Introduction
The improvements in in vitro culture systems for ovarian follicles, oocytes and embryos are of great relevance to increase efficiency of assisted reproduction techniques in mammalian species. However, lipid peroxidation and imbalance in the production and elimination of reactive oxygen species (ROS) represent the main barriers to having healthy oocytes and embryos after in vitro culture and cryopreservation (Soto-Heras and Paramio, Reference Soto-Heras and Paramio2020). In this sense, the addition of natural substances to the culture media has been an alternative to control the damages caused by excessive ROS (Paulino et al., Reference Paulino, Barroso, Silva, Barroso, Barbalho, Bezerra, Souza, Monte, Silva, Matos and Silva2022). L-carnitine is a water-soluble vitamin-like compound that is naturally produced and synthesized primarily from lysine and methionine in the liver to improve lipid breakdown and generate metabolic (Modak et al., Reference Modak, Alam, Islam, Paul, Akter, Hashem, Kabir and Moniruzzaman2022). According to Carrillo-González et al. (Reference Carrillo-González, Hernández-Herrera and Maldonado-Estrada2023), lipids are the most abundant reservoir of energy in bovine embryos, and triacylglycerol-containing lipid droplets represent the main stocks of fatty acids in oocytes. These authors showed that L-carnitine mobilizes fatty acids from oocyte cytoplasm to mitochondria, which results in β-oxidation and generation of energy. Additionally, acetyl-L-carnitine exhibits antioxidant effects and has beneficial effects on reproductive functions (Liu et al., Reference Liu, Head, Kuratsune, Cotman and Ames2004; Cheng and Chen, Reference Cheng and Chen2008; Aliabadi et al., Reference Aliabadi, Soleimani Mehranjani, Borzoei, Talaei-Khozani, Mirkhani and Tabesh2012; Agarwal et al., Reference Agarwal, Sengupta and Durairajanayagam2018). When administered exogenously, acetyl-L-carnitine has higher bioavailability than L-carnitine and regulates even the production of reproduction-associated hormones (Agarwal et al., Reference Agarwal, Sengupta and Durairajanayagam2018).
This review aims to show the role of L-carnitine on hypothalamus-pituitary-gonad-axis and to discuss its influence on lipid β-oxidation and oxidative stress during in vitro culture of ovarian follicles, oocyte maturation, embryo development and cryopreservation.
Oxidative stress
Free radicals are chemical specimens that have at least one unpaired electron in their outer orbitals, being highly reactive (Prevedello and Comachio, Reference Prevedello and Comachio2021). This characteristic enables the transfer of electrons between neighbouring molecules, causing changes in the molecular environment (Ferreira et al., Reference Ferreira, da Silva Ferreira, de Almeida Costa, de Lima Santos and da Silva2020; Martelli and Nunes, Reference Martelli and Nunes2014). The ROS are naturally produced by cellular metabolism and play an important physiological role, being involved in several processes, such as energy production, phagocytosis, intercellular signalling, regulation of cell growth, immunity, cell defence and synthesis of biological substances (Prevedello and Comachio, Reference Prevedello and Comachio2021). However, when ROS production exceeds its degradation, it causes oxidative stress, being responsible for various damages to DNA, proteins and phospholipids in different cell types (Simas et al., Reference Simas, Granzoti and Porsch2019). Controlling the production and neutralization of ROS is crucial for maintaining cellular integrity. In vivo, this control is performed through enzymatic and non-enzymatic antioxidant systems. Various endogenous enzymes, like catalase (CAT), peroxiredoxins (PRDX), superoxide dismutase (SOD) and glutathione reductase/peroxidase (GPX) constitute the endogenous antioxidant system (Souza et al., Reference Souza, Costa, Santos, Santos, Costa, Oliveira, Silva and Estevam2020), which are capable of inactivating the harmful effects of free radicals. The non-enzymatic system includes low molecular weight compounds such as L-carnitine, ascorbic acid, tocopherol, selenium, zinc, taurine, hypotaurine, carotene, lipoic acid and other thiol compounds such as cystine, cysteine, cysteamine and beta-mercaptoethanol (Crocomo et al., Reference Crocomo, Marques Filho, Landim-Alvarenga and Bicudo2012).
