Introduction
Alzheimer’s disease (AD) and vascular dementia (VaD) represent the leading causes of dementia, accounting for around 60-80% and 15% of all dementia cases, respectively. 1,Reference O’Brien and Thomas2 AD is characterized by the accumulation of misfolded amyloid-beta protein (Aβ plaques) and aggregation of tau protein (neurofibrillary tangles), which cause neurodegenerative changes that lead to memory deficits and executive dysfunctions as well as language, attention, or visuospatial impairments. Reference Scheltens, Blennow and Breteler3 By contrast, VaD is linked to variable cognitive impairments that depend on the specific sites and type of apparent cerebrovascular pathology. Reference O’Brien and Thomas2 The most common type is subcortical vascular dementia (SVaD), which is characterized by steno-occlusion of small vessels leading to ischemic white-matter (WM) lesions and subcortical lacunar infarctions. Reference O’Brien and Thomas2,Reference Pantoni4
Yet, evidence suggests that a large proportion of dementia cases are associated with multiple underlying pathologies and therefore cannot be identified as either purely AD or SVaD. Rather, they are referred to as “mixed” dementia (MixD) with the most common form being the coexistence of AD and vascular pathologies that exacerbates with age. Reference Chui and Ramirez-Gomez5,Reference Jørgensen, Aguayo-Orozco and Lademann6 For example, prospective amyloid imaging studies leveraging 11C-labeled Pittsburgh compound-B positron emission tomography (PiB-PET; an in vivo molecular imaging of Aβ) have found PiB positivity in ∼30% of SVaD participants. Reference Lee, Kim and Kim7,Reference Kim, Kim and Cho8 The presence of mixed AD and vascular pathologies has also been confirmed in a series of autopsy studies, including the Nun Study (39% of AD cases had at least one infarct), Reference Snowdon9 the Rochester Epidemiology Project (12% of dementia cases had combined AD and VaD), Reference Knopman, Parisi and Boeve10 the Rush Religious Orders Study and the Memory and Aging Project (38% of dementia cases had mixed AD and cerebral infarctions Reference Schneider, Arvanitakis and Bang11 ; 30.2% of probable AD cases had mixed AD and macroscopic infarcts Reference Schneider, Arvanitakis and Leurgans12 ), Jellinger and colleagues (16–20% of AD cases had additional cerebrovascular lesions), Reference Jellinger and Attems13 the Baltimore Longitudinal Study of Aging (35% of dementia cases associated with hemispheral infarcts alone or in conjunction with AD pathology), Reference Troncoso, Zonderman and Resnick14 the Hisayama Study (7.9% of dementia cases had neuropathological diagnosis of AD + VaD), Reference Matsui, Tanizaki and Arima15 the Honolulu-Asia Aging Study (14.2% of dementia cases had mixed Alzheimer and microvascular ischemic lesions), Reference White16 and De Reuck and colleagues (30% of dementia cases had mixed AD and cerebrovascular lesions). Reference De Reuck, Deramecourt and Cordonnier17 These findings suggest that dementia cases with mixed pathologies are relatively common.
Previous studies have shown different patterns of neuropsychological and neuropsychiatric outcomes among AD, SVaD, and suspected MixD subjects who were stratified based on clinical and imaging features (i.e. by means of fulfilling the diagnostic criteria of both AD and SVaD and/or having SVaD with amyloid positivity). For example, MixD performed significantly worse in global cognitive composite, attention, and visuo-construction scores compared to AD, Reference Dong, Gan and Tay18 as well as significantly worse in memory tasks compared to SVaD. Reference Lee, Kim and Kim7 Also, MixD showed less depressive symptoms and aberrant motor behaviors compared to VaD, but had marginally more agitation compared to AD. Reference Anor, O’Connor and Saund19 Therefore, although autopsy findings are essential to confirm the presence of mixed pathology, a biomarker-based characterization of the clinically defined population may help us distinguish suspected MixD from AD or SVaD.
To date, various magnetic resonance imaging (MRI) contrasts have been applied to characterize brain abnormalities associated with AD and VaD. For example, MRI-based morphometric studies have shown that AD is typically associated with atrophy of the medial temporal lobe structures (e.g. hippocampus and entorhinal cortex), temporal and parietal lobes as well as subcortical nuclei. Reference Scheltens, Blennow and Breteler3 In SVaD, the hippocampus and entorhinal cortex are less affected compared to AD although substantial cortical thinning can be found in the frontal and temporal lobe areas. Reference Kim, Seo and Kim20,Reference Kim, Ye and Yoon21 The primary MRI feature of SVaD is considered to be WM signal abnormalities (WMSA), visible as hyperintensities on T2-weighted or fluid-attenuated inversion recovery (T2-FLAIR) images, which presumably represent small vessel disease associated with chronic ischemia. Reference Fernando, Simpson and Matthews22 However, T2-hyperintense WMSA (T2-HyperWMSA) are also present in AD, especially around the parietal and occipital periventricular regions. Reference Smith, Johnson and Van Eldik23,Reference Lee, Viqar and Zimmerman24 Furthermore, non-conventional MRI techniques have been used to compare microstructural tissue properties in AD and VaD. For example, diffusion tensor imaging (DTI) studies have observed lower fractional anisotropy (FA; the degree of anisotropic water diffusion) and higher mean diffusivity (MD; the magnitude of water diffusion) values in most WM tracts in SVaD compared to AD. Reference Fu, Zhang and Chang25,Reference Kim, Kwon and Lee26 Recent studies have also investigated magnetic susceptibility alterations by means of quantitative susceptibility mapping (QSM), where elevated QSM levels were found in early AD and in VaD compared to controls especially within the subcortical nuclei structures. Reference Moon, Han and Moon27–Reference Sun, Ge and Han31 Such increased susceptibility measurements may be explained by demyelination and increased iron concentrations, Reference Moon, Han and Moon27,Reference Acosta-Cabronero, Williams and Cardenas-Blanco29–Reference Damulina, Pirpamer and Soellradl33 which may result from iron misregulation, accumulation, or neuronal loss during neurodegenerative pathogenesis. Reference Ke and Qian34,Reference Hernández-Torres, Wiggermann and Machan35
Nonetheless, brain abnormalities in individuals with suspected MixD remain to be elucidated. Conventional MRI findings suggest that global measures such as whole-brain volume, gray matter (GM) volume, or WM hyperintensities count/volume may not be well-suited for differentiating MixD from AD or SVaD as they share similar imaging features. Reference Du, Schuff and Chao36,Reference Jagust, Zheng and Harvey37 AD-specific changes in subcortical structures (e.g. hippocampal and amygdala shapes) may distinguish MixD from SVaD, Reference Kim, Kim and Cho8 but not from AD. Likewise, changes in WM tracts (e.g. DTI indices) may differentiate MixD from AD, Reference Rosenberg, Prestopnik and Knoefel38 but not from SVaD. Moreover, the scarcity of MixD imaging studies is likely due to the difficulty in recruiting appropriate participants, who are likely among the oldest of the old and are unlikely to tolerate MRI and/or PiB-PET sessions. This highlights the significance of well-focused hypotheses and careful selection of MRI contrasts and methods especially relevant to MixD.
