Reality orientation therapy has been associated with significant improvements in cognition and behaviour and with a reduced risk of admission to care among people with Alzheimer's disease (Reference Zanetti, Frisoni and De LeoZanetti et al, 1995; Reference Metitieri, Zanetti and GeroldiMetitieri et al, 2001; Reference Spector, Thorgrimsen and WoodsSpector et al, 2003). A meta-analysis of six controlled trials concluded that reality orientation should be considered as part of dementia care programmes, but also identified the need for large, well-designed multicentre trials (Reference Spector, Orrell and DaviesSpector et al, 2000a ). In addition, clinical trials of reality orientation published so far have not tested the effectiveness of this therapy in association with medication with cholinesterase inhibitors, nor evaluated the efficacy of programmes provided in the patient's own home (Reference Spector, Davies and WoodsSpector et al, 2000b ). Therefore, the aim of this randomised clinical trial was to evaluate the effectiveness of a long-term (25 weeks), home-based programme of reality orientation on cognitive function in a group of patients with Alzheimer's disease receiving treatment with cholinesterase inhibitors.
METHOD
The study was conducted in five Alzheimer Evaluation Units (Catholic University of the Sacred Heart, Rome; Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Centro San Giovanni di Dio, Fatebenefratelli, Brescia; Hospital San Eugenio, Rome; Hospital Opera Don Uva, Guidonia; Hospital San Giovanni Calibita Fatebenefratelli, Rome) participating in the CRONOS project, a national study sponsored by the Italian government with the intention of standardising prescriptions of cholinesterase inhibitors and assessing the effects of these drugs on defined outcomes in unselected individuals with Alzheimer's disease (Blanchetti et al, 2003).
People were considered suitable for participation in the study if they met the National Institute of Neurological and Communicative Diseases and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS–ADRA) criteria for probable Alzheimer's disease (Reference McKhann, Drachman and FolsteinMcKhann et al, 1984), scored between 14 and 27 on the Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975), did not present with major