During in vitro culture of different types of cells, the reduction of endogenous antioxidant protection linked to other factors, such as exposure to light and high concentrations of oxygen, favours a significant increase in ROS production (Alves et al., Reference Alves, Furtado, Nascentes, Rumpf and Tavares2019; Sadeesh et al., Reference Sadeesh, Shah, Balhara, Thirumaran, Yadav and Yadav2014) and oxidative stress, which has been reported as one of the main limitations of in vitro culture of various types of cells (Del Collado et al., Reference Del Collado, da Silveira, Oliveira, Alves, Simas, Godoy, Coelho, Marques, Carriero, Nogueira, Eberlin, Silva, Meireles and Perecin2017; Soto-Heras and Paramio, Reference Soto-Heras and Paramio2020). In excess, oxidative stress in granulosa cells results in follicular atresia (Saeed-Zidane et al., Reference Saeed-Zidane, Linden, Salilew-Wondim, Held, Neuhoff, Tholen, Hoelker, Schellander and Tesfaye2017) and has been reported as one of the main factors associated with poor quality of cultured ovarian follicles (Sá et al., Reference Sá, Bruno, Guerreiro, Cadenas, Alves, Cibin, Leal- Cardoso, Gastal and Figueiredo2018; Paulino et al., Reference Paulino, Barroso, Silva, Barroso, Barbalho, Bezerra, Souza, Monte, Silva, Matos and Silva2022). Due to damages caused by oxidative stress during in vitro culture, several studies have sought to develop protocols to minimize it (Cordeiro et al., Reference Cordeiro, Silva, Paulino, Barroso, Barrozo, de Lima Neto and Silva2023; Nascimento et al., Reference Nascimento, Azevedo, Barroso, Barrozo, Silva, Silva, Donato, Peixoto and Silva2022).
Effects of L-carnitine on hypothalamus-pituitary-gonadal axis
The L-carnitine influences the hypothalamus-pituitary-gonad axis and upregulates gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus and, consequently, induce depolarization of hypothalamic neuronal cells to increase secretory activity (Agarwal et al., Reference Agarwal, Sengupta and Durairajanayagam2018; Krsmanovic, et al., Reference Krsmanovic, Virmani, Stojilkovic and Catt1994). Also, L-carnitine has been reported to increase the levels of other hormones, like luteinizing hormone (LH), progesterone and oestradiol, while it decreases prolactin secretion in mammalian species (Agarwal et al., Reference Agarwal, Sengupta and Durairajanayagam2018; Genazzani et al., Reference Genazzani, Lanzoni, Ricchieri, Santagni, Rattighieri, Chierchia, Monteleone and Jasonni2011; Krsmanovic et al., Reference Krsmanović, Virmani, Stojilković and Catt1992). Figure 1 illustrates the effects of L-carnitine on the reproductive system of mammalian females.
