Coccidioidomycosis is a re-emerging infectious disease [Reference Kirkland and Fierer1] acquired by inhaling aerosolized spores of the soil-dwelling fungus Coccidioides immitis and C. posadasii. In the USA, coccidioides grows primarily in the desert soils of the San Joaquin Valley in California, southern Arizona, southern New Mexico and west Texas, and in scattered foci in coastal southern California, southern Nevada, and Utah [Reference Kirkland and Fierer1]. Symptomatic illness occurs in about 40% of persons infected with coccidioides [2]. Clinical manifestations range from influenza-like illness to progressive pulmonary disease and, in <1% of infections, potentially fatal disseminated disease. Disseminated disease occurs more often in persons who are immunocompromised, pregnant, or of black race or Filipino ancestry [2]. Older age, diabetes, and recent history of cigarette smoking are risk factors for severe pulmonary disease [Reference Rosenstein3]. Coccidioides infection usually confers immunity to re-infection [2].
From 2000 to 2006, incidence rates for coccidioidomycosis more than tripled in California, increasing from 2·4 to 8·0/100 000 statewide [2]. From 2000 to 2007, rates increased from 14·7 to 53·9/100 000 in the highly endemic San Joaquin Valley region [2]. A resurgence of coccidioidomycosis also occurred in California from 1991 to 1995 [2]. Environmental conditions favourable to fungal proliferation and airborne release, and increases in non-immune populations may contribute to periodic epidemic increases [Reference Kirkland and Fierer1, 2].
Point-source outbreaks of coccidioidomycosis are infrequently reported in the literature and have been associated with soil-disturbing activities in endemic areas such as archaeological digs, construction, military manoeuvres, and outdoor group activities [Reference Peterson4]. Persons who engage in these activities in coccidioides-endemic areas are at increased risk of infection. However, out-of-area persons, such as intermittent or temporary workers or travellers and their physicians and employers, may be unaware of these risks and waning epidemic cycles may be accompanied by decreased clinical suspicion and recognition of coccidioidomycosis.
We report here a point-source outbreak of coccidioidomycosis in a 12-person contract civilian crew following excavation and installation of an underground pipe on Camp Roberts Military Base in San Luis Obispo and Monterey counties in October 2007. This outbreak illustrates important epidemiological and clinical features of coccidioidomycosis and challenges to prevention and control of coccidioidomycosis in areas with episodic focal disease activity.
We reviewed company records to identify the work crew, the exposure period, and days worked during the exposure period. We collected from the crew demographic information, medical histories, signs and symptoms of illnesses with onset after first exposure to the outbreak site, and work activities at the outbreak site. Medical records were reviewed, as needed. We attempted to obtain acute-phase and convalescent-phase serum specimens and chest X-rays for all crew members. Acute- and convalescent-phase serum samples were assayed to detect coccidioidal immunoglobulin (Ig) M by enzyme immunoassay (EIA) and immunodiffusion (ID) and to detect coccidioidal IgG by ID and complement-fixing antibody titres (CF). Kern County Public Health Laboratory conducted ID and CF serological testing utilizing protocols and reagents similar to those of the University of California Davis Serology Laboratory [Reference Pappagianis and Zimmer5] except serum specimens were not concentrated prior to assay. In general, coccidioidal serological tests are highly sensitive although concentrating sera prior to assay increases sensitivity [Reference Peterson4, Reference Pappagianis and Zimmer5]. A commercial test kit (Meridian Bioscience Inc., USA) was used to detect IgM by EIA. Compared to ID, EIA has been found to be 100% sensitive and 96% specific but an isolated EIA reactive to IgM (with no other evidence of seroconversion) may be falsely positive [Reference Kaufman6].
We defined clinically compatible illness as one or more of the following: influenza-like signs and symptoms, pneumonia or other pulmonary lesion, erythema nodosum or multiforme rash, or involvement of the bones, joints, or skin by dissemination [7]. We defined serological confirmation of infection as evidence of coccidioidal IgM or IgG as described above and defined a positive CF titre as ⩾1:2. We considered laboratory results for a patient as equivocal if acute-phase IgM EIA assays were reactive and all other acute- and convalescent-phase assays were negative. We defined a serologically confirmed case as one with acute onset of clinically compatible illness and serological confirmation. We defined a clinical case as one with acute onset of clinically compatible illness and equivocal or negative laboratory test results.