aphasia or blindness, and had received pharmacological treatment with donepezil for at least 3 months. Figure 1 shows the trial profile, including data on people screened and excluded because they did not meet the eligibility criteria. Data on those not receiving pharmacological treatment with donepezil for at least 3 months were not collected. All patients participating in the CRONOS project were taking donepezil (the only cholinesterase inhibitor available in Italy at the time of the study) and were followed for at least 6 months.
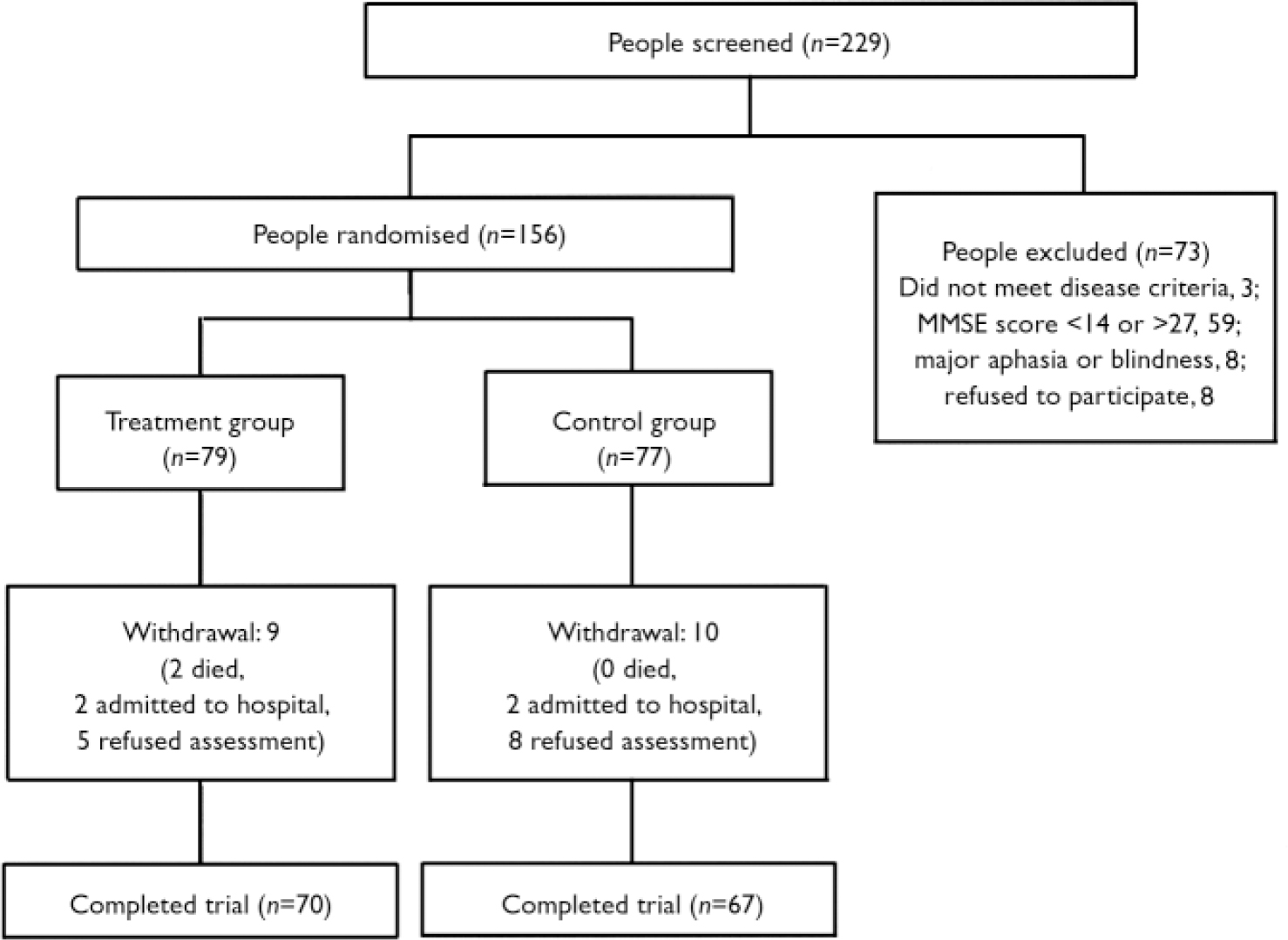
Fig. 1 Trial profile. MMSE, Mini-Mental State Examination.
A total of 156 eligible patients, enrolled in the participating centres between January 2002 and August 2002, were randomly assigned in a 1:1 ratio to receive either a reality orientation programme at home, provided by caregivers, or no treatment. Participants were allocated to the two study groups according to a computerised block randomisation process (block randomisation was used in order to keep the number of participants in the different groups closely balanced at all times).
Patients and caregivers participating in the study were assessed at baseline and at the 25-week follow-up (end of the study) by personnel unaware of group allocation. Patients’ assessments included demographic information; cognitive function, measured with the MMSE and the Alzheimer's Disease Assessment Scale – Cognition (ADAS–Cog; Reference Rosen, Mohs and DavisRosen et al, 1984); functional status, measured with the Barthel index (Reference Mahoney and BarthelMahoney & Barthel, 1965) and Instrumental Activities of Daily Living (IADL; Reference Lawton and BrodyLawton & Brody, 1969); behaviour (Neuropsychiatric Inventory; Reference Cummings, Mega and GrayCummings et al, 1994); and medications used. Caregivers’ assessment included demographic information; mood measured with the Hamilton Rating Scales for Depression (HRSD; Reference HamiltonHamilton, 1967) and Anxiety (HRSA; Reference HamiltonHamilton, 1959); quality of life (rated using the Medical Outcomes Study 36-item Short-Form General Health Survey (SF–36; Reference Tarlov, Ware and GreenfieldTarlov et al, 1989)); and burden of care (Caregiver Burden Inventory; Reference Novak and GuestNovak & Guest, 1989).
