Chronic inflammation and oxidative stress have been associated with increased endothelial dysfunction and insulin resistance, major risk factors for hypertension and subsequent CVD, which contribute to about 35–40 % of global deaths(Reference Jagannathan, Patel and Ali1,Reference Bansilal, Castellano and Fuster2) . Previous work has indicated that low intake of fruits, vegetables, nuts and whole grains is highly associated with increased risk of CVD and their related complications, resulting in elevated inflammatory markers, endothelial dysfunction and ensuing hypertension and atherosclerosis(Reference Chiu, Shen and Huang3,Reference Lim, Vos and Flaxman4) . Hence, consumption of a sufficient amount of fruits, vegetables and nuts is important to lower the risk of CVD and their related complications. Recently, many studies have indicated that increased consumption of nuts, especially pistachios, which have lower fat and energy content than other nuts, would considerably reduce the risk of CVD by preserving endothelial function(Reference Guasch-Ferré, Liu and Malik5–Reference Del Gobbo, Falk and Feldman7).
Pistachio trees (Pistacia vera L.) are popular nut-bearing trees that belong to the family Anacardiaceae and are commonly grown in the Middle East, Southwestern Asia and Southern Europe. Pistachios have been traditionally used as a folk remedy to treat various abnormal and disease conditions, such as hepatic and renal diseases as well as sexual dysfunction, owing to their high nutritive value and long shelf life(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8–Reference King, Blumberg and Ingwersen10). The major nutrients present in pistachios are unsaturated fats (MUFA and PUFA), proteins (essential amino acids), dietary fibre, minerals (K, Mg, P and vitamins D, E and K), phytosterols (stigmasterol, β-sitosterol and campesterol), xanthophyll carotenoids (lutein and anthocyanin) and phenolic acids(Reference Dreher6,Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8,Reference Terzo, Baldassano and Caldara11) . Pistachios exhibit a broad range of beneficial properties including antioxidant, anti-inflammatory, anti-diabetic, anti-hyperlipidaemic, anti-hypertensive, anti-obesity and anti-cancer properties due to the aforementioned phytonutrients(Reference Hernández-Alonso, Bulló and Salas-Salvadó12,Reference Gebauer, West and Kay13) .
A growing body of evidence demonstrates that moderate consumption of pistachios could reduce CVD risk by lowering inflammation and oxidative stress, thus improving endothelial function and glycaemic control(Reference Sari, Baltaci and Bagci9,Reference Terzo, Baldassano and Caldara11,Reference Sauder, McCrea and Ulbrecht14,Reference Kasliwal, Bansal and Mehrotra15) . Previous studies have considered the effect of nut consumption on CVD, but those studies presented subgroup analyses by nut type; additionally, because they did not specifically evaluate the effects of pistachios, some previous studies have missed the potential effects of pistachios alone(Reference Mohammadifard, Salehi-Abargouei and Salas-Salvadó16,Reference Neale, Tapsell and Guan17) . In fact, no systematic review and meta-analysis of randomised controlled trials (RCT) has been conducted to consider the efficacy of pistachios on inflammation, endothelial dysfunction and hypertension. Thereby, this study aimed to systematically investigate the effect of pistachios on anthropometric indices, inflammatory markers, endothelial dysfunction and blood pressure compared with control group, among adults, by conducting a systematic review and meta-analysis.
Methods
This meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)(Reference Moher, Liberati and Tetzlaff18) (online Supplementary Table S1) and follows the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions(Reference Higgins and Green19).
Ethics approval and consent to participate
All analyses were based on previous studies, so no ethical approval or patient consent were required.
Search strategy
The systematic literature search was performed using PubMed, Scopus, Cochrane Library and Web of Science databases with key search terms (combination of subject and free words): (Pistachio OR Pistachios OR Pistacia) AND (Intervention OR Intervention study OR Intervention studies OR Controlled trial OR Randomized OR Random OR Randomly OR Placebo OR Assignment OR Clinical trial OR Trial OR Randomized controlled trial OR Randomized clinical trial OR RCT OR Blinded OR Double blind OR Parallel OR Parallel study OR Parallel trial). The search period was up to 31 June 2019, and no limitation was imposed on the publication year and language to minimise publication and language bias. The search strategy used for the online databases is provided in online Supplementary Table S2. The literature search was enhanced by reviewing the citations of the articles considered eligible for the systematic review.
Study selection
After excluding duplicates through the reference manager software Endnote, version X6 (Thomson Reuters), two investigators separately screened the title and abstract of the records for eligibility and then assessed the full text of the retained records for final inclusion decisions. The PICOS (population, intervention, comparator, outcome, study design) criteria were used to establish study eligibility. We included studies that met the following criteria: (1) population (healthy and unhealthy adults); (2) intervention (pistachio consumption); (3) comparator (matched control group, that is, the only difference between the control and treatment groups was pistachio); (4) outcome (systolic blood pressure (SBP), diastolic blood pressure (DBP), flow-mediated dilation (FMD), C-reactive protein (CRP), TNF-α, IL-6, body weight (BW), BMI and waist circumference (WC)) and (5) study design (RCT, either parallel or crossover design). Articles were excluded if: (1) trials did not provide sufficient information for the outcomes in pistachio or control groups; (2) studies had follow-up time <1 week because based on previous meta-analyses(Reference Mohammadifard, Salehi-Abargouei and Salas-Salvadó16,Reference Fogacci, Cicero and Derosa20) , this time is too short to see the beneficial effects of pistachios. When multiple articles reported different outcomes from a single study, the article reporting the longest follow-up period was included in the review.