The crew included 12 persons (manual labourers, heavy equipment operators, and supervisors) who worked four 10-hour days per week with some variations from 8 to 17 October 2007 (Fig. 1). Eleven workers were men, 11 were white non-Hispanic, and one was Hispanic. The median age was 32·5 years (range: 19–64 years). Reported permanent residences were the states of: Idaho (3), Nevada (3), New Mexico (1), and California (5).
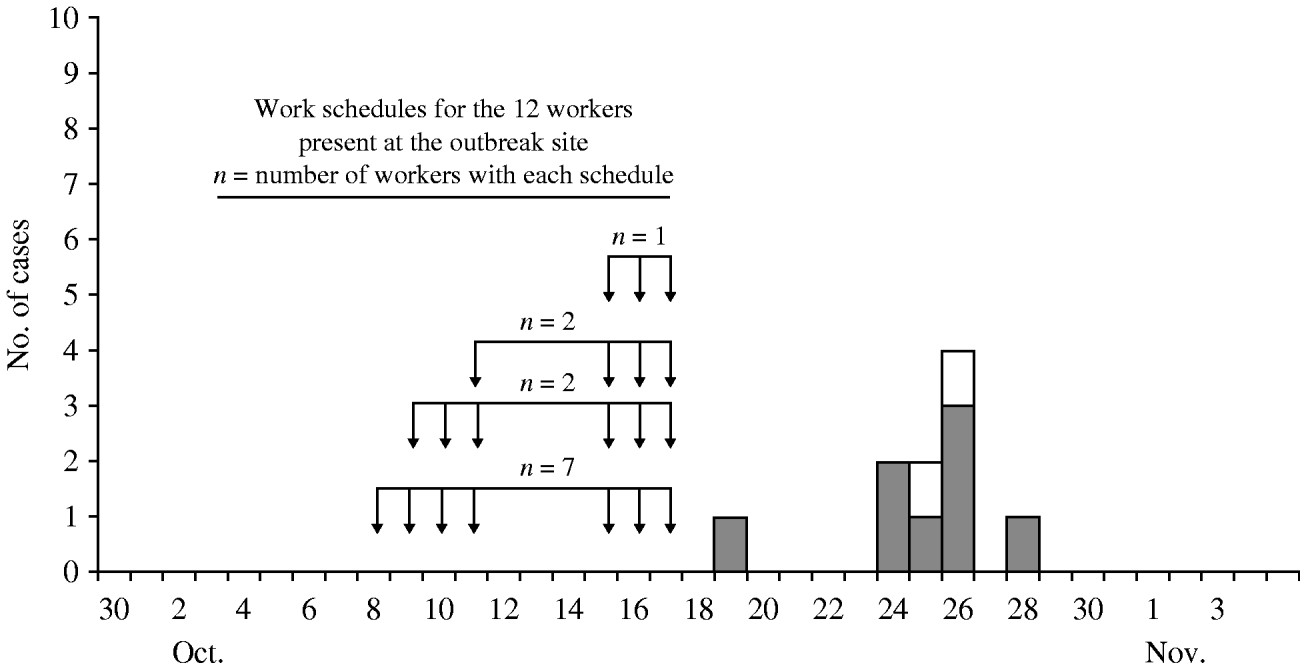
Fig. 1. Coccidioidomycosis outbreak cases at a military base in California, 2007, by illness onset date. □, Clinical cases; ![]() , serologically confirmed cases. Arrows indicate days worked.
, serologically confirmed cases. Arrows indicate days worked.