We conducted an exploratory study to assess potential brain abnormalities in mixed dementia using conventional and non-conventional MRI measures sensitive to changes in brain tissue properties. To investigate, we analyzed participants recruited at a single site who were classified in terms of clinical diagnosis of AD, SVaD, or MixD (i.e. fulfilling the diagnostic criteria of both AD and SVaD). We hypothesized that our MixD group would have characteristic MRI abnormalities due to contributions from both AD and SVaD pathologies. To investigate, we conducted region-of-interest (ROI)-based comparisons of MixD with either AD or SVaD in terms of 1) conventional measures including WMSA and DTI parameters as well as 2) novel susceptibility-weighted imaging-based metrics including QSM) and effective transverse relaxation rate (R2*). Our findings can provide a priori knowledge for future studies of imaging abnormalities in MixD.
Methods
Study Participants
A total of 17 participants were recruited through the University of British Columbia Clinic for Alzheimer’s Disease and Related Disorders. The participants were stratified into probable AD if they fulfilled the McKhann NIA-AA criteria, Reference McKhann, Knopman and Chertkow39 or SVaD if they fulfilled the criteria by Erkinjuntti, Reference Erkinjuntti, Inzitari and Pantoni40 or into the MixD subgroup if they fulfilled the diagnostic criteria of both AD and SVaD. This study was approved by the University of British Columbia Clinical Ethics Review Board. All experiments were performed in accordance with relevant guidelines and regulations. All participants provided written informed consent. Table 1 summarizes the baseline demographic, clinical, cognitive, and global brain volume characteristics for each diagnostic subgroup. We report the following global measures of clinical and cognitive functions: Clinical Dementia Rating Sum of Boxes (CDRSOB), Neuropsychiatric Inventory (NPI), Functional Activities Questionnaire (FAQ), Global Deterioration Scale (GDS), Finger Tapping Test (FTT), Motor Screening Task Standard Score (MOT), Montreal Cognitive Assessment (MoCA), and Mini-Mental State Exam (MMSE) total scores.
Table 1: Baseline demographic, clinical, cognitive, and global brain volume characteristics for in AD, MixD, and SVaD subjects

CDRSOB, Clinical Dementia Rating Scale Sum of Boxes; NPI, Neuropsychiatric Inventory; FAQ, Functional Activities Questionnaire; GDS, Global Deterioration Scale; FTT, Finger Tapping Test; MOT, Motor Screening Task Standard Score; MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Exam.
† FTT: 1 MixD missing.
†† MOT: 2 MixD, 1 SVaD missing.
* Neither age, age, education level, NPI, GDS, FTT, MOT, MoCA, MMSE nor baseline level of atrophy (whole-brain, cortical GM, and total WM) were significantly different among AD, SVaD, and MixD.
** SVaD had significantly lower CDRSOB scores compared to AD (p = 0.05) or MixD (p = 0.03), ANOVA and Tukey’s HSD post hoc testing.
*** SVaD had significantly lower FAQ total scores compared to AD (p = 0.01) or MixD (p = 0.01), ANOVA, and Tukey’s HSD post hoc testing.
Image Acquisition
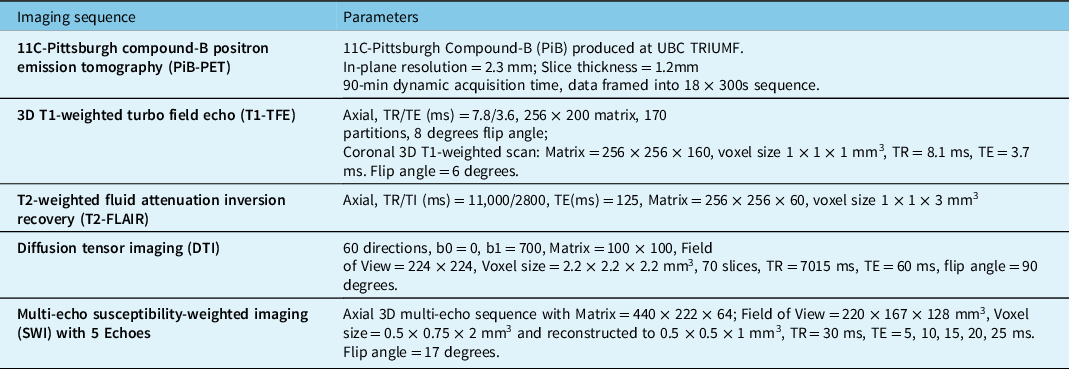
Participants were cross-sectionally scanned on a 3.0 T Philips Achieva MRI scanner (all N = 17 participants) and a GE Advance PET tomography (N = 15 due to two participants not consenting to PET scanning). Two PiB-PET data sets were further excluded from subsequent analysis due to low tracer dose or motion artifact (final PET N = 13; 4:5:4 AD:MixD:SVaD). The acquired sequences are outlined in Table 2.