Data extraction
Data were extracted from each trial and are presented in Table 1, which includes: (1) study characteristics (first author’s last name and year of publication, location of the study, sample size and study design); (2) participants’ information (sex, mean age, mean BMI and health status); (3) intervention details (duration of treatment, dosage of intervention) and (4) main results. When data were not provided in publications, we attempted contacting the authors for further information. One author (O. A.) extracted the relevant data from included trials, and the second author (E. G.) checked the extracted data. Any disagreements were discussed until consensus was met.
Table 1. Characteristics of included studies in meta-analysis

R, randomised; CG, control group; CO, crossover; F, female; M, male; PA, parallel; NR, not reported.
Risk of bias assessment
Study quality was evaluated independently by two reviewers (O. A. and E. G.) using Cochrane risk of bias tool(Reference Higgins, Altman and Gøtzsche30). Each RCT was given one of three rankings, ‘high risk’, ‘low risk’ or ‘unclear risk’, in each of the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias. For all studies, each item was described as having a low risk of bias, a high risk of bias or an unclear risk of bias. Trials were considered low risk when each independent domain was rated as low risk. Any domain rated as unclear or high risk increased the overall risk score. If the scores awarded by the two investigators differed, a third investigator (A. H.) assessed the study in question.
Statistical analysis
STATA software (version 11.0; Stata Corporation) was used for all statistical analyses. The change in means and standard deviation of the outcomes of interest between intervention and control groups was used to calculate effect sizes (mean difference, MD) with 95 % CI to compare reported outcomes across studies by meta-analysis. In studies in which mean change was not directly reported, it was calculated by the subtraction of the post-intervention data from the baseline value. If standard deviation of the MD was not reported, it was calculated using the following formula: sd = square root ((sd pre-treatment)2 + (sd post-treatment)2 – (2 × R × sd pre-treatment × sd post-treatment))(Reference Follmann, Elliott and Suh31). To ensure the meta-analysis was not sensitive to the selected correlation coefficient (R 0·5), all analyses were repeated using correlation coefficients of 0·2 and 0·8. When standard error was reported in place of standard deviation, we converted it to standard deviation for further analyses: sd = se × sqrt (n), n = number of subjects. Random effects models were used to pool the data to account for the potential heterogeneity introduced by the variation in location, follow-up time, population and dosage presented in the various studies. Statistical heterogeneity among studies was evaluated using the I 2 statistical value, and an I 2 value >50 % was regarded as having substantial heterogeneity(Reference Higgins and Green19). To find the potential sources of between-study heterogeneity, we carried out subgroup analyses based on baseline BMI (overweight or obese), intervention duration (≥12 or <12 weeks) and health status (healthy (without any disease) or unhealthy). A sensitivity analysis using the leave-one-out method was also performed to assess the effect of each study on the overall effect size. The potential publication bias in each analysis was assessed using Egger’s regression test. A P value of <0·05 was considered statistically significant.
Results
Study selection
Of the 1553 papers identified in the preliminary search, 476 duplicate articles were removed and 1077 papers remained for evaluation based on title and abstract. Among them, nineteen papers were selected for full-text assessment. After further perusal, twelve eligible studies(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8,Reference Sari, Baltaci and Bagci9,Reference Gebauer, West and Kay13,Reference Kasliwal, Bansal and Mehrotra15,Reference Sheridan, Cooper and Erario21–Reference West, Gebauer and Kay23,Reference Gulati, Misra and Pandey25–Reference Carughi, Bellisle and Dougkas29) were found. Additionally, one article was included from a manual search(Reference Wilson, Young and Anderson24). Finally, thirteen articles(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8,Reference Sari, Baltaci and Bagci9,Reference Gebauer, West and Kay13,Reference Kasliwal, Bansal and Mehrotra15,Reference Sheridan, Cooper and Erario21–Reference Carughi, Bellisle and Dougkas29) were eligible for our systematic review and meta-analysis. These studies were detected by a process that is shown in Fig. 1.

Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of study selection process.