In each of the participating centres, caregivers in the intervention group were trained by a team including physicians, psychologists and therapists to deliver a programme of reality orientation in patient's own home. They were also provided with a manual of instruction on this therapy and specific schedules for each session. During the training meeting, a brief history of reality orientation therapy and results obtained by the use of this approach in previous studies were presented. Next the manual was read and discussed in order to answer questions raised by caregivers and to resolve any doubts. In addition, caregivers were given a detailed explanation of how to approach and stimulate the patients both during the reality orientation session and informally during the day. Finally, a simulated therapy session was presented.
Caregivers were instructed to provide three orientation sessions per week, for 25 consecutive weeks. Each session lasted about 30 min and consisted of an organised, intensive cognitive training during which the caregiver gradually presented information such as date, time and location. In the first part of the session attention was directed to personal, time and space orientation; following this, topics of general interest such as historical events and famous people, attention, memory and visuospatial exercises were introduced. Patients were prompted to give either spontaneous or cued answers, with the aid of calendars, clocks and notes. Besides the formal reality orientation sessions, caregivers were also invited to stimulate and involve patients in reality-based communication two or three times throughout the day informally, focusing on personal, time and space orientation and discussing news or topics of general interest.
The appropriate local research ethics committees granted approval. After hearing an explanation of the study, patients and caregivers who agreed to participate gave written informed consent.
We calculated that a sample size of 142 participants allows for detection of a difference of 2 points in MMSE score between study groups, with 80% power and at a 0.05 level of type I error. This calculation assumed, on the basis of a previous observation (Reference Metitieri, Zanetti and GeroldiMetitieri et al, 2001), a common standard deviation of 4 and a 10% withdrawal rate.
Statistical analysis
Differences in baseline characteristics between the treatment group and the control group in categorical parameters were tested using Fisher's exact test. Differences between continuous variables were assessed by analysis of variance (ANOVA) comparisons for normally distributed parameters; otherwise, the Kruskal–Wallis test was used. Data were analysed based on intention to treat. Analysis of covariance (ANCOVA) was performed to compare the change in outcome measures between treatment and control group. Analyses were adjusted for baseline value of the outcome measure. In additional analyses, we calculated the number of participants needed to be treated for 1 patient to achieve one of the following outcomes: an improvement of 4 or more points in the ADAS–Cog score, or any improvement in the ADAS–Cog score (change in score 40). The number needed to treat (NNT) was calculated using the formula described by Cook & Sackett (Reference Cook and Sackett1995). Finally, to explore whether the effect of the intervention on cognitive outcomes (MMSE and ADAS–Cog) differed according to baseline cognitive status, we repeated ANCOVA comparisons separately for patients with baseline MMSE scores below 20 (n=60; moderate dementia) and those with scores of 20 or over (n=77; mild dementia). These analyses were adjusted for baseline value of the outcome measure. A value of P<0.05 (two-tailed) was considered statistically significant. All analyses were performed using the Statistical Package for the Social Sciences (SPSS for Windows, version 10.0).
RESULTS
The mean age of the 156 patients participating in the study was 75.8 years (s.d.=7.1); there were 113 (72%) women in the sample and Alzheimer's disease was diagnosed on average 2.0 (s.d.=1.5) years before study entry. Baseline characteristics of patients and caregivers according to study group are presented in Table 1. Seventy of 79 patients in the treatment group and 67 of 77 patients in the control group completed the study (Fig. 1). Only two patients died during the follow-up period and four were admitted to institutional care.
