Introduction
Identifying causes of death and assessing their prevalence is fundamental in understanding species’ population dynamics, and targeting the reduction of mortality in endangered populations. A good understanding of the causes and rates of mortality is therefore of vital importance for the conservation of endangered species.
Compared to other demographic parameters of wildlife populations, mortality is difficult to estimate (McCallum Reference McCallum2000) due to difficulties in capturing and monitoring animals, which usually results in small sample sizes, and the uncertainty about the fate of a large proportion of tracked individuals. Nevertheless, the increasing use of remote tracking devices and the improvement in capture techniques has facilitated mortality and survival studies in free-ranging animals (Krebs Reference Krebs1999, Kenward Reference Kenward2001, Millspaugh and Marzluff Reference Millspaugh and Marzluff2001).
While natural threats are less subject to human control, sources of anthropogenic mortality can be challenging to manage (Loss et al. Reference Loss, Will and Marra2012). This is the case with mortality caused by overhead power lines, wind farms and buildings, or hunting and illegal killing (Erickson et al. Reference Erickson, Johnson and Young2005, Tourenq et al. Reference Tourenq, Combreau, Lawrence, Pole, Spalton, Xinji, Al Badani and Launay2005, Loss et al. Reference Loss, Will and Marra2014, Silva et al. Reference Silva, Palmeirim, Alcazar, Correia, Delgado and Moreira2014).
The Little Bustard Tetrax tetrax (Linnaeus, 1758) is currently classified as globally ‘Near Threatened’ (BirdLife International 2016). Its present breeding distribution is fragmented and concentrated in two main regions: one centered in south-eastern European Russia and Kazakhstan, and a second in the Iberian Peninsula, France, Sardinia and Morocco (Cramp and Simmons Reference Cramp and Simmons1980, del Hoyo et al. Reference Del Hoyo, Elliott and Sargatal1996, Palacín and Alonso Reference Palacín and Alonso2009, BirdLife International 2016). Over recent decades the population has been declining mainly in its western range. In western France, for example, between the 1980s and late 1990s the population suffered an estimated decline of 92% (Jolivet Reference Jolivet1997). Because of this declining trend, the species is classified as ‘Vulnerable’ in the Iberian Peninsula (Madroño et al. Reference Madroño, González and Atienza2004, Cabral et al. Reference Cabral, Almeida, Almeida, Ferrand de Almeida, Oliveira, Palmeirim, Queiroz, Rogado and Santos-Reis2005) and is considered a priority species under the European Union Wild Birds Directive (2009/147/EC), which has led to the designation of many steppe areas as Special Protection Areas (SPA).
The Iberian Peninsula holds the main stronghold of the species in Europe (Iñigo and Barov Reference Iñigo and Barov2010). The Spanish population was estimated between 29,000 and 48,000 individuals (García de la Morena et al. Reference García de la Morena, Bota, Ponjoan and Morales2006), and the Portuguese population was estimated at 17,500 males (Silva and Pinto Reference Silva and Pinto2006). Over the last 10 years, alarming declines have been reported for several regions of Iberia, such as Catalonia, Central Spain and Extremadura, with regional losses between 50% and 70% of the breeding population (Morales et al. Reference Morales, Garcia de la Morena, Delgado and Traba2006a, Reference Morales, Traba and Arroyo2015, De Juana Reference De Juana2009, Mañosa et al. Reference Mañosa, Bota, Estrada and Cuscó2015).
A number of factors are contributing to the Little Bustard’s decline. A major overall threat is agricultural intensification, which leads to habitat loss and degradation (e.g. Goriup Reference Goriup, Tucker and Heath1994, Morales et al. Reference Morales, Bretagnolle and Arroyo2005a, Reference Morales, Suarez and Garcia de la Morena2006b, García et al. Reference García, Suárez-Seoane, Miguélezc, Osborne and Zumalacárreguie2007). In Iberian landscapes, agricultural intensification leads to the decreasing use of traditional crop rotation systems and suppression of fallow land, which is a key breeding habitat for the species (Martínez Reference Martínez1994, Morales et al. Reference Morales, García and Arroyo2005b, Moreira et al. Reference Moreira, Silva, Estanque, Palmeirim, Lecoq, Pinto, Leitão, Alonso, Pedroso, Santos, Catry, Silva, Henriques and Delgado2012). In France, nest destruction during harvesting has also been reported as a main factor of decline (Inchausti and Bretagnolle Reference Inchausti and Bretagnolle2005).