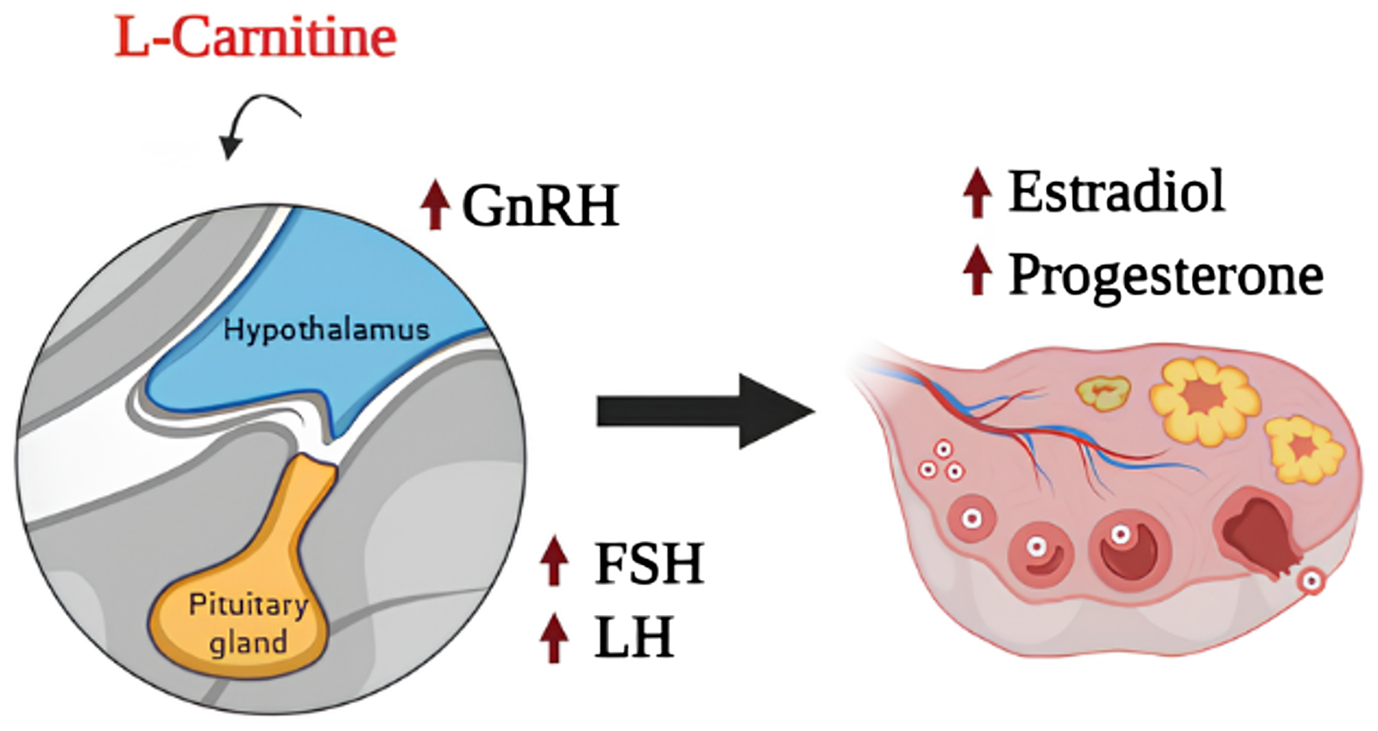
Figure 1. Effects of L-carnitine on hypothalamus-pituitary gonad axis.
The L-carnitine and its primary ester have direct effects against oxidative stress, minimizing cell death by apoptosis and maintaining cellular energy (Agarwal et al., Reference Agarwal, Sengupta and Durairajanayagam2018; Abdelrazik et al., Reference Abdelrazik, Sharma, Mahfouz and Agarwal2009; Infante et al., Reference Infante, Tschanz, Shaw, Michaud, Lawrence and Brenna2002; Vanella et al., Reference Vanella, Russo, Acquaviva, Campisi, Di Giacomo, Sorrenti and Barcellona2000). To minimize oxidative stress, L-carnitine can also be used in combination with other antioxidant commonly known for quenching free radicals such as vitamins (C, E and β-carotene), and some metalloenzymes, including GPx, CAT and SOD (Nimse and Pal, Reference Nimse and Pal2015). Thus, due to its energy generation property combined with its antioxidant property, L-carnitine has been studied for use in reproductive technologies, including in vitro culture of ovarian follicles, in vitro maturation, in vitro embryo production and cryopreservation.
Effects of L-carnitine on in vitro follicle development and oocyte maturation
The role of L-carnitine in ovarian follicles in vitro is still little explored. Dunning and Robker (Reference Dunning and Robker2012) reported that L-carnitine did not alter survival, growth or differentiation of mouse secondary follicles in vitro. However, it significantly increased β-oxidation and markedly improved fertilization rate and blastocyst development. Recently, Modak et al. (Reference Modak, Alam, Islam, Paul, Akter, Hashem, Kabir and Moniruzzaman2022) reported that L-carnitine increased the rate of oocytes in metaphase II (MII) stage from early antral follicles cultured in vitro. Furthermore, the presence of L-carnitine decreased the rate of degeneration and even promoted the formation of structures similar to antrum after the in vitro culture of buffalo oocyte granulosa complexes.