Table 1 Characteristics of participants at study entry
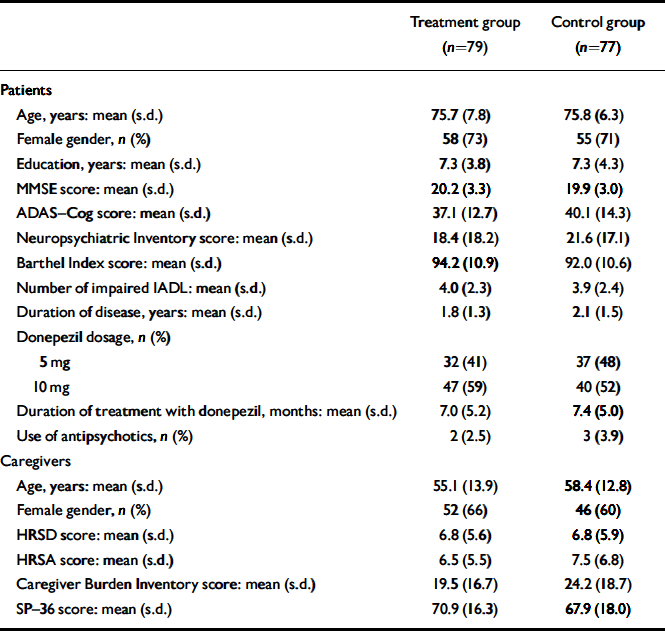
| Treatment group (n=79) | Control group (n=77) | |
|---|---|---|
| Patients | ||
| Age, years: mean (s.d.) | 75.7 (7.8) | 75.8 (6.3) |
| Female gender, n (%) | 58 (73) | 55 (71) |
| Education, years: mean (s.d.) | 7.3 (3.8) | 7.3 (4.3) |
| MMSE score: mean (s.d.) | 20.2 (3.3) | 19.9 (3.0) |
| ADAS—Cog score: mean (s.d.) | 37.1 (12.7) | 40.1 (14.3) |
| Neuropsychiatric Inventory score: mean (s.d.) | 18.4 (18.2) | 21.6 (17.1) |
| Barthel Index score: mean (s.d.) | 94.2 (10.9) | 92.0 (10.6) |
| Number of impaired IADL: mean (s.d.) | 4.0 (2.3) | 3.9 (2.4) |
| Duration of disease, years: mean (s.d.) | 1.8 (1.3) | 2.1 (1.5) |
| Donepezil dosage, n (%) | ||
| 5 mg | 32 (41) | 37 (48) |
| 10 mg | 47 (59) | 40 (52) |
| Duration of treatment with donepezil, months: mean (s.d.) | 7.0 (5.2) | 7.4 (5.0) |
| Use of antipsychotics, n (%) | 2 (2.5) | 3 (3.9) |
| Caregivers | ||
| Age, years: mean (s.d.) | 55.1 (13.9) | 58.4 (12.8) |
| Female gender, n (%) | 52 (66) | 46 (60) |
| HRSD score: mean (s.d.) | 6.8 (5.6) | 6.8 (5.9) |
| HRSA score: mean (s.d.) | 6.5 (5.5) | 7.5 (6.8) |
| Caregiver Burden Inventory score: mean (s.d.) | 19.5 (16.7) | 24.2 (18.7) |
| SP—36 score: mean (s.d.) | 70.9 (16.3) | 67.9 (18.0) |
The mean duration of follow-up was 25.4 weeks (s.d.=5.0). Table 2 compares changes in patient and caregiver outcomes between the two study groups, after adjusting for the baseline value of the outcome measure examined. Reality orientation appeared to have an additive beneficial effect on cognition: in the treatment group MMSE scores showed a slight improvement (0.2 points, s.e.=0.4) compared with a decline of 1.1 points (s.d.=0.4) in the control group (P=0.02). Similarly, the ADAS–Cog score improved by 0.4 points (s.e.=0.8) in the treatment group and declined by 2.5 points (s.e.=0.8) in the control group (P=0.01). No significant difference between study groups was observed for ratings on the Neuropsychiatric Inventory (P=0.23), Barthel Index (P=0.18) and number of impaired IADL (P=0.34). In addition, we did not observe any significant difference for scores on the Neuropsychiatric Inventory sub-scales (data not shown).