The attack rate for clinical illness was 83·3% (10/12). Symptomatic workers reported acute onset of cough (10), chest pain (10), fever (9), night sweats (8), myalgia and/or arthralgia (7), headache (5), and rash (4). Illness onsets ranged from 19 to 28 October 2007 (Fig. 1). Seven cases had abnormalities on chest radiograph including infiltrates (2), bilateral infiltrates and dense consolidations (1), bilateral dense consolidation and right pleural effusion (1), perihilar pneumonia (1), infiltrates and multiple granulomata (1), and infiltrates and pleural effusion (1). One case was hospitalized for 2 days. One white non-Hispanic case was diagnosed with disseminated disease involving the skin. Medical histories were unremarkable except for reports of diabetes (1) and asthma (2). Five cases smoked cigarettes during their exposure period.
The attack rate for serologically confirmed disease was 66·7% (8/12). Eight of 10 symptomatic workers had serological confirmation. All eight had EIAs reactive to IgM and positive CF antibody titres; highest CF titres per case were 1:2 (1), 1:16 (5), 1:32 (1), and 1:64 (1). Two symptomatic workers without serological confirmation reported clinically compatible but mild symptoms including low fever (2), cough (2), chest pain (2), fatigue (2), and night sweats (1). Both had normal chest radiographs; serological test results were negative for one worker and equivocal for the second worker. This latter worker declined additional serological testing using concentrated sera. Acute-phase sera for the crew were collected a median of 14·0 days (range 4–23 days) post-onset and convalescent-phase sera were collected a median of 44·5 days (range 37–56 days) post-onset.
Seven of 10 symptomatic workers sought treatment from at least 21 physicians. There was no pre-designated occupational medicine physician or clinic; most patients sought care at local emergency departments or urgent care clinics. Seven cases received a course of antibiotics and five received antifungal medications. As of 1 March 2008, the ten symptomatic cases missed 0 (3), 10 (1), 30 (1), 40 (1), 60 (1), 450 (1), 510 (1), and 560 (1) hours of work, one worker was assigned to 160 hours of light duty, and two workers remained on disability leave.
The pipe trench was excavated mechanically and was compacted and backfilled mechanically and manually. The soil was wetted with water but dust suppression was reduced when extensive sections of sandy soil were encountered. Equipment operators routinely kept cab doors open thereby bypassing air-conditioning and filtration systems. All workers assisted with manual labour tasks including shovelling dirt, as needed. Although many of the workers had been trained in and fit-tested for respirator use, none used respiratory protection. Workers, company management, and the camp's Operations Manager all reported being unaware, prior to the outbreak, of any risk of exposure to coccidioides at Camp Roberts.
On 3 March 2008, 1 of 2 remaining disease-free workers developed acute-onset night sweats and haemoptysis. On 10 March 2008, this patient was diagnosed with pneumonia, and serology and sputum culture specimens collected on that date were positive (i.e. serum was EIA IgM reactive and ID IgG non-reactive, CF antibody titre was 1:2; and sputum demonstrated C. immitis by genetic probe). This diagnosis followed his exposure to the outbreak site from 18 February to 4 March 2008. It remains unclear whether this worker used respiratory protection, including an N-95 respirator, during this 2008 exposure.
This point-source outbreak of coccidioidomycosis occurred in previously healthy civilian construction workers who excavated soil at Camp Roberts Military Base. The epidemic curve, descriptive epidemiology, and laboratory data from the investigation are consistent with a single point-source exposure. Camp Roberts is west of the highly endemic San Joaquin Valley and was originally sited as a military base to avoid exposure to coccidioides among trainees. However, an outbreak in military recruits occurred at the camp in the 1940s [Reference Shelton8] and fungus was isolated from dust-laden air samples in the 1950s [Reference Hoggan9]. This current outbreak was notable for the rapid onset and high proportion of symptomatic infections, significant morbidity including one case of disseminated infection, and lengthy periods of disability. Factors that likely contributed to the outbreak included soil excavation in an endemic area, and the lack of information and education among workers and their contract employer about the risk of coccidioidomycosis in this endemic area. Specifically, despite the availability of N-95 respirators, workers did not know that respiratory protection was needed. Several decades between outbreaks at this camp may have contributed to decreased awareness of the risk of coccidioides exposure in the area.