Study characteristics
Eligible studies were conducted in the USA(Reference Gebauer, West and Kay13,Reference Sheridan, Cooper and Erario21–Reference Wilson, Young and Anderson24,Reference Nieman, Scherr and Luo26,Reference Sauder, McCrea and Ulbrecht28,Reference Carughi, Bellisle and Dougkas29) , Turkey(Reference Sari, Baltaci and Bagci9), India(Reference Kasliwal, Bansal and Mehrotra15,Reference Gulati, Misra and Pandey25) , Spain(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8) and Iran(Reference Parham, Heidari and Khorramirad27). Studies were published between 2007 and 2019. Eight studies(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8,Reference Sari, Baltaci and Bagci9,Reference Gebauer, West and Kay13,Reference Sheridan, Cooper and Erario21,Reference West, Gebauer and Kay23,Reference Nieman, Scherr and Luo26–Reference Sauder, McCrea and Ulbrecht28) contained a parallel design, and five(Reference Kasliwal, Bansal and Mehrotra15,Reference Li, Song and Nguyen22,Reference Wilson, Young and Anderson24,Reference Gulati, Misra and Pandey25,Reference Carughi, Bellisle and Dougkas29) contained a crossover design. Among the included studies, one study was conducted in men only(Reference Nieman, Scherr and Luo26), while another study(Reference Carughi, Bellisle and Dougkas29) was conducted in women only. The majority of studies included both sexes(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8,Reference Sari, Baltaci and Bagci9,Reference Gebauer, West and Kay13,Reference Kasliwal, Bansal and Mehrotra15,Reference Sheridan, Cooper and Erario21–Reference Gulati, Misra and Pandey25,Reference Parham, Heidari and Khorramirad27,Reference Sauder, McCrea and Ulbrecht28) . In total, 563 subjects were enrolled in these studies(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8,Reference Sari, Baltaci and Bagci9,Reference Gebauer, West and Kay13,Reference Kasliwal, Bansal and Mehrotra15,Reference Sheridan, Cooper and Erario21–Reference Carughi, Bellisle and Dougkas29) . The mean age of the participants ranged from 22 to 60 years, and the mean BMI ranged from 21 to 35 kg/m2. Trial duration ranged from 2 to 24 weeks. Pistachio supplementation dosage varied from 25 to 126 g/d in these studies, comprising 10–20 % of the total daily energy intake. These studies were conducted on subjects with moderate hypercholesterolaemia(Reference Sheridan, Cooper and Erario21), CVD(Reference Gebauer, West and Kay13), obesity(Reference Li, Song and Nguyen22), dyslipidaemia(Reference Kasliwal, Bansal and Mehrotra15,Reference West, Gebauer and Kay23) , the metabolic syndrome(Reference Gulati, Misra and Pandey25) and type 2 diabetes(Reference Parham, Heidari and Khorramirad27,Reference Sauder, McCrea and Ulbrecht28) , as well as on individuals who were overweight(Reference Wilson, Young and Anderson24), cyclists(Reference Nieman, Scherr and Luo26) or healthy(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8,Reference Sari, Baltaci and Bagci9,Reference Carughi, Bellisle and Dougkas29) . General characteristics of eligible studies are summarised in Table 1.
Risk of bias assessment
Random allocation of participants was shown in all included studies except for one(Reference Sari, Baltaci and Bagci9). The method of random sequence generation has been described in four trials(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8,Reference Li, Song and Nguyen22,Reference Gulati, Misra and Pandey25,Reference Parham, Heidari and Khorramirad27) , whereas the other trials have an unclear risk of bias(Reference Sari, Baltaci and Bagci9,Reference Gebauer, West and Kay13,Reference Kasliwal, Bansal and Mehrotra15,Reference Sheridan, Cooper and Erario21,Reference West, Gebauer and Kay23,Reference Wilson, Young and Anderson24,Reference Nieman, Scherr and Luo26,Reference Sauder, McCrea and Ulbrecht28,Reference Carughi, Bellisle and Dougkas29) . Allocation concealment of one trial(Reference Sauder, McCrea and Ulbrecht28) has been reported, while the other studies(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8,Reference Sari, Baltaci and Bagci9,Reference Gebauer, West and Kay13,Reference Kasliwal, Bansal and Mehrotra15,Reference Sheridan, Cooper and Erario21–Reference Parham, Heidari and Khorramirad27,Reference Carughi, Bellisle and Dougkas29) indicated unclear risk of bias. Risk of bias regarding blinding of participants and personnel indicated high risk of bias in all of the trials(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8,Reference Sari, Baltaci and Bagci9,Reference Gebauer, West and Kay13,Reference Kasliwal, Bansal and Mehrotra15,Reference Sheridan, Cooper and Erario21–Reference Carughi, Bellisle and Dougkas29) . Low risk of bias has been indicated in three trials(Reference Gebauer, West and Kay13,Reference Sheridan, Cooper and Erario21,Reference Sauder, McCrea and Ulbrecht28) based on blinding of outcome assessment. All of the included studies(Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora8,Reference Sari, Baltaci and Bagci9,Reference Gebauer, West and Kay13,Reference Kasliwal, Bansal and Mehrotra15,Reference Sheridan, Cooper and Erario21–Reference Carughi, Bellisle and Dougkas29) showed low risk of bias based on incomplete outcome data and selective reporting. Details of the risk of bias assessment are described in Fig. 2 and online Supplementary Table S3.