Table 2: PET and MRI acquisition protocols
PET Processing
Reconstructed PET imaging data were frame-to-frame realigned and motion-corrected using AIR. Reference Woods, Cherry and Mazziotta41 A PiB-PET template in the Montreal Neurological Institute (MNI) 305 space was created by averaging a set of PiB-PET images from a separate cohort of healthy control subjects whose MRI images had been warped to the SPM MNI305 template. For each subject, the mean PiB-PET image was normalized to the PiB-PET template via non-linear regularization (16 non-linear iterations and 8 mm smoothing) and affine regularization. Then, all the PiB-PET frames were transformed to the MNI space using the corresponding parameters. Standardized uptake value ratio (SUVR) 40 to 90 min post-injection was calculated by normalizing SUV (tracer concentration/(injected dose/body weight)) images to the cerebellar cortex SUV.
To explore the distribution of Aβ on PET among our participants, we calculated the average SUVR (for minutes 40 to 90) within the following bilateral ROIs grouped by cortical and subcortical regions: frontal (orbitofrontal and medial prefrontal cortex), parietal (angular gyrus, superior parietal, precuneus, and supramarginal gyrus), temporal (lateral temporal gyrus, middle temporal gyrus, medial temporal gyrus, and temporal pole), occipital (occipital cortex, medial occipital), sensory-motor cortex, cingulate gyrus (posterior and anterior cingulate gyrus), perirolandic region, and subcortical nuclei (thalamus, caudate and putamen).
MRI Processing – Preprocessing of T1-Weighted Images
T1-weighted images were visually checked for quality and then processed using the FreeSurfer 6.0 cross-sectional pipeline, which provides cortical reconstruction and volumetric segmentation. Reference Fischl and Dale42,Reference Fischl, Salat and Busa43 We utilized a version implemented in the “Cloud Engine Resource for Accelerated Medical Image Computing for Clinical Applications” (CERAMICCA; https://ceramicca.ensc.sfu.ca) portal. All FreeSurfer outputs were manually examined and corrected for errors.
The outputs from the FreeSurfer volumetric pipeline were used to obtain the whole-brain, cortical GM, and total WM volumes for each participant. Further, FreeSurfer-derived subcortical GM nuclei and hippocampal/amygdala volumetric outputs were combined with the large deformation diffeomorphic metric mapping (LDDMM Reference Beg, Miller and Trouvé44 )-based label propagation method Reference Khan, Wang and Beg45 to produce segmentations of subcortical structures (caudate, putamen, pallidus, thalamus, and hippocampus/amygdala) for each participant. To account for individual variations in head size, we also calculated total intracranial volume (ICV) for each participant using a multi-atlas label fusion method. Reference Ma, Popuri and Bhalla46
MRI Processing – WM Structural Abnormalities
T2-HyperWMSA were identified on T2-FLAIR images using in-house software based on the following steps: 1) manual identification of pathological hyperintensities by a neuroradiologist, who marked each lesion with one or more seed points, 2) intensity thresholding based on T2-FLAIR signal intensity distribution, 3) removal of hyperintensities not containing any seed points. While the lesion identification step was manual, the rest was automated. For each participant, the T2-FLAIR image and the T2-HyperWMSA mask were 6-parameter rigid body registered to the T1-weighted scan using FSL-FLIRT. Reference Jenkinson, Bannister and Brady47
T2-HyperWMSA that also appear hypointense on the corresponding T1-weighted image (i.e. T1-HypoWMSA) are considered as areas of more severe WM injury, as suggested by more aberrant DTI values and their association with faster conversion from mild cognitive impairment (MCI) to AD. Reference Riphagen, Gronenschild and Salat48,Reference Dadar, Maranzano and Ducharme49 We additionally identified T1-HypoWMSA on T1-weighted images as markers that may be more specific to tissue damage in the WM. They were defined for each participant as follows: The voxels within the T2-HyperWMSA mask with the corresponding T1-weighted image intensity of 65% or lower than the surrounding normal-appearing white-matter (NAWM) voxels.
The burden and distribution of WMSAs were assessed in terms of ROIs, which included total and lobar (frontal, parietal, occipital, and temporal) WM masks. The lobar ROIs were defined on T1-weighted images by combining the corresponding Desikan-Killiany atlas labels through FreeSurfer’s “mri_annotation2label” and “mri_aparc2aseg” functions. Reference Desikan, Ségonne and Fischl50 For each ROI, we obtained the ICV-normalized volume of T2-HyperWMSA (i.e. (T2-HyperWMSA∩ROI voxels) over (ICV voxels) * 100), which was used as an index of apparent WM tissue abnormalities within the specific ROI. The same was repeated for T1-HypoWMSA.