Adult survival has also been pointed out as one of the most important demographic parameters affecting Little Bustard population viability (Morales et al. Reference Morales, Bretagnolle and Arroyo2005a, Inchausti and Bretagnolle Reference Inchausti and Bretagnolle2005), highlighting the importance of knowing mortality rates as well as their underlying causes. There is, however, a great dearth of information on these topics. Exceptions include the study by Schulz (Reference Schulz1987), who found that predation is the most common natural cause of death for Little Bustard. In fact, its behaviour seems to be determined, in great measure, by an anti-predator strategy that includes flocking outside the breeding season and selecting habitats with a vegetation structure that provides both cover and visibility (Silva et al. Reference Silva, Pinto and Palmeirim2004, García de la Morena Reference García de la Morena2016). Collisions with overhead power lines have also been described as an important source of non-natural mortality (Silva et al. Reference Silva, Pinto and Palmeirim2010, unpubl. data). A rough estimate based on the number of dead birds found next to power lines indicated that 1.5% of the Portuguese national population could be killed annually by these structures (Silva et al. Reference Silva, Pinto and Palmeirim2014). Other sources of human induced mortality, such as illegal killing, have also been identified as threats, but have never been quantified (Iñigo and Barov Reference Iñigo and Barov2010).
The purpose of this paper is to assess the causes of adult mortality and estimate the relative importance of each of the identified causes in the Iberian Peninsula. This information is essential to outline a future strategy aiming to set a conservation plan to reverse the decline of this threatened steppe-land bird. Based on a joint effort, all tracking data on adult Little Bustards collected by the Iberian research teams over a period of 12 years were used for this paper.
Methods
Study area
The study was carried out in the Iberian Peninsula, within several areas holding important populations of Little Bustard, namely north-eastern Iberia (Catalonia and Aragón), central Iberia (Madrid, Castilla-La Mancha, Castilla-León) and south-western Iberia (Extremadura in Spain and Alentejo in Portugal). In central and north-eastern Iberian areas, the agricultural landscape is dominated mainly by dry cereal farmland of varying degrees of intensification (Mañosa et al. Reference Mañosa, Bota, Estrada and Cuscó2015, Morales et al. Reference Morales, Traba and Arroyo2015). Conversely, in south-western Iberia the landscape is characterised by larger fields and considerable amounts of grasslands, especially in Portugal (Moreira et al. Reference Moreira, Silva, Estanque, Palmeirim, Lecoq, Pinto, Leitão, Alonso, Pedroso, Santos, Catry, Silva, Henriques and Delgado2012).
Data collection
We collected mortality data from 151 adult individuals that were tagged in several areas of the Iberian Peninsula (Figure 1) from 2001 to 2013. A total of 52 females and 99 males were tracked by different technologies in different areas: VHF tracking, PTT GPS and PTT Doppler, and with different data acquisition frequencies (Table 1). Life expectancy of the transmitters ranged between one and three years for VHF tracking and above three years for solar PTT GPS and PTT Doppler. For males, the capture was made with a stuffed female used as a decoy, with trap loops around it. Individuals that were attracted and fell into the traps were fitted with harnesses with transmitters attached. Females were captured with the funnel trap method (Ponjoan et al., Reference Ponjoan, Bota and Mañosa2010). In all cases the tracking device was fitted to the bird using Teflon ribbon harnesses (Kenward Reference Kenward2001). When a tracking signal was lost, or when the mortality sensor was activated or indicated immobility for a long period of time (Burnside et al. Reference Burnside, Collar, Scotland and Dolman2016), the individual’s last position was field-checked. The individual’s status was set to censored (lost to follow-up) if no carcass, remains or transmitter were subsequently found, or when the tag reached the end of its functional time. In these cases, the individual was accounted as alive until the last day of appearance. Instead, if a carcass, a feather spot or a transmitter were located, the likely cause of death was assessed based on the signs found on the remains or the transmitter, combined with local evidence and the circumstances surrounding the place and time of death. Causes of death were categorised as human or natural. Anthropogenic deaths were classified as (a) collision with overhead transmission lines, if the bird was found near or underneath overhead transmission power lines with clear signs of trauma; (b) vehicle collision, if the carcass was found next to a road, with hard trauma injury, no evident signs of illegal killing nor near power lines; or (c) illegal killing, if the carcass was found with pellets or showing pellet wounds; transmitter with pellet impacts or Teflon ribbon showing pellet marks.