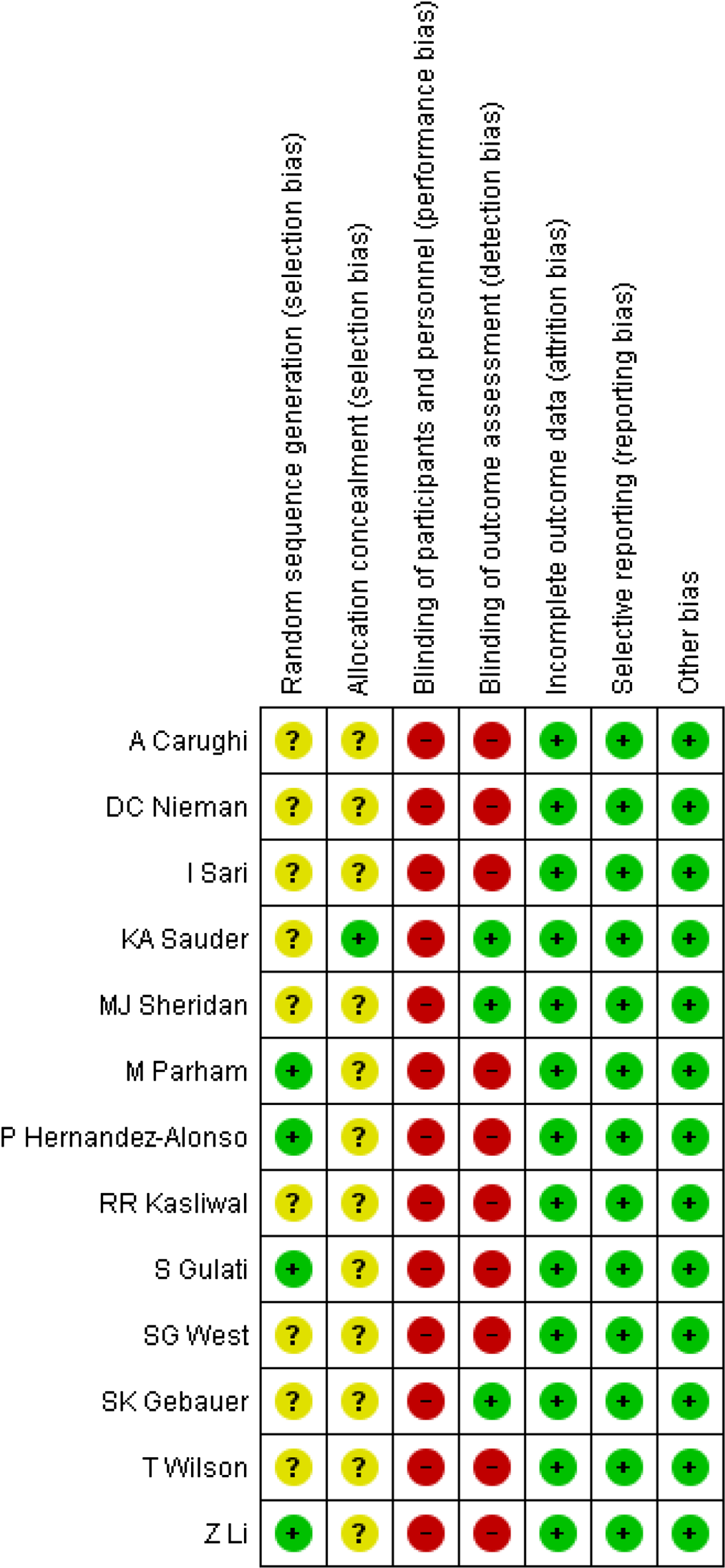
Fig. 2. Risk of bias assessment of included studies.
Meta-analysis
Effect of pistachio supplementation on anthropometric indices
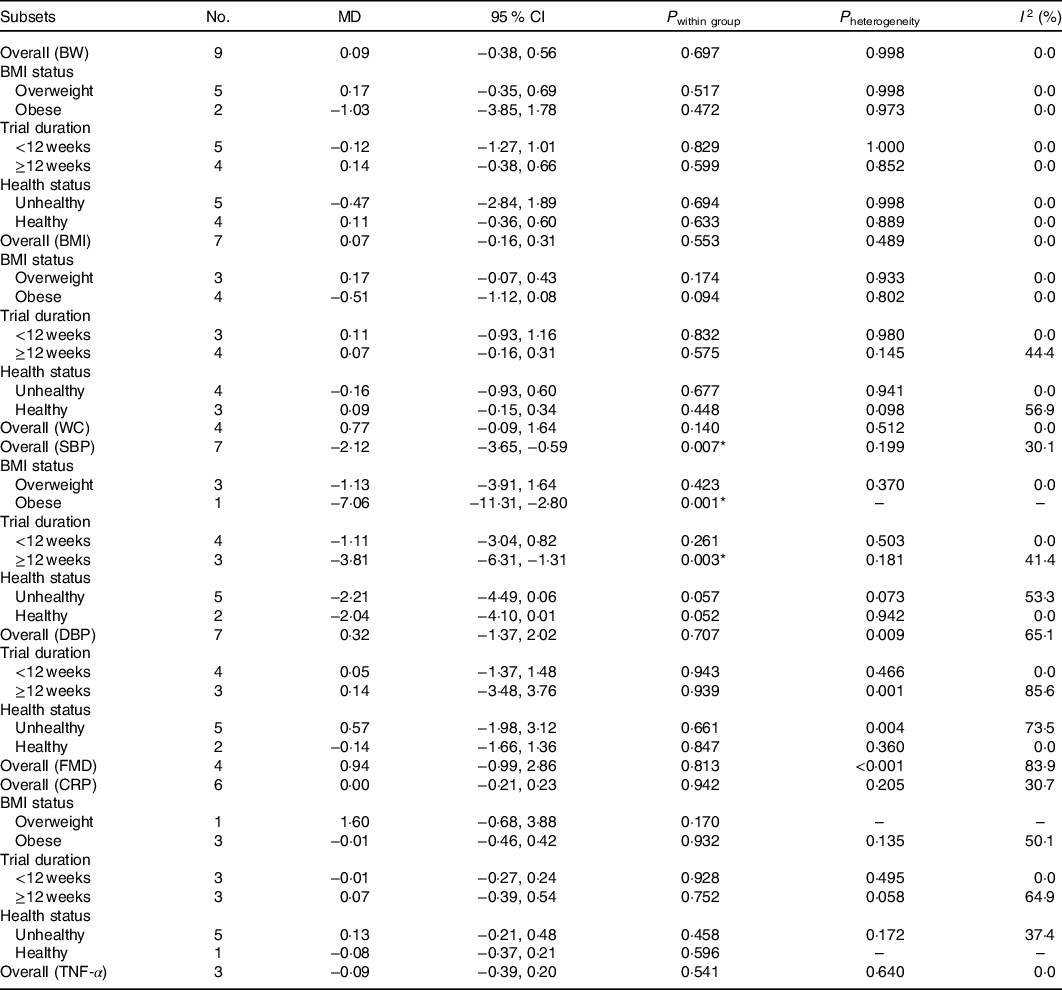
Trials investigating the effects of pistachios on anthropometric indices (BW, BMI and WC) as desired outcomes included four studies (four effect sizes), while the effects of pistachios on BW, BMI and WC were evaluated in seven studies (seven effect sizes). Fig. 3 represents the forest plot of the pooled effect of pistachios on BW, BMI and WC. The results showed that pistachios did not have a significant effect on BW (MD: 0·09 kg, 95 % CI −0·38, 0·69, P = 0·697), and there was a lack of significant heterogeneity among the studies (I 2 0·0 %, P = 0·998). Furthermore, BMI was not significantly affected by pistachios (MD: 0·07 kg/m2, 95 % CI −0·16, 0·31, P = 0·553), and there was no evidence of heterogeneity among the studies (I 2 0·0 %, P = 0·489). Finally, pistachios did not confer a change in WC within the studies (MD: 0·77 cm, 95 % CI −0·09, 1·64, P = 0·140), and there was a lack of heterogeneity (I 2 0·0 %, P = 0·512). Upon further subgroup analyses, the results were unchanged and can be found in Table 2.