For further analyses, we defined normal-appearing gray matter (NAGM) and white-matter (NAWM) ROIs as follows: 1) segmentation of GM and WM on T1-weighted images using SPM12 (https://www.fil.ion.ucl.ack.uk/spm/), 2) creation of GM and WM masks using binarization threshold of >0.5, and 3) subtraction or addition of the T2-HyperWMSA mask from the GM and WM masks, respectively.
DTI images were processed using the FSL Diffusion Toolbox. Reference Jenkinson, Beckmann and Behrens51,Reference Behrens, Woolrich and Jenkinson52 Briefly, the steps included 1) manual checking of the diffusion data, 2) susceptibility-induced distortion correction using “topup”, 3) brain extraction on the corrected non-diffusion weighted b0 images using “bet,” 4) eddy currents and subject motion correction using “eddy,” and 5) voxel-wise fitting of the eddy-corrected diffusion tensor data using “dtifit.”
For comparison, we assessed the average FA and MD values within major WM tracts based on the ICBM-DTI-81 WM labels atlas. Reference Mori, Oishi and Jiang53 While the atlas comprises 48 WM tract labels, they were combined to reduce the number of comparisons. However, we assessed the regions overlapping with T2-HyperWMSA and the normal-appearing regions separately, in order to detect differences in WM microstructure that are not captured by WM hyperintensities.
MRI Processing – QSM and R2*
SWI images were processed using in-house software to produce maps of QSM as well as R2*, which is a related measure that is correlated with iron in deep GM and myelin and iron in WM. Reference Hu, Rajendran and Lapointe54,Reference Kames, Wiggermann and Rauscher55 QSM was reconstructed using a rapid two-step dipole inversion algorithm. Reference Kames, Wiggermann and Rauscher55 Maps of R2* relaxation rates were computed by fitting a mono-exponential decay function to the multi-echo data Reference Denk and Rauscher56 after correction for signal decay due to background inhomogeneities. Reference Fernández-Seara and Wehrli57
We evaluated the average QSM and R2* values within the predefined T2-HyperWMSA and T1-HypoWMSA ROIs as well as within major subcortical nuclei (caudate, putamen, pallidus, thalamus, hippocampus, and amygdala). For this, the WMSAs and the FreeSurfer+LDDMM-derived subcortical nuclei ROIs were 6-parameter rigid body registered to the corresponding SWI images.
Statistical Analysis
All analyses were conducted using SAS PROC GLM and JMP software (SAS, Cary, NC). Analysis of variance (ANOVA) and Tukey’s HSD post hoc tests were used to identify differences between groups in age, education, CDRSOB, NPI, FAQ, GDS, MoCA, MMSE, and baseline global brain volume measures.
Due to the further reduced sample size in our PiB-PET analysis, we did not conduct formal statistical analyses. Instead, we reported the individual SUVR data within the 19 predefined ROIs with a reference threshold to determine PiB-PET positivity within the region. As our PET imaging protocol followed that of the Alzheimer’s Disease Neuroimaging Initiative (ADNI), we provided an ADNI literature threshold of 1.465. Reference Mormino, Kluth and Madison58
T2-HyperWMSA, T1-HypoWMSA, and SWI measures were analyzed using a GLM to assess subgroup differences while adjusting for age and sex (model: MRI_measure ∼ subgroup + age + sex). DTI-based measures were analyzed using a similar GLM with additional ICV-corrected WM volume term to account for the effect of atrophy (model: MRI_measure ∼ subgroup + age + sex + volume). Similar as above, we examined whether the omnibus F-test was significant and then used Dunnett’s test to conduct post hoc multiple comparisons with MixD as the control group (2 total: MixD vs. AD; MixD vs. SVaD).
Additionally, DTI, QSM, and R2* measures were analyzed using two-tailed paired t-tests to assess differences in values between normal-appearing and WMSA areas (T2-HyperWMSA for DTI; T2-HyperWMSA and T1-HypoWMSA for QSM and R2*).
Correction for multiple comparisons: For the GLM analyses, we have used a hierarchical approach where we first performed the omnibus F-test to assess whether a group difference exists in a given measure among MixD, AD, and SVaD. If the omnibus test was not significant, we stopped the analysis as there was no evidence of rejecting the null hypothesis. However, if the omnibus test was significant (F-statistic < 0.05), we proceeded with post hoc pairwise group comparisons using Dunnett method with MixD as the control group. Two comparisons were made against MixD (MixD vs. AD and MixD vs. SVaD), with the p-values adjusted for multiple comparisons. We assumed that the dependent variables (multi-modal MRI measures) were strongly correlated with each other, as they were each acquired from the same participant. For the paired t-tests, we have used the Bonferroni method to adjust the p-values for the simultaneous pairwise comparisons (WMSA vs. normal-appearing WM for each group, leading to 3 comparisons per measure). We assumed that the comparisons were independent, resulting in the significance threshold of p = 0.004.
Results
Baseline Characteristics
Our MixD participants tended to have older age and lower MoCA scores than the other participants, although not statistically significant. Overall, neither age, education level, NPI, GDS, FTT, MOT, MoCA, MMSE nor baseline level of atrophy (whole-brain, cortical GM, and total WM) were significantly different among the subgroups. However, both AD and MixD had significantly higher CDRSOB and FAQ total scores compared to SVaD participants. Table 1 summarizes the findings.
Description of PiB-PET SUVR Data
Figure 1 presents the individual PiB-PET SUVR data in the 19 ROIs, grouped by cortical and subcortical regions. AD participants had above-threshold SUVR in most regions, except in the temporal pole, perirolandic, medial occipital, and medial temporal regions. The majority of MixD participants (60-80%) had above-threshold SUVR in all regions, except in the temporal pole, medial temporal, and occipital regions. The proportion of above-threshold SVaD participants was relatively more varied and region-dependent.