The outbreak's high attack rate and small cohort precluded us from quantifying the infection risk associated with specific work activities. Work done closest to the ditch and in the wake of freshly excavated soil was probably associated with the greatest exposure to dust and spores. However, coccidioides spores are extremely small (2–5 μm), are easily made airborne, settle slowly from air, and can penetrate the deep lung including alveoli [Reference Fisher, Bultman and Pappagianis10, Reference Schmelzer and Tabershaw11]. Small numbers of spores can cause infection in humans and animals [Reference Fisher, Bultman and Pappagianis10]. Therefore, once the site soil was disrupted, non-immune workers were likely to be at risk for infection.
Serological tests are important diagnostic tools for coccidioidomycosis but may be negative early in the disease or in persons with insufficient antibody production [Reference Pappagianis and Zimmer5]. We identified two clinically ill cases without documented seroconversion. These cases may have had false-negative assay results (e.g. antibody production may have been below the detection threshold of the test), or true-negative results (e.g. the symptoms arose from some other febrile respiratory illness). Although these cases have a lower level of diagnostic certainty, they demonstrated compatible, albeit mild clinical symptoms in the context of an outbreak with a high percentage of demonstrated serological conversions.
Prevention and control of coccidioidomycosis, especially in large outdoor areas associated with episodic focal activity, are challenging. Focal coccidioides growth or accumulation sites may be small and unevenly distributed within endemic areas [Reference Kirkland and Fierer1, Reference Fisher, Bultman and Pappagianis10]. Environmental sampling contributes little towards assessing exposure risk, and exposure risks can be reduced but not eliminated by soil decontamination [Reference Fisher, Bultman and Pappagianis10] or dust suppression [Reference Fisher, Bultman and Pappagianis10, Reference Smith12]. Therefore, workers should be well-informed of the risks regarding their activities. There are currently no specific U.S. federal standards or guidelines on preventing occupational or community-acquired coccidioidomycosis. General measures to minimize high-risk occupational exposure hazards have been suggested, including risk disclosure, education, effective respiratory protection, and appropriate medical surveillance and supervision [Reference Fisher, Bultman and Pappagianis10, Reference Schmelzer and Tabershaw11]. Effective respiratory protection is critical but additional research is needed on the prevention effectiveness of respiratory protection and risk-reduction strategies for potentially high-exposure outdoor activities. Ideally, significant soil disruption in focal areas of high risk should be avoided altogether or restricted to persons with known immunity to coccidioides. However, there is currently no commercially available skin test to identify immune persons. The utility of antifungal prophylaxis for preventing disease has been minimally studied in persons who are HIV-infected and living in endemic areas [Reference Woods13] and not yet studied in non-immune persons at risk for potentially high occupational exposures. An effective vaccine would be the optimal preventive solution but is, as yet, unavailable.
In conclusion, we documented a point-source outbreak of coccidioidomycosis during a period of epidemic disease activity in California. This outbreak provides further evidence that Camp Roberts is a coccidioides-endemic area. Employer, military, and volunteer organizations that send personnel into any area ever associated with an outbreak, even if there has been no known exposure in that area for decades, should ensure personnel have access to all available risk- reduction measures. U.S. federal guidelines on preventing occupational coccidioidomycosis, additional research on the prevention effectiveness of various respiratory protection measures, and an effective vaccine would fill important gaps in coccidioidomycosis prevention and control. Clinicians and public health authorities should maintain situational awareness about coccidioidomycosis, especially during epidemic periods and in persons who intermittently travel to and participate in potentially high-exposure activities in endemic areas.
ACKNOWLEDGEMENTS
The authors thank the outbreak investigation team: Dr Gregory Thomas, former Health Officer, Marilynn McDermott, Public Health Nurse, and Janelle Gorman, former Public Health Nurse, San Luis Obispo Public Health Department; Dr Thomas Maier, former Director, Kern County Public Health Laboratory. The authors also thank Dr Raul J. Cano, Environmental Biotechnology Institute, California Polytechnic State University; and Dr Demosthenes Pappagianis, University of California, Davis for their technical advice on the investigation.
DECLARATION OF INTEREST
None.



