 38 ). To analyse this mechanism and its physiological consequences would require further studies.
The results of the present study confirm that choline is required in the maternal diet during lactation, not only for maternal and pup growth but also to maintain maternal immune function, both T cell and innate immunity. The current recommendation of 1 g choline/kg diet in a high-fat diet appears sufficient to maintain T-cell function; however, there may be some benefits to innate immunity of feeding 2·5 g choline/kg. Exceeding this minimal recommendation by 6-fold (in the form of a choline salt) was associated with a higher concentration of choline in breast milk and negative effects on maternal and pup body weight and resulted in increased activation of T-cell function when stimulated via the T-cell receptor. The translation of this higher intake to humans is not possible as the current Institute of Medicine recommendation is that of an adequate intake, but suggests that there may be concern if women were to use very high amounts of supplement choline salts during lactation.
Acknowledgements
The authors would like to acknowledge the technical assistance of Nicole Coursen, Kelly-Ann Leonard, Yeping Xiong, Peter Iglinski and Howe-Ming Yu and Yuan Yuan Zhao.
The present study was funded by the Natural Science and Engineering Council of Canada (grant numbers NSERC RGPIN 121610 and 386652) and Quality Food for Health grant from the ALMA, Alberta Innovates Biosolutions and the Egg Farmers of Alberta (grant number 2012Q005R). This work was further supported by the Women and Children's Health Research Institute (E. D. L., Graduate Studentship and the Queen Elizabeth II Graduate Scholarship), from the Canadian Institutes of Health Research Postdorctoral Fellowship, Fonds de Recherche en Santé du Québec and Izaak Walton Killam Memorial Postdoctoral Fellowships (all C. R.). None of the funders had any role in the design and analysis of the study or in the writing of this article.
None of the authors has any conflict of interest to declare.
The authors' contributions are as follows: N. S. D. conducted the study, analysed and interpreted the results, and wrote the manuscript with C. J. F.; C. J. F., R. L. J. and J. M. C. formulated the research questions, designed the study and secured the funding for the study; S. G., E. D. L., M. R. R. and C. R. helped in method development, conducting the study or the analysis of the results. All authors reviewed and edited the manuscript.