In mouse oocytes, L-carnitine acts through the electrogenic force of voltage-gated Na+ channels and it is transported by Na+/organic cationic transporter-2 (OCTN-2) to oocytes (Infante et al. Reference Infante, Tschanz, Shaw, Michaud, Lawrence and Brenna2002; Dunning and Robker Reference Dunning and Robker2012). In the oocyte, L-carnitine is converted to acetyl-L-carnitine by carnitine palmitoyltransferase-I (CPT-I) in the mitochondria and can act on the endoplasmic reticulum, mitochondria and even in ooplasm (Mingorance et al., Reference Mingorance, Rodriguez-Rodriguez, Justo, Herrera and de Sotomayor2011) (Figure 2). Various studies have shown that L-carnitine optimizes glucose metabolism by transferring fatty acids to the mitochondria and facilitating β-oxidation since lipid metabolism is one of the primary regulators of oocyte maturation (Stojkovic et al., Reference Stojkovic, Machado, Stojkovic, Zakhartchenko, Hutzler, Gonçalves and Wolf2001; Dunning et al., Reference Dunning, Cashman, Russell, Thompson, Norman and Robker2010; Agarwal et al., Reference Agarwal, Sengupta and Durairajanayagam2018). Within the oocyte, L-carnitine is converted to acetyl-L-carnitine and keeps glucose metabolism through the citric acid cycle and, consequently, increases energy production (Infante et al., Reference Infante, Tschanz, Shaw, Michaud, Lawrence and Brenna2002; Agarwal et al., Reference Agarwal, Sengupta and Durairajanayagam2018) (Figure 2). The L-carnitine reduces pyruvate entry into the citric acid cycle and transports palmitate and other long-chain fatty acids to facilitate their utilization through β-oxidation (Dunning et al., Reference Dunning, Cashman, Russell, Thompson, Norman and Robker2010) (Figure 2). The L-carnitine reduces the levels of palmitate in the endoplasmic reticulum by transferring it to mitochondria or by eliminating it from where it can cause oocyte lipotoxicity by oxidative stress (Agarwal et al., Reference Agarwal, Sengupta and Durairajanayagam2018). L-carnitine increases the proportion of mature oocytes with uniform mitochondrial distribution and supports in vitro oocyte maturation and embryonic development in mice and pigs (Zare et al., Reference Zare, Masteri Farahani, Salehi, Piryaei, Ghaffari Novin, Fadaei Fathabadi, Mohammadi and Dehghani-Mohammadabadi2015; Somfai et al., Reference Somfai, Kaneda, Akagi, Watanabe, Haraguchi, Mizutani, Dang-Nguyen, Geshi, Kikuchi and Nagai2011). Chankitisakul et al. (Reference Chankitisakul, Somfai, Inaba, Techakumphu and Nagai2013) showed that L-carnitine increases the rate of bovine embryo production after in vitro maturation and subsequent vitrification of oocytes. Marin et al. (Reference Marin, Nogueira da Costa, di Paula Bessa Santana, Baia de Souza, Rolim Filho, da Silva Cordeiro and Ohashi2020) also reported that L-carnitine increased oocyte competence during buffalo oocyte maturation in the absence of foetal bovine serum.

Figure 2. Direct effects of L-carnitine on oocytes of mammals.
In vivo, L-carnitine can stabilize the mitochondrial membrane and protect DNA against ROS-induced damage in oocytes of women with polycystic ovary syndrome (PCOS) (Mohd Shukri et al., Reference Mohd Shukri, Norhayati, Badrin and Abdul Kadir2022; Ismail et al., Reference Ismail, Hamed, Saso and Thabet2014; Fenkci, et al., Reference Fenkci, Fenkci, Oztekin, Rota and Karagenc2008). Using a mouse model of PCOS, oral administration of acetyl-L-carnitine alleviated ovarian dysfunction associated with that syndrome through its antioxidant/glycation activity and mitochondria potentiation (Di Emidio et al., Reference Di Emidio, Rea, Placidi, Rossi, Cocciolone, Virmani, Macchiarelli, Palmerini, D’Alessandro, Artini and Tatone2020).