Figure 1: PiB-PET average SUVR data in the 19 ROIs, grouped by cortical and subcortical regions. Each data point represents each individual participant, with boxplots showing quantile statistics. Red dashed lines represent the PET positivity threshold of 1.465.
MRI Results – White-Matter Hyperintensities and Hypointensities
Figure 2 provides representative FLAIR images depicting the presence of brain atrophy and WM hyperintensities in the AD, SVaD, and MixD participants. T2-HyperWMSA proportions, adjusted for age and sex, were significantly higher in the MixD subgroup versus the AD subgroup (p = 0.0067) but not versus the SVaD subgroup (p = 0.11). Assessing the regional distribution, the effect was significant in the frontal lobe (MixD vs. AD p = 0.0094; MixD vs. SVaD p = 0.07) and the occipital lobe (MixD vs. AD p = 0.026; MixD vs. SVaD p = 0.92) but not in the other lobes.

Figure 2: Examples of the T2-FLAIR image for AD (Left), SVaD (Middle), and MixD (Right) that demonstrates the presence of brain atrophy and white-matter signal abnormalities (hyperintensities).
T1-HypoWMSA proportions were significantly higher in the MixD subgroup versus both the AD (p = 0.01) and the SVaD (p = 0.04) subgroups. The effect was dominant in the frontal lobe (MixD vs. AD p = 0.02; MixD vs. SVaD p = 0.02) but not in the other lobes. Table 3 compares the ICV-adjusted volumes of T2-HyperWMSA and T1-HypoWMSA among the subgroups and provides post hoc findings for significant comparisons. Figure 3 shows the frequency maps of WM hyperintensities (T2-HyperWMSA) that have been normalized to the MNI 152 template for easier visualization.

Figure 3: Frequency maps of T2-HyperWMSA for AD (Left), SVaD (Middle), and MixD (Right). The maps are spatially normalized to the MNI152 template.
Table 3: Proportions of FLAIR white-matter hyperintensities (T2-HyperWMSA) and T1w-hypointensities (T1-HypoWMSA) within total white-matter and bilateral lobar regions of interest

* Pairwise comparisons were conducted only if omnibus F-test was significant at p < 0.05. Pairwise p-values were corrected for multiple comparisons using Dunnett’s post hoc test with MixD as the control group. Subgroup comparisons were adjusted for age and sex.
MRI Results – White-Matter DTI
Comparisons among AD, SVaD, and MixD: Significant subgroup effects in FA and MD values were noted within the total and NAWM tracts. Within both ROIs, the SVaD subgroup had significantly lower FA values versus MixD (total: p = 0.017; normal-appearing: p = 0.0082) while the AD and MixD comparison was not significant (total: p = 0.36; normal-appearing: p = 0.91). MD values were not significantly different among the subgroups. Table 4 compares the FA and MD values among the subgroups and provides post -hoc findings for significant comparisons.
Table 4: Diffusion tensor imaging measures within WMSA and normal-appearing white-matter regions of interest
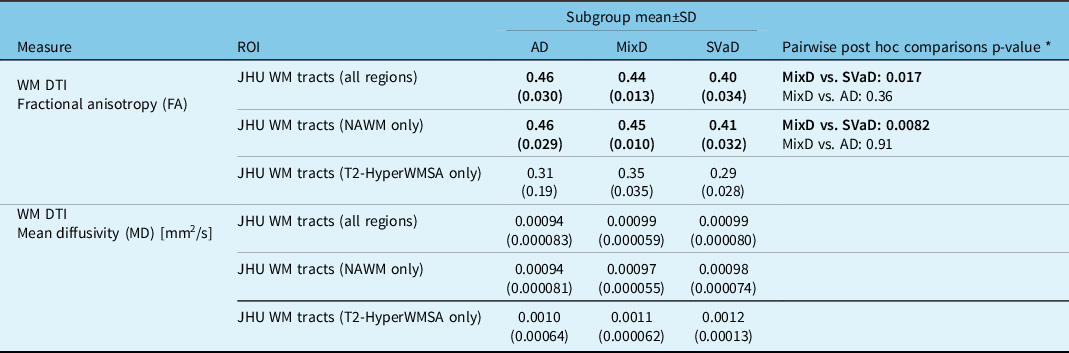
* Pairwise comparisons were conducted only if omnibus F-test was significant at p < 0.05. Pairwise p-values were corrected for multiple comparisons using Dunnett’s post hoc test with MixD as the control group. Subgroup comparisons were adjusted for age and sex.
Comparisons between normal-appearing and WMSA areas: For MixD, paired Bonferroni-corrected t-tests indicated significantly lower FA (p < 0.0003) and higher MD (p < 0.002) values within T2-HyperWMSA versus NAWM. For SVaD, T2-HyperWMSA had significantly lower FA (p = 0.01) but comparable MD (p = 0.17) values compared to NAWM. For AD, both FA (p = 0.53) and MD (p = 1.0) values were not significantly different between T2-HyperWMSA and NAWM.
MRI Results – White-Matter and Subcortical Nuclei SWI
Comparisons among AD, SVaD, and MixD: QSM values within the assessed ROIs were not significantly different among the subgroups. However, R2* measures within the T2-HyperWMSA areas were significantly lower in the MixD subgroup versus both AD (p = 0.03) and SVaD (p = 0.04). Other regions showed insignificant differences. Table 5 compares the QSM and R2* values among the subgroups and provides post hoc findings for significant comparisons. Figure 4 shows the examples of the QSM and R2* images with the subcortical nuclei ROI masks overlaid.