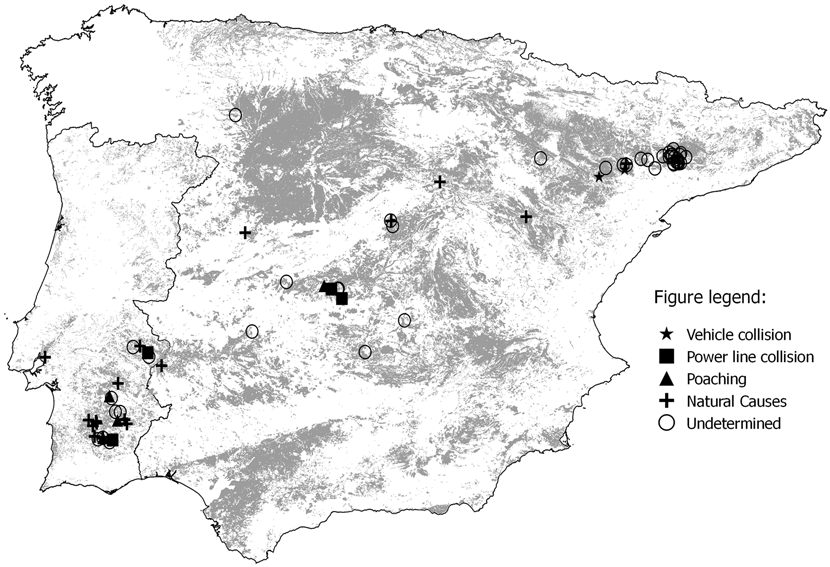
Figure 1. Symbols indicate the location of dead individuals recorded between 2001 and 2013 used in the cause-specific analysis. The areas in light grey represent the potential habitat for the Little Bustard, i.e. land uses most frequented by the species (Martínez, Reference Martínez1994, Silva et al. Reference Silva, Palmeirim, Alcazar, Correia, Delgado and Moreira2004, 2007, Morales et al. Reference Morales, Suarez and Garcia de la Morena2006b).
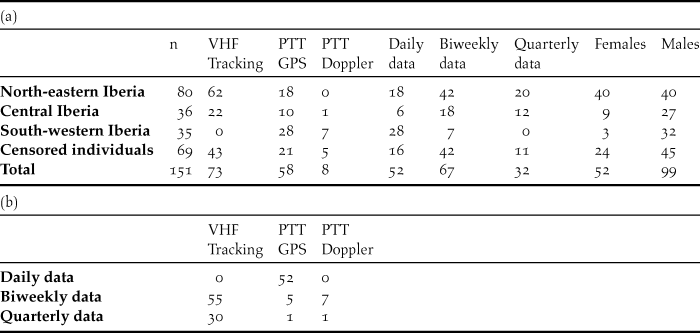
Table 1. (a) Number of birds, tracking methods, data frequency and sex per region; censored individuals. (b) Number of individuals per tracking method and data frequency.