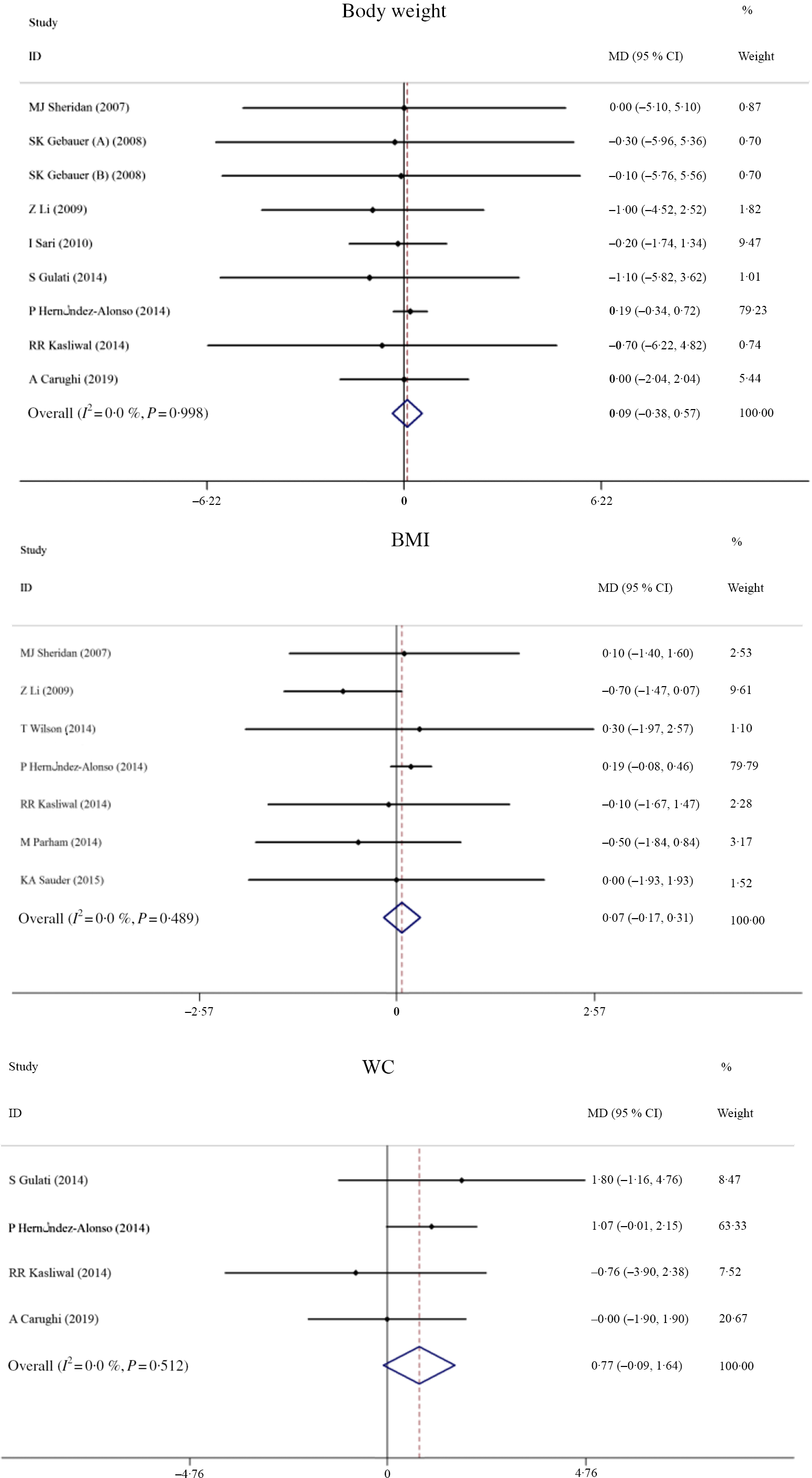
Fig. 3. Forest plot of the effect of pistachio consumption on anthropometric indices. MD, mean difference; WC, waist circumference.