Figure 4: Examples of the QSM (top row) and R2* (bottom row) images from AD (Left), SVaD (Middle), and MixD (Right) participants. Subcortical nuclei masks are overlaid in blue and yellow colors.
Table 5: Quantitative susceptibility mapping and R2* relaxation measures within WMSA and subcortical structures regions of interest

* Pairwise comparisons were conducted only if omnibus F-test was significant at p < 0.05. Pairwise p-values were corrected for multiple comparisons using Dunnett’s post hoc test with MixD as the control group. Subgroup comparisons were adjusted for age and sex.
Comparisons between normal-appearing and WMSA areas: Paired Bonferroni-corrected t-tests indicated nonsignificant difference in susceptibility (AD p = 1.0; MixD p = 1.0; SVaD p = 1.0) but significantly lower R2* (AD p = 0.0015; MixD p < 0.0003; SVaD p < 0.0003) values within T2-HyperWMSA versus NAWM. Results were similar between T1-HypoWMSA and NAWM (susceptibility: AD p = 0.17; MixD p = 0.16; SVaD p = 0.52; R2*: AD p = 0.009; MixD p < 0.0003; SVaD p < 0.0003).
Discussion
We conducted an exploratory analysis of brain imaging abnormalities in clinically diagnosed MixD, AD, and SVaD participants in terms of ROI-based comparisons of conventional and non-conventional MRI metrics. PiB-PET positivity was observed within the AD-relevant regions for most MixD and AD participants. MRI characteristics of our MixD participants included 1) significantly higher proportion of WM structural abnormalities, mainly within the frontal lobe, as indicated by T2-HyperWMSA (versus AD) or T1-HypoWMSA (versus AD and SVaD); 2) significantly higher DTI FA values within major NAWM tracts versus SVaD, but not versus AD; and 3) significantly lower R2* values within the T2-HyperWMSA areas compared to both AD and SVaD.
Overview of PiB-PET SUVR Data
We used PiB-PET SUVR to explore the regional distribution of Aβ pathology among our study participants, who were stratified based on clinical diagnostic criteria. Reference McKhann, Knopman and Chertkow39,Reference Erkinjuntti, Inzitari and Pantoni40 It is recognized that in AD, 11C-PiB radiotracer binding is consistently elevated in regions including the prefrontal cortex, precuneous, posterior cingulate cortex, lateral parietal cortex, lateral temporal cortex, and striatum. Reference Villemagne and Chételat59–Reference Laforce and Rabinovici61 Indeed, all of AD and most (60–80%) of MixD participants had SUVR above the threshold in all of those regions. SVaD participants had relatively lower uptake level in the AD-related regions, except for posterior cingulate cortex and angular gyrus. This may be consistent with an increased PiB-uptake level in the precuneous and posterior cingulate cortex observed in patients after subcortical ischemic stroke. Reference Yasuno, Kajimoto and Ihara62 Furthermore, elevated posterior cingulate and occipital uptake level may indicate an occipital-predominant pattern observed in SVaD, Reference Jang, Park and Jang63 although this needs to be verified in future work with larger samples.
MRI – White-matter Signal Abnormalities
T1-HypoWMSA was able to differentiate MixD from both AD and SVaD, indicating a higher burden of presumably more severely injured tissue in terms of lower signal on T1-weighted MRI. It is unlikely that these T1-HypoWMSA represented cavitated lacunes, which appear hypointense on T2-FLAIR images and were not included in the T2-HyperWMSA masks. Potential sources of T1-HypoWMSA could have included recent small subcortical infarcts or microbleeds associated with cerebral amyloid angiopathy (CAA), Reference Wardlaw, Smith and Biessels64 both of which are prominent in MixD. Furthermore, the pathological specificity of hypointensities on T1 gradient-echo sequences (including ours) is relatively low, unlike those on spin-echo sequence which have been histopathologically validated as a marker of severe tissue destruction. Reference van Walderveen, Kamphorst and Scheltens65 Further work is warranted to evaluate the clinical relevance of the hypointensity-based measure, including its origin and association with cognitive impairment.
The MixD subgroup had significantly higher overall burden of T2-HyperWMSA compared to AD and marginally but not significantly higher compared to SVaD. It is known that AD and VaD have characteristic regional distributions of WM hyperintensities, with different localizations of hyperintensities associated with cognitive impairment as well as conversion to dementia. Reference Yoshita, Fletcher and Harvey66–Reference Gootjes, Teipel and Zebuhr68 In our AD subgroup, the occipital, parietal, and frontal lobes were more affected by T2-HyperWMSA. This is consistent with the findings of higher posterior preponderance (e.g. posterior periventricular and the splenium of the corpus callosum), Reference Yoshita, Fletcher and Harvey66 as well as frontal lobar burden in AD. Reference Mortamais, Reynes and Brickman67–Reference Holland, Smith and Csapo69
For VaD, studies have shown increased burden of WM hyperintensities in the frontal and parietal lobes, Reference Gootjes, Teipel and Zebuhr68 which is likely related to the anterior preponderance of WM hyperintensities in ischemic stroke patients. Reference Schirmer, Giese and Fotiadis70,Reference Wen and Sachdev71 Moreover, posterior cerebral artery territories are also affected after ischemic stroke, Reference Schirmer, Giese and Fotiadis70 contributing to the heterogeneous nature of VaD. Accordingly, the parietal, frontal, and occipital lobes were mostly affected by T2-HyperWMSA in our SVaD subgroup.