Natural deaths corresponded to predation events, and for most cases it was possible to distinguish predation by birds of prey from predation by mammals. In the presence of broken bones, or feathers still attached to the remains or without remaining feather quills, or even bite marks on the tag’s plastic cover, the predator was presumably a mammal (Brown et al. Reference Brooks, Bonyongo and Harris2003, Fraigneau Reference Fraigneau2008). In contrast, if the carcass was left whole, with feathers with remaining broken section quill around it, a bird of prey was the probable predator (Brown et al. Reference Brown, Ferguson, Lawrence and Lees2003). In cases when there was information on whether the predated carcass was near a power line of road, the cause of dead was assigned to collisions with the latter, as many dead carcasses resulting from collisions are scavenged. If the cause of death was not clearly identified, it was set to “undetermined” or, in case of obvious signs of human manipulation (birds with Teflon ribbon cuts or manipulation or buried tags without signs of the carcass), to “undetermined with human manipulation”.
Given that it is not always possible to distinguish between a transmitter failure/end of natural battery life or actual death, mortality rate estimation was carried out using two models: model 1 – where we followed the classification above and censored individuals were not considered to be mortalities (Pollock and Winterstein Reference Pollock and Winterstein1989) being accounted until the last day of appearance; and model 2 – where all censored cases were classified as undetermined deaths.
Because the Little Bustard in particular is susceptible to capture myopathy (Marco et al. Reference Marco, Mentaberre, Ponjoan, Bota, Mañosa and Lavín2006, Ponjoan et al. Reference Ponjoan, Bota, De La Morena, Morales, Wolff, Marco and Mañosa2008), which can affect the bird’s mobility up to 11 days after capture and consequently rendering it vulnerable to predation (Ponjoan et al. Reference Ponjoan, Bota, De La Morena, Morales, Wolff, Marco and Mañosa2008), we only analysed mortality events that occurred after a first tracking period of 25 days to avoid confounding effects between natural death and capture myopathy. Our total sample of 151 tracked animals does not include those cases.
Data analysis
The date of death or disappearance was assumed to be the median time between the date of the last live record and the date when the carcass was found (range 0–67 days, mean = 2.32, SD = 8.97). Censored individuals were not considered to be mortalities (Pollock and Winterstein Reference Pollock and Winterstein1989) and were only accounted for until the last day of appearance.
Transmitter weight can influence animal mortality, and consequently affect the final study results (Wilson and McMahon Reference Wilson and McMahon2006, Brooks et al. Reference Brooks, Bonyongo and Harris2008, Casper Reference Casper2009). Backpack transmitters, like those used in our study, fitted with a full harness, have the advantage of not affecting the bird’s balance (Irvine et al. Reference Irvine, Leckie and Redpath2007). The medium weights of the backpacks (i.e. transmitter plus harness) was 4.54% (SD ± 0.69) of the weight of the bustards, therefore below the maximum recommended 5% of body weight for harness mounts (Kenward Reference Kenward2001). In order to evaluate if there was an bias effect of the tracking device weight on mortality, we carried out a Kaplan-Meier analysis (Kaplan and Meier Reference Kaplan and Meier1958), and a Log-Rank Test (Harrington and Fleming Reference Harrington and Fleming1982), to compare the distributions of the backpack weights.
Descriptive statistics were used to characterise the relative prevalence of anthropogenic and natural deaths in the adult population. The Heisey and Fuller estimator (Heisey and Fuller Reference Heisey and Fuller1985) was used to compute annual mortality rates, i.e. the percentage of the population that died each year due to each type of causal factor. When extended for multiple causes of death, the Heisey and Fuller estimator states that the daily mortality rate due to a particular death cause (j) is the probability that an animal alive at the beginning of a day in interval i dies during the day due to this cause. The maximum likelihood estimator of this probability m ij is the number of deaths in interval i due to cause j (y ij), divided by the total number of transmitter days in the interval (xi): ![]() ${\hat{m}_{ij}} = {y_{ij}}/{x_i}$. The probability that an animal dies from cause j during the interval i (M ij) is the sum of the probabilities that it survives to a particular day, and then dies on that day from cause j. This result is expressed as
${\hat{m}_{ij}} = {y_{ij}}/{x_i}$. The probability that an animal dies from cause j during the interval i (M ij) is the sum of the probabilities that it survives to a particular day, and then dies on that day from cause j. This result is expressed as ![]() ${M_{ij}} = {m_{ij}} + {s_i}{m_{ij}} + s_i^2{m_{ij}} + \ldots + {s^{\left( {{L_i} - 1} \right)}}{m_{ij}} = \left( {{{{m_{ij}}} \over {1 - {s_i}}}} \right)(1 - s_i^{{L_i}})$, where s i is the daily survival rate for the interval and L i is the length in days of interval i. This estimator was computed using the software MICROMORT 1.3 (Heisey and Fuller Reference Heisey and Fuller1985).