Table 2. Overall and subgroup analyses of pistachio consumption on body weight (BW), BMI, waist circumference (WC), flow-mediated dilation (FMD), systolic blood pressure (SBP), diastolic blood pressure (DBP), C-reactive protein (CRP) and TNF-α
(Mean differences (MD) and 95 % confidence intervals)
Effect of pistachio supplementation on flow-mediated dilation and blood pressure
The effects of pistachio supplementation on FMD were determined by pooling effect sizes from three studies with four effect sizes (the study of West et al. (Reference West, Gebauer and Kay23) included two intervention arms). The results indicated a non-significant effect of pistachio supplementation on FMD (MD: 0·94 %, 95 % CI −0·99, 2·86, P = 0·813); however, there was significant heterogeneity between studies (I 2 83·9 %, P < 0·001) (Fig. 4). The overall effect of pistachios on DBP was not significant (MD: 0·32 mmHg, 95 % CI −1·37, 2·02, P = 0·707), but there was considerable heterogeneity between studies (I 2 65·1 %, P = 0·009). The results indicated that pistachios could reduce SBP (MD: −2·12 mmHg, 95 % CI −3·65, −0·59, P = 0·007), and the included studies lacked considerable heterogeneity (I 2 30·1 %, P = 0·199) (Fig. 5). These results were found in studies lasting ≥12 weeks (MD: −3·81 mmHg, 95 % CI −6·31, −1·31, P = 0·003) that included obese subjects (MD: −7·06 mmHg, 95 % CI −11·31, −2·80, P = 0·001) (Table 2).
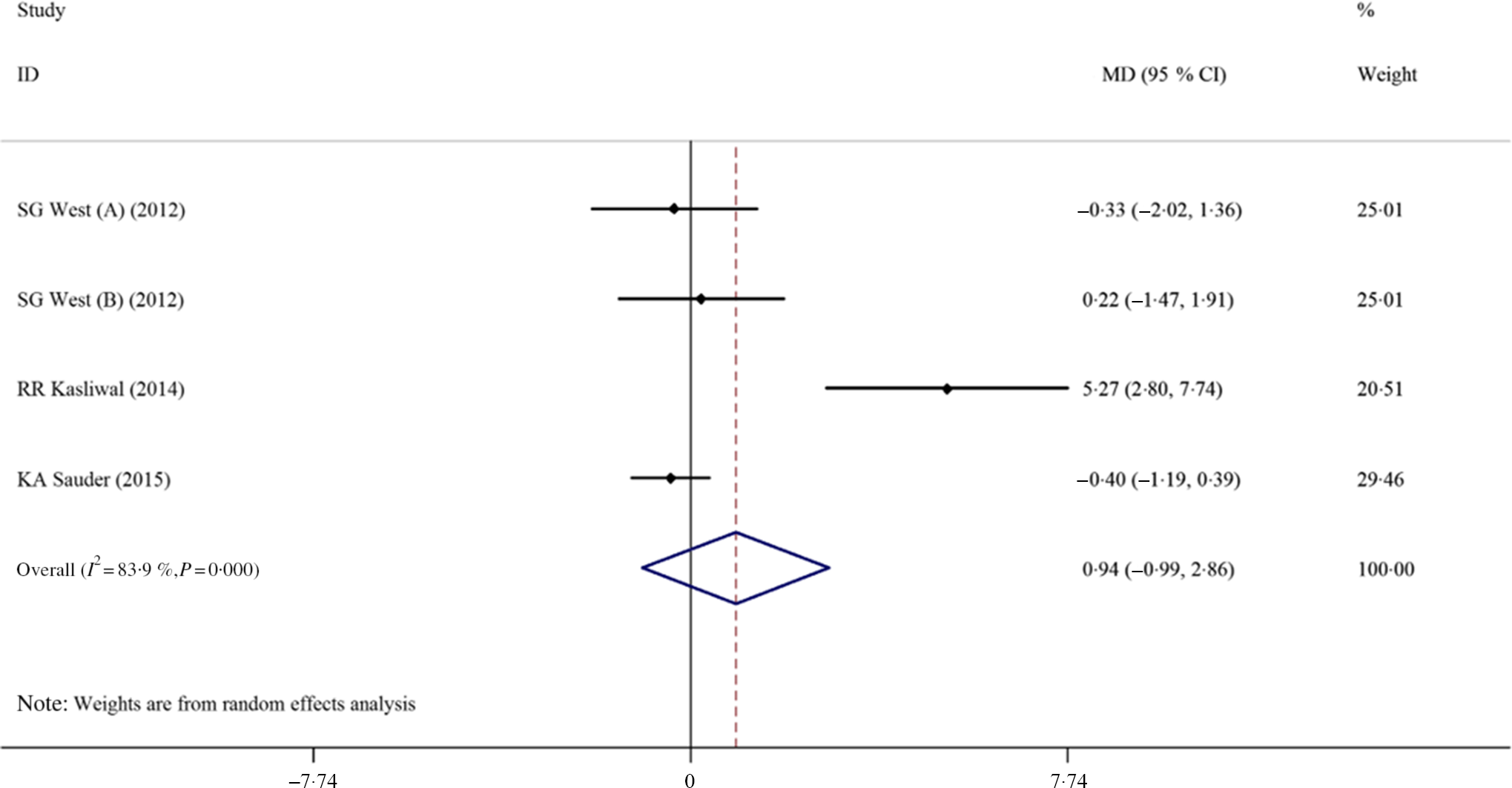
Fig. 4. Forest plot of the effect of pistachio consumption on flow-mediated dilation. MD, mean difference.