Table 2 Change in patients’ and caregivers’ outcomes between baseline and follow-up (positive values signify improvement)
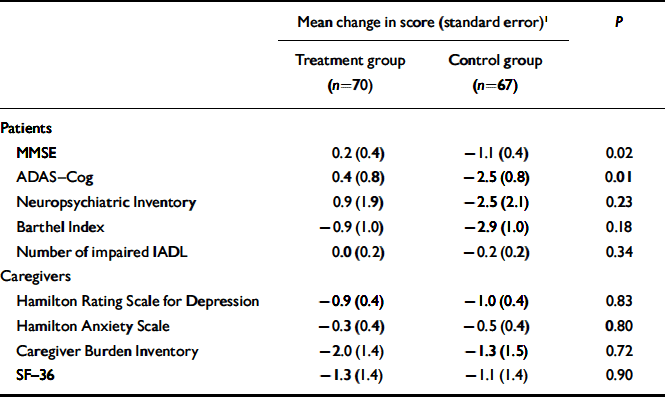
| Mean change in score (standard error)1 | P | ||
|---|---|---|---|
| Treatment group (n=70) | Control group (n=67) | ||
| Patients | |||
| MMSE | 0.2 (0.4) | -1.1 (0.4) | 0.02 |
| ADAS—Cog | 0.4 (0.8) | -2.5 (0.8) | 0.01 |
| Neuropsychiatric Inventory | 0.9 (1.9) | -2.5 (2.1) | 0.23 |
| Barthel Index | -0.9 (1.0) | -2.9 (1.0) | 0.18 |
| Number of impaired IADL | 0.0 (0.2) | -0.2 (0.2) | 0.34 |
| Caregivers | |||
| Hamilton Rating Scale for Depression | -0.9 (0.4) | -1.0 (0.4) | 0.83 |
| Hamilton Anxiety Scale | -0.3 (0.4) | -0.5 (0.4) | 0.80 |
| Caregiver Burden Inventory | -2.0 (1.4) | -1.3 (1.5) | 0.72 |
| SF—36 | -1.3 (1.4) | -1.1 (1.4) | 0.90 |
There was no difference between the two caregiver groups in terms of their decline in scores on the HRSD, HRSA, Caregiver Burden Inventory and SF–36 (Table 2). No significant difference between the groups was observed in Caregiver Burden Inventory and SF–36 sub-scales (data not shown).
Overall, 19% of participants in the treatment group and 11% in the control group improved by 4 or more points in the ADAS–Cog score. When calculating the NNT for this outcome, we found that 14 people needed to be treated in order for 1 to benefit. Similarly, 45% of participants in the treatment group and 36% in the control group showed an improvement in the ADAS–Cog score (change in score >0). In this case, 11 people needed to be treated in order for 1 to benefit.
In additional analyses we explored the effect of treatment on cognitive outcomes in patients with moderate dementia (n=60) and mild dementia (n=77). Among patients with moderate dementia, treatment was associated with an improvement in both MMSE score (1.1 points, s.e.=0.7) and ADAS–Cog (1.8 points, s.e.=1.2), compared with a decline in these measures observed among patients in the control group (MMSE score –0.4, s.e.=0.6, P=0.12 v. treatment group; ADAS–Cog –1.8, s.e.=1.1, P=0.03 v. treatment group). Among patients with mild dementia, both MMSE and ADAS–Cog scores declined during the study period, but this change was less marked in the intervention group than in the control group: MMSE score: treatment group –0.4 (s.e.=0.4), control group –1.7 (s.e.=0.4), P=0.03; ADAS–Cog score: treatment group –0.8 (s.e.=1.0), control group –2.8 (s.e.=1.1), P=0.18. No significant interaction was observed between dementia group and treatment on change in MMSE score (P=0.83) and ADAS–Cog score (P=0.40).