${M_{ij}} = {m_{ij}} + {s_i}{m_{ij}} + s_i^2{m_{ij}} + \ldots + {s^{\left( {{L_i} - 1} \right)}}{m_{ij}} = \left( {{{{m_{ij}}} \over {1 - {s_i}}}} \right)(1 - s_i^{{L_i}})$, where s i is the daily survival rate for the interval and L i is the length in days of interval i. This estimator was computed using the software MICROMORT 1.3 (Heisey and Fuller Reference Heisey and Fuller1985).
Results
Out of the 151 tracked individuals followed over a total of 76,182 radio-tracking days, and after censoring individuals whose transmitter stopped working, we recorded 82 mortality events and 69 censored individuals that entered the analysis - a summary of the data is presented as a Kaplan-Meier survival curve (Figure 2). Cases of possible failed transmitters were a small percentage (8.6%). Predation was responsible for 26% of the total recorded mortality. Birds of prey were the main predators identified, with a prevalence of 55.6%, while mammals were responsible for 38.9% of predation events. Cases classified as natural deaths but for which it was not possible to recognize the type of predator corresponded to 5.6%. Anthropogenic death causes represented 18% of the detected mortality events. Illegal killing was the most common source of anthropogenic mortality (10%), followed by power line collision (6%) and vehicle collision (2%). Undetermined mortality corresponded to 57% of the mortality cases.
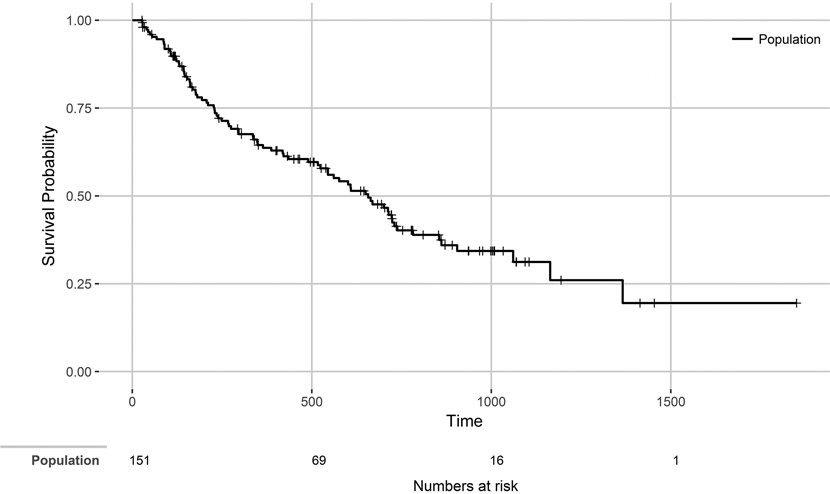
Figure 2. Plot of Kaplan-Meier product limit estimator of survival for the 151 Little Bustards with 82 mortality events. Time is displayed in days and the number of birds at risk at day 0, 500, 1,000 and 1,500 is represented below.