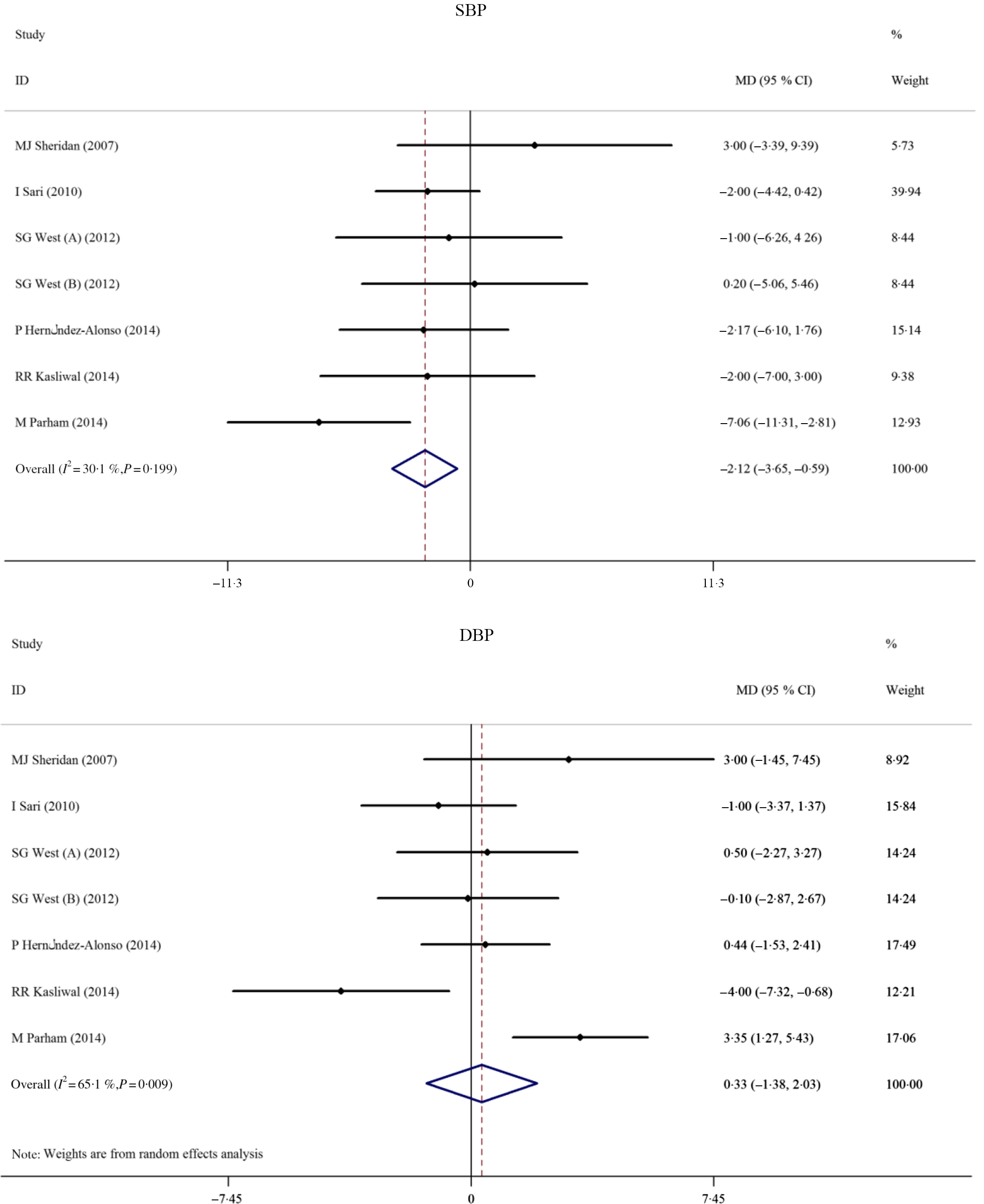
Fig. 5. Forest plot of the effect of pistachio consumption on blood pressure. SBP, systolic blood pressure; MD, mean difference; DBP, diastolic blood pressure.
Effect of pistachio supplementation on inflammation
The effects of pistachio intake on CRP and TNF-α were evaluated by pooling effect sizes from six and three studies, respectively. The results showed that pistachio consumption had no significant effect on CRP (MD: 0·00 mg/l, 95 % CI −0·21, 0·23, P = 0·942) with no evidence of heterogeneity between studies (I 2 30·7 %, P = 0·205). Additionally, pistachios did not significantly affect TNF-α (MD: −0·09 pg/ml, 95 % CI −0·38, 0·20, P = 0. 0·541), and there was a lack of considerable heterogeneity between studies (I 2 0·0 %, P = 0·640). Results can be found in Fig. 6. Due to the lack of adequate effect sizes, the subgroup analysis was performed only for the CRP; however, the results were not different in the analysis of subgroups (Table 2). Furthermore, the lack of eligible studies evaluating the effects of pistachios on IL-6(Reference Sari, Baltaci and Bagci9,Reference Nieman, Scherr and Luo26) did not allow for meta-analysis, as at least three eligible studies are needed for performing meta-analysis.

Fig. 6. Forest plot of the effect of pistachio consumption on inflammatory markers. CRP, C-reactive protein; MD, mean difference.
Publication bias
To identify whether publication bias was present, Egger’s regression test was considered. The results did not reveal significant bias in the publications regarding BMI (P = 0·248), WC (P = 0·308), SBP (P = 0·244), DBP (P = 0·497), FMD (P = 0·312), CRP (P = 0·221) or TNF-α (P = 0·692). However, Egger’s regression test did identify potential publication bias for BW (P = 0·010).