DISCUSSION
Major findings
Our study shows that among patients with Alzheimer's disease, a home-based programme of reality orientation therapy provided by the patients’ caregivers can enhance the effects of cholinesterase inhibitors on cognitive function and that this effect is independent of baseline cognitive status. This intervention does not seem to modify caregivers’ psychological status and quality of life.
Our results confirm and extend to the long term the beneficial effects of reality orientation on cognitive function reported by previous trials of shorter duration (Spector et al, Reference Spector, Orrell and Davies2000a , Reference Spector, Thorgrimsen and Woods2003), and suggest an additive effect of reality orientation when combined with anticholinesterase therapy. This effect may be explained through cognitive stimulation by caregivers, which made participants more able to communicate effectively, and to respond to the environment and to other people, by reinforcing questioning, thinking and interacting ability. In addition, this cognitive training may have improved patients’ self-esteem through their increased ability to retain information and continuous encouragement by caregivers (Reference Spector, Thorgrimsen and WoodsSpector et al, 2003).
The size of the effect of the reality orientation programme on MMSE score (1.3 points) was smaller than we predicted; however, Jonsson et al (Reference Jonsson, Lindgren and Wimo1999) have shown that a difference of even 1 point in MMSE score is associated with a substantial reduction in the cost of caring for patients with dementia. In addition, we failed to show any significant effect on functional and behavioural changes. Thus, if the benefit of our intervention is evaluated in the light of the main objective of psychosocial rehabilitation, its value may be limited. However, it has been suggested that the Barthel Index, IADL and behavioural measures may have low sensitivity to detect mild functional and behavioural changes related to cognitive stimulation programmes and that more sensitive outcomes (i.e. functional performance measures) might provide a superior method for longitudinal assessments (Reference Zanetti, Frisoni and De LeoZanetti et al, 1995; Reference Onder, Penninx and LapuertaOnder et al, 2002). In this context, it should be noted that a recent Cochrane review concluded that reality orientation may have a beneficial effect on behaviour (Spector et al, 2002a), but only one of the individual trials included demonstrated a significant difference in this outcome (Reference Baines, Saxby and EhlertBaines et al, 1987). In line with these findings, previous studies on cholinesterase inhibitor therapy alone showed a lack of improvement in carers’ and patients’ functional and behavioural outcomes, despite a positive effect on cognition (Reference Trinh, Hoblyn and MohantyTrinh et al, 2003; Reference Courtney, Farrell and GrayCourtney et al, 2004).
Comparison with other reality orientation programmes
This is, to our knowledge, the first randomised trial to assess the effectiveness of a home-based reality orientation programme delivered by caregivers. This study combines a formal reality orientation approach, based on lessons given by caregivers on a regular basis during the week, with an informal approach, based on repetition of orientation information at all times throughout the day with no fixed schedule. Most of the clinical trials published so far evaluated the efficacy of formal therapy given in classrooms where groups of patients met in specialised centres on a regular basis to engage in orientation-related activities. Compared with this latter approach, which provides only a ‘mass teaching’ of generic orientation skills, a home-based programme of formal and informal reality orientation delivered by caregivers offers the advantage of a more individualised treatment (Reference Spector, Orrell and DaviesSpector et al, 2000a ). In addition, continuous stimulation and orientation to reality during the day might be a good way to retain what has been learned during formal lessons, leading to an increment in the effect of the formal therapy. In this context, it is noteworthy that our home-based programme did not significantly worsen the psychological status and quality of life of caregivers, suggesting that the higher burden of care related to the sessions was counterbalanced by the improvements experienced by patients.