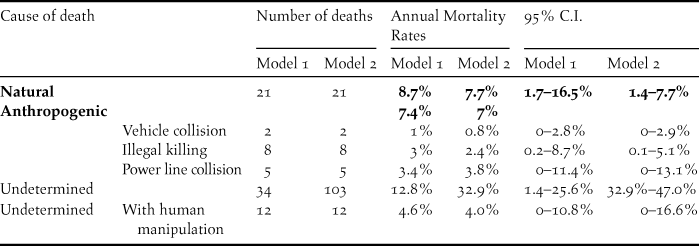
Using the Heisey and Fuller estimator, the estimated overall annual survival rate was 66.5% (C.I. 95%: 48–82%) for model 1 and 48.3% (C.I. 95%: 32–66%) for model 2. Annual cause-specific mortality rates are presented in Table 2. Both models produced similar results for cause-specific mortality rates - the yearly mortality rate of Little Bustards due to anthropogenic causes was 7% while 8% died from natural causes. Power line collision represented 3.4–3.8% of population deaths, followed by illegal killing (3–2.4%) and vehicle collision (1–0.8%) (Table 2). The estimates presented above correspond to model 1 and model 2, respectively. The Log-Rank Test did not detect a statistically significant relation between mortality and the weight of the tracking device (χ2 = 0.6, df = 1, P = 0.436).
Table 2. Annual cause-specific mortality rates and 95% confidence intervals estimated using Heisey and Fuller method (1985) for adult Little Bustards Tetrax tetrax in the Iberian Peninsula. Annual rates were computed based on a 12-year period (2001–2013).
Discussion
This is the first time that mortality rates of adult Little Bustard were estimated based on a relatively large sample of tagged birds. Previous estimates, used for the development of demographic models in France, were based on resighting data of colour-ringed birds (Inchausti and Bretagnolle Reference Inchausti and Bretagnolle2005), and might therefore be subject to different sorts of biases. This new dataset is particularly important since it relates to the species’ stronghold in Europe, contributing to an assessment of its global conservations needs.
Animal tracking allowed us not only to estimate mortality rates, but also to obtain some information on the causes of death. Although we were not able to determine with absolute certainty the cause of death of many birds, the evidence was usually enough to assess the most probable cause of death and, at the very least, to distinguish between anthropogenic and predation-related mortality. The fact that we did not find any significant effect of the weight of the transmitters on the survival of the tracked Little Bustards, indicates that our results were not affected by the tagging protocol. Although with the current tracking equipment, failure is not common, it does occur. We were not able to distinguish between possible transmitter failure and natural end of battery life and both situations were treated as censored cases. In any case, when censored data were classified as mortality by unknown causes, they yielded similar mortality estimates compared to model 1, showing that possible undetermined censored data did not represent a source of bias in the analysis. White and Garrott (Reference White and Garrott1990) refer that a low number of possible transmitter failures will not influence the analysis. Premature cessation of transmission (defined by us as < 365 days) occurred in 13 cases (8.6%).
Here we adopted a simple approach to distinguish anthropogenic from natural mortality. It is also important to note that we are assuming that the identification of the causes of death is reliable, even though it is a probable cause of death, in the absence of necropsy. In addition, we also assume that the probability of detecting anthropogenic death is the same as of detecting natural death.