Effects of L-carnitine on in vitro embryo development
The ROS may originate in the embryo itself or from exogenous sources. During oocyte in vitro fertilization (IVF), strategies to reduce ROS production, such as addition of free radical scavengers and lowering the oxygen tension are important for improving the fertility potential in assisted reproductive technologies (Agarwal et al., Reference Agarwal, Durairajanayagam and du Plessis2014). The ROS are involved in defective embryo development and retardation of embryo growth and induce cell membrane damage, DNA damage and apoptosis (Volpe et al., Reference Volpe, Villar-Delfino, Dos Anjos and Nogueira-Machado2018). Apoptosis results in fragmented embryos, which have limited potential to implant and therefore, result in poor fertility rates (Agarwal et al., Reference Agarwal, Durairajanayagam and du Plessis2014).
The antioxidant capacity of L-carnitine might account for its preferential use to improve in vitro oocyte and embryo development. The treatment of porcine embryos with antioxidants improved blastocyst production (Castillo-Martín, et al., Reference Castillo-Martín, Bonet, Morató and Yeste2014). Moreover, the presence of L-carnitine in the culture medium was associated with increased cleavage and blastocyst rates in porcine species (Lowe et al., Reference Lowe, Bartolac, Bathgate and Grupen2017). Finally, the supplementation of L-carnitine to bovine embryo culture medium has been shown to scavenge ROS within two-cell stage embryos (Takahashi et al., Reference Takahashi, Inaba, Somfai, Kaneda, Geshi, Nagai and Manabe2013).
Effects of L-carnitine on cryopreservation of ovarian follicles, oocytes and embryos
The cryopreservation technique allows the sub-zero storage of tissues or cells by dramatically reducing natural cellular biochemical processes for extended periods of time (Vining et al., Reference Vining, Zak, Harvey and Harvey2021). Currently, cryopreservation is a modern and safe method which assists in the preservation of genetic material from follicles, oocytes and embryos in human and animals (Sekhon et al., Reference Sekhon, Lee, Flisser, Copperman and Stein2018), but the cells can still be seriously damaged during cryopreservation (Truong, et al., Reference Truong, Harvey and Gardner2022; Spijkers et al., Reference Spijkers, Lens, Schats and Lambalk2017; Barsky et al., Reference Barsky, St Marie, Rahil, Markenson and Sites2016; Beyer and Griesinger, Reference Beyer and Griesinger2016). Unfortunately, frozen follicles, oocytes and embryos are still reported to contain a higher proportion of apoptotic cells compared to their non-frozen counterparts, with freezing procedures generally associated with triggering apoptotic cell death (Vining, et al., Reference Vining, Zak, Harvey and Harvey2021). Exposure to high concentrations of cryoprotectants, osmolarity and rapid temperature changes during cryopreservation have been shown to affect gamete and embryo physiology (Somoskoi et al., Reference Somoskoi, Martino, Cardone, Lacalandra, Dell’Aquila and Cseh2015; Dalcin et al., Reference Dalcin, Silva, Paulini, Silva, Neves and Lucci2013), as well as their gene expression (Sahraei et al., Reference Sahraei, Shahhoseini and Movaghar2018; Monzo et al., Reference Monzo, Haouzi, Roman, Assou, Dechaud and Hamamah2012). These deleterious effects are strongly associated with the occurrence of oxidative stress during cryopreservation.