Sensitivity analysis
A sensitivity analysis was conducted to evaluate the contribution of each study to the overall estimated effect size. The pooled effect was not significantly changed by excluding individual studies reporting BW, BMI, WC, DBP, FMD or CRP. However, by removing one study(Reference Parham, Heidari and Khorramirad27), the overall effect of pistachios on SBP was altered (MD: −1·39 mmHg, 95 % CI −3·02, 0·24). Additionally, removing the studies by Kasliwal et al. (Reference Kasliwal, Bansal and Mehrotra15) and Carughi et al. (Reference Carughi, Bellisle and Dougkas29) significantly altered the effect of pistachios on WC. Results after the removal of each study were (MD: 0·89 cm, 95 % CI 0·00, 1·79) and (MD: 0·97 cm, 95 % CI 0·00, 1·94). Furthermore, by eliminating one(Reference Sari, Baltaci and Bagci9) of two studies, the effect of pistachios on IL-6 was significantly changed (MD: −0·16 pg/ml, 95 % CI −0·68, 0·36).
Discussion
To the best of our knowledge, the current systematic review and meta-analysis is the first to consider the evidence for pistachios to reduce risk for CVD-related factors, including SBP and DBP, endothelial dysfunction and inflammation. This systematic review and meta-analysis included thirteen RCT and 563 participants. The pooled sample sizes present in the studies in our review are somewhat small. Overall, pistachio consumption is supported for reductions in SBP, but no effects were detected for DBP, FMD, CRP or TNF-α. Due to a high level of heterogeneity in FMD and DBP parameters, findings must be interpreted with great caution.
Our results indicated that pistachio consumption is effective at lowering SBP, especially in trials with a follow-up period of ≥12 weeks. The reduction in SBP reported by the current meta-analysis is modest. However, even a 2 mmHg decrease in SBP would reduce the risk for stroke and myocardial infarction by 4 %(Reference Selmer, Kristiansen and Haglerød32). A previous meta-analysis that considered the effects of tree nuts, peanuts and soya nuts on blood pressure found that nuts, as a whole, did not significantly lower SBP, but pistachios did lower SBP(Reference Mohammadifard, Salehi-Abargouei and Salas-Salvadó16). In the current meta-analysis, beneficial effects on SBP were detected among obese individuals consuming pistachios for at least 12 weeks’ duration. Our findings support previous findings that pistachios did not produce a significant change in FMD(Reference Fogacci, Cicero and Derosa20). Additional studies have also reported that pistachios exert beneficial effects on hypercholesterolaemia, including a reduction in total cholesterol, LDL and TAG as well as an increase in HDL(Reference Lippi, Cervellin and Mattiuzzi33,Reference London, Pawlak and Colby34) . Taken together, the available literature suggests that pistachios may promote cardiovascular health.
Mechanistic insight into the effects of pistachios on SBP has not been fully elucidated, but plausible explanations have been suggested. Previous work suggests that phytosterols, MUFA and arginine might be the most beneficial compounds for promoting a reduction in SBP, whereas lutein, β-carotene and γ-tocopherol may produce a larger effect on IL-6. Nevertheless, the synergistic benefits of these nutrients and many others, such as fibre, plant proteins, antioxidants, flavonoids and other micronutrients, likely contribute to the overall benefits garnered from pistachio consumption(Reference Mohammadifard, Salehi-Abargouei and Salas-Salvadó16). Additional work to provide mechanistic insight into the role that pistachios may play in CVD reduction, and particularly reductions in SBP, is warranted.
Furthermore, attention should be given to the total energy provided by pistachios, which includes about 564 kcal (2360 kJ) per 100 g(Reference Dreher6). However, we showed here that pistachio consumption did not affect any of anthropometric indices. While other studies have acknowledged that nuts are energy-dense foods, current scientific evidence does not suggest an association between nut consumption and weight gain(Reference Flores-Mateo, Rojas-Rueda and Basora35). On the contrary, pistachio consumption has been associated with a lower risk of obesity, which is likely due to associated feelings of satiety and fullness that may potentially decrease consumption of unhealthy snacks(Reference Bes-Rastrollo, Wedick and Martinez-Gonzalez36,Reference Freisling, Noh and Slimani37) .
While benefits of pistachios were found in the present systematic review and meta-analysis, these results should be interpreted with some caution. The potential risk of bias from random allocation was evident in seven of the thirteen studies due to a lack of transparency, and one study did not include random allocation. The risk of bias from blinding participants and study personnel was suggested systematically. Furthermore, somewhat small sample sizes and short study durations present in the studies in our review could influence the overall results. Heterogeneity was also observed in participant characteristics, particularly health status, and in background and control diets. In addition, the present study was not registered in the International Prospective Register of Systematic Reviews (PROSPERO), which may be a limitation as well. However, this review and meta-analysis was designed and performed according to the Cochrane guidelines.
In conclusion, the cardio-protective effects of pistachios are supported by previous work and probable mechanisms of action have been suggested. Of interest, demonstrable reductions in SBP were found in the current analysis. To more clearly understand the effectiveness of pistachios in the improvement of cardiovascular health, additional work is needed.
Acknowledgements
The present work did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
O. A. and E. G. designed and conceived the study, searched databases, screened articles and extracted data. E. G. performed the statistical analyses. O. A. and E. G. interpreted the results and drafted the manuscript with contributions from A. H. All authors reviewed and commented on subsequent drafts of the manuscript. M. S. C. critically revised the manuscript. O. A. and E. G. have the primary responsibility for the final content.
The authors declare that they have no competing interests.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520004523