We estimated an overall annual survival rate of adult Little Bustards of just 67%, similar to the 68–72% estimated for western France when the population was declining between 1998 and 2003 (Inchausti and Bretagnolle Reference Inchausti and Bretagnolle2005). It is also lower than in other vulnerable Eurasian bustards: Great Bustard Otis tarda with 90% (Palacín et al. Reference Palacín, Alonso, Martín and Alonso2017) and Houbara bustard Chlamydotis undulata with 75–86% in non-hunting areas (Hardouin et al. Reference Hardouin, Hingrat, Nevoux, Lacroix and Robert2015). As lower confidence intervals seem highly unlikely to represent the reality of the Little Bustard’s survival rates, the upper confidence intervals of both models in terms of annual survival rate (model 1: 82% and model 2: 66%), probably indicate the best-case estimates for the survival of this species. Given that only 54% of the chicks seem to survive to become adult (Schulz Reference Schulz1987), the overall annual population mortality (considering both juvenile and adult together) is likely to be higher. Productivity is a key demographic parameter for the viability of animal populations. A productivity of less than one fledging per female a year could lead to a decline in the Little Bustard population of 15% a year (Bretagnolle et al. Reference Bretagnolle, Villers, Denonfoux, Cornulier, Inchausti and Badenhausser2011). Other factors are likely to worsen this scenario in areas with more intensive agriculture, such as low productivity (Bretagnolle et al. Reference Bretagnolle, Villers, Denonfoux, Cornulier, Inchausti and Badenhausser2011, Lapiedra et al. Reference Lapiedra, Ponjoan and Gamero2011), or a biased sex ratio towards males (Inchausti and Bretagnolle Reference Inchausti and Bretagnolle2005, Morales et al. Reference Morales, Bretagnolle and Arroyo2005a, Reference Morales, Traba, Carriles, Delgado, Paula and García de la Morena2008), that may result in a decrease in productivity due to female shortage (Tarjuelo et al. Reference Tarjuelo, Delgado, Bota, Morales, Traba, Ponjoan, Hervás and Mañosa2013). Morales et al. (Reference Morales, Bretagnolle and Arroyo2005a) used simulations to show that in declining populations like those in western France, adult survival values close to 70% result in population survival probabilities of 60%, a low value for a species with ‘Near Threatened’ classification. Additional analyses are needed to assess how this level of mortality could be affecting the Iberian Peninsula population dynamics.
Anthropogenic mortality of the Iberian Little Bustard population was, surprisingly, almost as high as the annual mortality recorded by predation. These high estimated mortality rates are consistent with the species’ overall declining trend (Morales et al. Reference Morales, Traba and Arroyo2015).
Power line collision was the main anthropogenic cause of mortality identified in both models, estimated to cause the death of 3.4% (model 1) and 3.8% (model 2) of the population, yearly. These values could reach up to 11.4% and 13.1% in the highest intervals of model 1 and model 2, respectively. A comparison with previous studies (Infante et al. Reference Infante, Neves, Ministro and Brandão2005, Jenkins et al. Reference Jenkins, Smallie and Diamond2010, Silva et al. Reference Silva, Santos, Queirós and Leitão2010) shows that the annual rate of mortality due to collisions with power lines is one of the highest ever recorded for a particular species. Previous estimates of mortality caused by linear infrastructure are based on counts of Little Bustard carcasses found next to power lines or roads, and may thus be biased by factors like removal by scavengers, difficult detection and limited search area (APPLIC, 2012). Survival analysis using tracked birds provide more robust estimates of mortality rates as it is subject to fewer biases (e.g. Naef-Daenzer et al. Reference Naef-Daenzer, Korner-Nievergelt, Fiedler and Grüebler2017).
Illegal killing was identified as the second main threat to Little Bustard’s survival, with an estimated annual mortality rate of 2.4 (model 2) and 3% (model 1) by this cause. These values could reach up to 8.7% in the highest interval of the most conservative model. Even though this threat was identified as important by Iñigo and Barov (Reference Iñigo and Barov2010), its importance has never been quantified. There are few references to Little Bustard hunting tradition or illegal killing in Iberia (Smith Reference Smith1868), in contrast to other Otididae species such as the Great Bustard or Houbara Bustard, for which there is a long and well documented hunting tradition (Palacín and Alonso Reference Palacín and Alonso2009, Sehhatisabet et al. Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012, Brochet et al. Reference Brochet, Van Den Bossche, Jbour, Ndang’Ang’a, Jones, Abdou, Al-Hmoud, Asswad, Atienza, Atrash, Barbara, Bensusan, Bino, Celada, Cherkaoui, Costa, Deceuninck, Etayeb, Feltrup-Azafzaf, Figelj, Gustin, Kmecl, Kocevski, Korbeti, Kotrošan, Laguna, Lattuada, Leităo, Lopes, López-Jiménez, Lucić, Micol, Moali, Perlman, Piludu, Portolou, Putilin, Quaintenne, Ramadan-Jaradi, Ružić, Sandor, Sarajli, Saveljić, Sheldon, Shialis, Tsiopelas, Vargas, Thompson, Brunner, Grimmett and Butchart2016). In fact, hunting and illegal killing have been identified as one of the main negative factors affecting the population viability of both species in areas of North Africa and Asia (Tourenq et al. Reference Tourenq, Combreau, Lawrence, Pole, Spalton, Xinji, Al Badani and Launay2005, Alonso et al. Reference Alonso, Palacín, Martín, Mouati, Arhzaf and Azizi2005), in spite of legal protection of the Great Bustard in Morocco.