Effect of reality orientation and baseline cognitive status
We have previously shown that baseline cognitive function may influence response to reality orientation therapy (Reference Zanetti, Oriani and GeroldiZanetti et al, 2002). In this study both patients with mild dementia and those with moderate dementia significantly benefited from the intervention, even if the more severely affected patients had a clear gain in terms of MMSE and ADAS–Cog points, compared with stabilisation in scores on these measures observed among the patients with milder dementia. This favourable response in patients with more severe dementia seems in line with the reported effects of anticholinesterase treatment. Indeed, Farlow et al (Reference Farlow, Hake and Messina2001) showed that rate of dementia progression predicts response to cholinesterase inhibitors, and that patients with more rapidly progressive disease might be particularly likely to benefit from treatment with these agents. However, we cannot exclude the possibility that our findings might be explained by a ceiling effect in this less compromised group, whose scores in the outcome measures were already elevated at baseline.
Reality orientation and anticholinesterase therapy
This is the first trial to evaluate the effect of reality orientation in combination with cholinesterase inhibitor therapy. Participants entering the study had already received treatment with donepezil for at least 3 months, the time required for the maximum effect of this drug to be reached (Reference Feldman, Gauthier and HeckerFeldman et al, 2001). This criterion was applied to ensure the selection of a group of participants receiving donepezil on a stable basis, to reduce the number of people leaving treatment because of side-effects, and to avoid the beneficial response to donepezil observed at the beginning of treatment confounding or hiding the effects of reality orientation. It is therefore not surprising that patients in the control group declined in cognitive outcomes despite treatment with donepezil, since they had started taking this drug on average 7 months before entering the study, by which time the maximum effect of cholinesterase inhibitor therapy would be waning.
Limitations
A major limitation of this study is that the reality orientation programme was administered by caregivers, with no guarantee that patients were receiving the intervention as intended in the study protocol. However, a poor adherence to study protocol would have led to a dilution of the effects, resulting in an underestimation of the benefits of the intervention. In addition, it could be argued that the beneficial effect of the intervention we observed may be related to an improvement in social contact or attention in the intervention group, and that the programme should have been compared with other psychosocial approaches. However, a review by Spector et al (Reference Spector, Davies and Woods2000b ) showed that there are no differences in the effect of alternative activities (either no treatment or alternative social therapy) offered to control groups during trials of reality orientation. Therefore, we concluded from our study that the qualities of reality orientation, rather than merely the therapeutic effect of social contact and attention, may affect patients’ outcomes. Another limitation relates to the fact that data on adherence to donepezil treatment were not collected. During the study period participants might have stopped or changed their dosage of donepezil, and this could have influenced their level of cognition. Finally, the rate of withdrawal in our study was 12% (19 of 156 patients), which is comparable to previous observations with shorter follow-up periods.
In conclusion, our study shows that a home-based programme of reality orientation provided by caregivers improves cognitive function, enhancing the effect of anticholinesterase treatment alone. Future studies are needed to explore whether the benefit of reality orientation may be extended to physical function and behaviour. The addition of non-pharmacological therapy should be recommended in patients receiving cholinesterase inhibitors.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ An individual programme of reality orientation provided by trained caregivers to patients in their own home has a beneficial effect on cognitive function in people with Alzheimer's disease.
-
▪ Reality orientation has an additive effect on cognitive function when combined with anticholinesterase therapy.
-
▪ The execution of a domicilliary programme of reality orientation does not seem to modify caregivers’ psychological status and quality of life.
LIMITATIONS
-
▪ The difference in Mini-Mental State Examination scores between the treatment and control groups, although statistically significant, is lower than predicted for sample size calculation.
-
▪ Adherence of the caregivers to delivery of the home programme was not assessed.
-
▪ Despite a positive effect on cognitive function, no significant difference between the treatment and control groups was detected in functional and behavioural outcomes.
Acknowledgements
The study was supported by a grant from the Italian Ministry of Health (grant number ICS 030.13/RA00.64). We thank Martina Buonomo and Francesca Arcangeli for their help in neuropsychological evaluations and caregiver education.






eLetters
No eLetters have been published for this article.