Production of ROS during the vitrification of gametes may be a crucial mediator of damage to proteins and DNA (Costa et al., Reference Costa, Vasconcelos, Azevedo, de Assis, Paulino, Silva, SILVA and Batista2022; Zhang et al., Reference Zhang, Yao, Liu, Ren, Xiang and Wang2020). Disturbances in the oxidative metabolism and damage in cell membranes are other important stress factors related to vitrification. Together, these effects decrease glutathione (GSH) levels, alter expression of regulatory genes and are associated with decreasing maturation rate and developmental competence of follicles, oocytes and embryos after cryopreservation (Berteli et al., Reference Berteli, Vireque, Da Luz, Borges, Ferreira and Navarro2022; Zare et al., Reference Zare, Rezaei and Mohammadi2022; Costa et al., Reference Costa, Vasconcelos, Azevedo, de Assis, Paulino, Silva, SILVA and Batista2022; Wu et al., Reference Wu, Pan, Qazi, Yang, Guo, Yang, Zhang, Zeng, Zhang, Han, Meng and Zhou2019; Pan et al., Reference Pan, Yang, Wu, Qazi, Liu, Han, Meng and Zhou2018). Furthermore, cryopreservation of oocytes or embryos has been reported to cause mitochondrial dysfunction, such as changes in membrane potential and reduced adenosine triphosphate (ATP) production (Gualtieri et al., Reference Gualtieri, Kalthur, Barbato, Di Nardo, Adiga and Talevi2021; Iwata, Reference Iwata2021). However, the detrimental effects of cumulative stress have been shown to be partly improved by adding antioxidants in vitrification media, such as L-carnitine. Some reports have already demonstrated that L-carnitine plays an important role in attenuating the deleterious effects of oxidative stress on cryopreserved follicles. For instance, Zhang et al. (Reference Zhang, Wang, Yao, Zhang, Zhang, Chen, Fu and Zhang2015) observed lower rates of apoptosis and malondialdehyde, as well as higher levels of oestradiol in mice ovarian follicles cryopreserved in situ. These results were translated into increased follicular survival. Zolini et al. (Reference Zolini, Carrascal-Triana, Ruiz de King, Hansen, Alves Torres and Block2019) demonstrated that the addition of L-carnitine in embryo culture medium improved post-thaw cryotolerance but had no effect on pregnancy and implantation rate after transfer of cryopreserved bovine embryos.
The L-carnitine is well known for its role in β-oxidation, ATP production and decreasing the lipid content during embryo development, providing improved cryo-survivability (Truong et al., Reference Truong, Soh and Gardner2016). In buffaloes, the addition of L-carnitine to the medium significantly benefits embryonic developmental competence after vitrification, as evidenced by the high cleavage rate and the formation of morulae and blastocysts. Improving the cryotolerance of buffalo embryos directly after thawing may be through increased lipid metabolism (El-Sokary et al., Reference El-Sokary, El-Naby, Hameed, Mahmoud and Scholkamy2021). Furthermore, L-carnitine acts as an antioxidant blocking degenerative changes arising from oxidative stress during embryonic development (Bhakty et al., Reference Bhakty, Kaiine, Karjan and Setiadim2021). Lowe et al. (Reference Lowe, Bartolac, Bathgate and Grupen2017) reported that the antioxidant capacity of L-carnitine was associated with the increased cleavage rate and the improved cryotolerance of resultant porcine blastocysts. Supplementation of L-carnitine to bovine embryo culture medium has been shown to scavenge ROS within two-cell stage embryos, antagonize the cryodamage and enhance the cryotolerance of blastocysts (Takahashi et al., Reference Takahashi, Inaba, Somfai, Kaneda, Geshi, Nagai and Manabe2013). It is plausible to suggest that L-carnitine may be used to improve the freezing survival of oocytes or embryos (Li et al., Reference Li, Wu, Ma, Zhang, Sun, Qi and Ma2023). Table 1 shows some effects of L-carnitine during in vitro culture of follicles, oocytes and embryos in different species.
Table 1. Effects of L-carnitine during in vitro culture of follicles, oocytes and embryos in different species

Final considerations
Many factors inherent to the oocyte itself and in vitro culture environment determine the chance of having a complete follicular development with success in the acquisition of oocyte competence in vitro. A culture system, with different combinations of hormones, growth factors and mainly antioxidant factors, at each stage of growth, is necessary to allow the follicles to present an adequate size in a long-term culture period. This review shows that L-carnitine can be used to regulate oxidative stress and lipid β-oxidation during in vitro culture of ovarian follicles, oocyte maturation, embryo production and cryopreservation, especially due to stimulation energy generation combined with its antioxidant properties.
Competing interests
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Funding
This work was supported by the National Council for Scientific and Technological Development (CNPq, Brazil, Grant No. 407992/2021-9) and Coordination for the Improvement of Higher Education Personnel (CAPES).