As regards predation, even though birds of prey were responsible for most of the identified cases, it was not possible to ascertain statistically if they were more important predators than mammals. However, it should be noted that the Little Bustard in Iberia is dependent on man-made landscapes and farming practices. Changes in landscapes or how these habitats are managed can play a decisive role in the rate of predation (Whittingham and Evans, Reference Whittingham and Evans2004). For example, overgrazed pastures may lead to birds being more exposed to predation due to reduced vegetation cover. Also, more intensified irrigation crops may lead to higher rates of disturbance and, finally, increased urbanisation of the rural landscape may promote increasing numbers of human-associated predators. Therefore, our designated “natural” mortality may be highly dependent on how the habitat is being transformed and/or managed.
Our work shows how poorly understood and previously unknown threats are affecting the survival of the most important Little Bustard population of Western Europe. Reducing anthropogenic mortality can thus have a major positive impact on the species’ viability, particularly for populations that show low breeding productivity (Lapiedra et al. Reference Lapiedra, Ponjoan and Gamero2011, Bretagnolle et al. Reference Bretagnolle, Villers, Denonfoux, Cornulier, Inchausti and Badenhausser2011). Anthropogenic deaths seem to have higher importance than what was initially foreseen in this species. Low awareness of the species’ conservation status among hunters is a problem, and the unfavourable population trends should be better communicated. The unexpectedly high impact of collision with power lines highlights the importance of adapting the overhead electric power line network to conservation needs. This may include the relocation of existing hazardous power lines and the routing of new ones away from areas with greater collision risk (Silva et al. Reference Silva, Palmeirim, Alcazar, Correia, Delgado and Moreira2014). New power line designs that minimise collision risk, should also be considered, including underground cabling (Raab et al. Reference Raab, Schütz, Spakovszky, Julius and Schulze2012, Silva et al. Reference Silva, Santos, Queirós and Leitão2010, Reference Silva, Palmeirim, Alcazar, Correia, Delgado and Moreira2014, Barrientos et al. Reference Barrientos, Ponce, Palacín, Martín, Martín and Alonso2012, Alcazar Reference Alcazar2013). Legislation should be drawn up at national level to ensure the integration of these preventive measures into the design of new power lines nationwide and not limited to the Natura 2000 network.
Acknowledgements
The Portuguese tracking data from 2000 and 2005 was funded by the European Union program INTERREG III-A - FAUNATRANS project, and between 2009 and 2012 was funded by EDP S.A. “Fundo EDP Biodiversidade”. Tracking data provided by the Autonomous University of Madrid was funded by projects REN 2000-0765 and CGL2004-06147-C02-02 of the Spanish Ministry of Science. University of Barcelona and Forest Science Center of Catalonia tracking program was funded by projects CGL2004-06147-C02-01 and CGL2009-13029/BOS of the Spanish Ministry of Science, and the companies REGSEGA, ASG and Infrastructures de la Generalitat de Catalunya, SAU. Aragón tracking data was funded by the Dirección General de Conservación del Medio Natural del Departamento de Agricultura, Ganadería y Medio Ambiente del Gobierno de Aragón. JM was funded by Grant SFRH/BD/114683/2016 from Fundação para a Ciência e Tecnologia. JPS was funded by Grant SFRH/BPD/72311/2010 from Fundação para a Ciência e Tecnologia.