Our MixD subgroup was characterized by the greatest T2-HyperWMSA burden, which were most prominent in the frontal and parietal WM lobes, accounting for around 1.2% and 0.6% of the ICV, respectively. Intriguingly, the frontal lobe involvement, in terms of T1-HypoWMSA, was significantly greater compared to both SVaD and AD. Whether this implies AD- or CAA-mediated exacerbation of vascular disturbances requires further verification, as WM abnormalities on MRI can represent different underlying etiologies. Future work is warranted to determine if the subgroups have different types or accelerated time courses of vascular events.
MRI – DTI
Microstructural WM tissue properties were assessed using DTI parameters FA and MD. It is known that FA is reduced and MD is elevated in aging-related WM hyperintensities compared to NAWM, Reference Maniega, Valdés Hernández and Clayden72 rendering it a non-specific marker of WM injury associated with axonal damage, demyelination, gliosis, or inflammation. As such, widespread DTI abnormalities have been reported in SVaD relative to AD, especially in the genu of the corpus callosum and prefrontal cortex WM tracts (e.g. anterior thalamic radiations and inferior fronto-occipital fasciculi). Reference Fu, Zhang and Chang25,Reference Sugihara, Kinoshita and Matsusue73–Reference Palesi, De Rinaldis and Vitali75 On the other hand, in AD, temporal lobe, hippocampus, and the splenium of the corpus callosum are more affected than in SVaD. Reference Fu, Zhang and Chang25,Reference Palesi, De Rinaldis and Vitali75 Therefore, it was unexpected that our MixD, with extensive WM hyperintensities, resembled AD in terms of DTI parameters with significantly higher FA and marginally lower MD values within NAWM compared to SVaD. Several factors may explain this finding. First, progression of brain atrophy or WM lesion formation is related to loss of WM microstructural integrity, such that decreases in FA and increases in MD can be observed in “vulnerable” NAWM regions that are likely to convert into WM lesions (e.g. hyperintensities on T2-FLAIR) in the future. Reference Vernooij, de Groot and van der Lugt76 This is a dynamic process, and it is possible that our MixD and SVaD subgroups were in different stages in terms of ongoing cerebrovascular events. For example, our SVaD participants may have had a higher average proportion of vulnerable normal-appearing WM compared to MixD, and this would have led to reduced FA in SVaD at the time of MRI visits. Second, different levels of extracellular fluid accumulation, due to vasogenic edema or other factors, may have confounded the DTI estimates. For example, a recent study suggested that “free water (FW)”, a DTI-based proxy for tissue water content, is increased in NAWM of VaD and MixD compared to AD or healthy controls. Reference Ji, Pasternak and Liu77 It is possible that edema accumulation may have applied pressure to adjacent WM fibers, resulting in a denser parallel alignment and a concomitant, arbitrary increase in FA. Reference Pasternak, Sochen and Gur78 Third, the effect of crossing-fibers on FA values might have been different between MixD and SVaD, due to different regional distribution of WM hyperintensities that affect the crossings, or different pattern of degeneration of fiber bundles that constitute the crossings. A future longitudinal study will be helpful to identify these WM changes and to determine whether the pattern of progression differs between SVaD and MixD or AD.
MRI – QSM and R2*
Previous studies have reported abnormally elevated susceptibility values in AD, VaD, and subcortical vascular MCI, particularly within the caudate, putamen, and hippocampus, compared to healthy controls. Reference Moon, Han and Moon27,Reference Kim, Park and Rhee28,Reference Sun, Ge and Han31 Although we did not have healthy controls to compare against, the susceptibility measures were likely elevated in all of our participants due to AD or vascular-related pathology. We did not observe group differences in susceptibility within the test subcortical nuclei structures, which is in line with a previous comparison where the authors did not observe significant subcortical QSM differences between AD and VaD. Reference Moon, Han and Moon27 These findings suggest that a similar level of iron accumulation within the assessed subcortical ROIs is present in all three dementia subtypes, even though the mechanisms of the accumulation may differ. For example, the QSM values in AD and MixD may have been influenced by amyloid accumulation and neurofibrillary tangles. Reference Spotorno, Acosta-Cabronero and Stomrud79,Reference Ayton, Fazlollahi and Bourgeat80
Within T2-HyperWMSA, the MixD subgroup had significantly lower R2* compared to both AD and SVaD, while the group differences in the QSM values were nonsignificant. As tissue water content is strongly negatively associated with R2* but not with QSM, this observation likely represents relatively elevated fluid accumulation (e.g. edema) within the T2-HyperWMSA areas of MixD. Tissue edema is commonly associated with disrupted tissue matrix, microglia activation, and blood–brain barrier (BBB) disruption. For instance in AD, CAA is linked to BBB disruption, infarcts, and WM changes, along with inflammatory and immune responses. Reference Sweeney, Sagare and Zlokovic81,Reference Magaki, Tang and Tung82 BBB permeability is also increased in people with subcortical vascular disease, Reference Taheri, Gasparovic and Huisa83 where factors like hypoperfusion and thromboembolism lead to inflammatory responses. Reference Venkat, Chopp and Chen84 It is possible that MixD represents an additive or synergistic combination of both conditions, manifesting as a relatively higher degree of edema within the abnormal WM tissue. Whether this actually points to increased neuroinflammatory responses in MixD is not possible to confirm using MRI alone and needs to be verified using peripheral inflammatory biomarkers. Reference Park, Han and Mook-Jung85
Summary and Study Limitations
Our findings have several implications for future study of imaging markers for MixD. First, most of our MixD participants were also PiB-positive with uptake patterns similar to those observed in AD. This implies that the stratification based on clinical severity is correlated with abnormal amyloid deposition and may corroborate the evaluation of potential MixD cases when amyloid PET imaging is not feasible. Second, an evaluation of tissue integrity-related MRI measures may help better characterize the status of tissue damage in MixD. WMSA observed in our MixD participants may also have been due to causes other than small vessel disease, for example demyelination or inflammatory activity. Our study included DTI and QSM measures, which may represent microstructural changes during demyelination or axonal degeneration. While we did not find strong evidence of DTI changes or elevated QSM within the WMHs of our MixD group, we suggest that potential demyelination within WMSAs needs to be further investigated using MRI measures sensitive to myelin changes, such as myelin water imaging. Reference MacKay and Laule86 In addition, T2-HyperWMSAs of our MixD participants had a higher proportion of areas with reduced intensity on T1-weighted images and lower average values on R2* maps. These findings suggest that WMSAs in MixD may be further affected by underlying pathology, such as greater axonal loss and/or fluid accumulation, compared to those in SVaD or AD. Future research will benefit from the inclusion of inflammatory markers in evaluating the implications of imaging data.
There were several limitations to our study. First, our AD, SVaD, and MixD groups were based on clinical diagnoses, but autopsy findings are crucial to confirm the actual presence of mixed pathology. Clinical and neuropathological diagnoses are not necessarily correlated, Reference Brayne, Richardson and Matthews87,Reference Savva, Wharton and Ince88 and we cannot exclude that some of our participants could have been misclassified. Second, the clinical diagnosis criteria used in our study included visual rating scales of periventricular and deep white-matter hyperintensities. Reference McKhann, Knopman and Chertkow39,Reference Erkinjuntti, Inzitari and Pantoni40 This implies that our SVaD and MixD groups were classified based on the same imaging criteria, and therefore, the total T2-HyperWMSA burden would have been “pre-destined” to possess less sensitivity to distinguish the two groups. We have alleviated this limitation by investigating the lobar distribution of WMSA volumes, which detail location and size of WM lesions, as well as incorporating the T1-HypoWMSA, which may be more specific to tissue damage in the WM. Third, we acknowledge that our sample size was very small and our findings should be interpreted with caution. While we presented imaging characteristics of our MixD group using various MRI contrasts and methods, it is likely that some of the comparisons did not have sufficient power to detect true between-group differences. Yet, we believe that our exploratory findings can provide practical a priori information for future studies investigation MixD population. Fourth, MixD likely represents a heterogeneous spectrum that depends on the relative influence of and interaction between AD and cerebrovascular pathologies. Reference Emrani, Lamar and Price89 However, the small sample size prevented us from including a subgroup interaction term in the statistical models, which would be necessary to examine the possible synergistic exacerbation of abnormalities in MixD. Also, a larger sample size is necessary to evaluate the correlation between imaging and neuropsychological findings, especially those involving region-specific or domain-specific measures. Given the practical difficulties of recruiting participants with suspected MixD, a multi-site pooling of data may be required to conduct these types of analyses. Fifth, several participants were missing PiB-PET scans, which prevented us from stratifying participants based on positive/negative PiB-PET binding. Integration of amyloid-specific biomarkers would have reinforced the assignment of the suspected MixD status. Sixth, a longitudinal design is needed to compare the progression of imaging abnormalities, such as whether the conversion of NAWM into WMSA (and the accompanying DTI parameter changes) is accelerated in MixD. The same design is also favored to track the rates of brain atrophy that may be additionally contributed by vascular factors.
Conclusions
Our exploratory analyses suggest that MixD patients present characteristic abnormalities, including higher burden of MRI signal alterations within the frontal lobar areas and potentially elevated tissue water content within the abnormal WM areas than in “pure” AD or SVaD. Future studies are warranted to expand our findings in a larger sample, to investigate the underlying etiologies, and to determine the clinical relevance of these characteristics.
Acknowledgements
This study was supported by an Alzheimer Society of Canada grant (#14-33) to Ging-Yuek Robin Hsiung.
Hyunwoo Lee acknowledges support from a CIHR Fellowship.
Author Contributions
HL: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing – Original Draft, Visualization.
VW: Software, Investigation, Writing – Review & Editing, Visualization.
AR: Software, Investigation, Writing – Review & Editing.
CK: Software, Writing – Review & Editing.
MFB: Software, Investigation, Writing – Review & Editing.
KP: Software, Investigation, Writing – Review & Editing.
RT: Software, Investigation, Writing – Review & Editing.
KL: Software, Investigation, Writing – Review & Editing.
CJ: Investigation, Writing – Review & Editing.
VS: Software, Investigation, Writing – Review & Editing.
ES: Software, Investigation, Writing – Review & Editing.
JP: Investigation, Writing – Review & Editing.
GYRH: Conceptualization, Methodology, Investigation, Resources, Writing – Review & Editing, Supervision, Project administration, Funding acquisition.
Disclosures
Hyunwoo Lee, Alexander Rauscher, Christian Kames, Mirza Faisal Beg, Karteek Popuri, Roger Tam, Kevin Lam, Claudia Jacova, Elham Shahinfard, Vesna Sossi, and Jacqueline Pettersen have no conflict of interest/disclosures to report.
Vanessa Wiggermann serves as a board member for NeuroImage: Clinical.
Ging-Yuek Robin Hsiung has received research support as a clinical trials site investigator from Anavax, Biogen, Eli Lilly, and Roche, and has received research grants from the CIHR, Alzheimer Society of Canada, and NIA/NIH. Dr Hsiung is supported by the Ralph Fisher Professorship in dementia research from the Alzheimer Society of British Columbia.











