INTRODUCTION
The increase in life expectancy of adults infected with human immunodeficiency virus (HIV) has contributed to an emerging patient category in the USA, namely HIV-positive elderly persons [Reference Grabar1]. Although similarities in the immunological decline associated with HIV infection and ageing have been noted [Reference Kalayjian2, Reference Appay and Rowland-Jones3], the extent to which HIV influences the normal ageing process and vice versa remains to be determined [Reference Effros4]. HIV-infected individuals are nevertheless recognized as being at increased risk for several age-related conditions, such as cancer, coronary artery disease, diabetes, and dementia [Reference Simone and Appelbaum5, Reference Frame6]. While numerous studies have examined the metabolic or neurological complications of ageing with HIV [Reference Valcour7–Reference Lawrence and Major9], fewer studies have assessed the impact of HIV on other major causes of morbidity and mortality in older persons, such as respiratory infections.
Pneumonia and influenza (P&I) is the sixth-ranked cause of hospitalization among Medicare beneficiaries in the USA [10]. HIV-infected persons of all ages are at heightened risk for P&I [Reference Neuzil11, Reference Lin and Nichol12], although older persons may be particularly vulnerable to hospitalization and death resulting from P&I [Reference Sureka13]. Consistent with the general disease trends in HIV-infected individuals [Reference Crum14, Reference Mocroft15], several studies have documented reduced incidence and case-fatality associated with P&I following the introduction of highly active antiretroviral therapy (HAART) [Reference Sogaard16, Reference Neuzil17]. However, the extent to which HAART has been effective in reducing P&I hospitalization and case-fatality in older persons is unclear. We hypothesize that such individuals are at dual risk for poor outcomes related to P&I due to the compounding effects of HIV-related immunosuppression and age-related immunosenescence. Using Medicare records for the entire US population aged ⩾65 years, we compared the temporo-demographic trends and clinical outcomes of P&I hospitalization in HIV-positive and -negative seniors, over a 14-year period spanning the introduction of HAART.
METHODS
Data
We abstracted all claims containing codes for P&I from the Centers for Medicare and Medicaid Services (CMS) Medicare Provider Analysis and Review database for the period January 1991 to December 2004. Each record provided information on age, gender, race, date of admission, discharge destination, up to ten diagnostic codes [based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes] and diagnosis-related group (DRG). A hospitalization was defined as being related to P&I if the claim included codes for P&I (ICD 480-487) in any of the ten diagnostic fields. Of the records containing a P&I diagnosis, records containing a concurrent diagnosis of HIV infection were identified. Due to changes in HIV coding during the study period, the presence of ICD codes for AIDS, AIDS-related complex and other HIV diseases (042–044), asymptomatic HIV infection (V08), HIV-2 infection (079·53), or inconclusive HIV test results (795·8 or 795·71) or DRG codes relating to HIV infection (488–490) in any of the ten diagnostic fields were used to define the presence of HIV infection.
Records were aggregated by age group (65–74, 75–84, ⩾85 years), gender, race (specifically, white and black), calendar year, calendar month, HIV status and HIV-care period, as appropriate to the analysis (see below). We defined two HIV-care periods as follows: (1) pre-HAART, spanning the period before the Food and Drug Administration's approval of the first protease inhibitor in December 1995 [Reference Walensky18]; and (2) HAART era, during which HAART was widely available in the USA. Due to pronounced seasonality of P&I, HIV-care periods were synchronized with influenza year to improve statistical efficiency. Influenza year was defined as the period from 1 July to 30 June of the following year, as described previously [Reference Thompson19, Reference Cohen20]. Thus, pre-HAART was defined as the period between 1 July 1991 and 30 June 1995; HAART era was defined as the period between 1 July 1997 and 30 June 2004. To be conservative, the period 1 July 1995 to 30 June 1997 was deemed to be a transitional period, marking the time during which HAART became increasingly available.
Analysis
Demographic characteristics
P&I counts were converted into annual rates, using the Medicare beneficiary population as the denominator [21]. Because data on HIV prevalence among Medicare beneficiaries were not available, the fraction of admissions representing HIV-positive persons was calculated with respect to the total number of P&I hospitalizations. Differences in age, gender and race composition by HIV status were assessed using non-parametric Mann–Whitney U tests and Pearson's χ2 test.
Temporo-demographic trends
To observe the temporal trends in P&I hospitalization in HIV-positive and -negative persons, monthly time-series were generated. P&I hospitalization rates were calculated as outlined above. Linear interpolation was used to adjust the denominator for population growth among Medicare beneficiaries. Trend was assessed using generalized log-linear models adapted for seasonality by incorporating harmonic functions [Reference Thompson22, Reference Wenger and Naumova23]. Results were expressed as a relative risk (RR), indicating the relative change in P&I hospitalization across the 14-year period.
To examine if the temporal trend differed by age, we developed a model that described temporal and demographic changes simultaneously. The number of HIV-positive patients/100 000 P&I hospitalizations for each single year of age and for each calendar year was predicted using a nonlinear additive model. The model provided a smoothed fit based on a non-parametric loess procedure with a small window (1/10; equivalent to the average of two consecutive years), as follows:
where Z ij is the number of HIV-positive patients, corrected for changes in P&I hospitalization and ageing in the Medicare beneficiary pool, as follows:
where N ij is the number of P&I hospitalizations of HIV-positive persons of age i in year j; T ij is the number of P&I hospitalizations representing all patients of age i in year j; P ij is the number of Medicare beneficiaries of age i in year j; i=65–85 years (age range was reduced to avoid the spurious high rates due low denominator of older age groups); and j=1991–2004. Predicted values of Z ij were plotted against age and chronological time in years to assess temporo-demographic trends.
In-patient case-fatality
In-patient case-fatality was estimated by calculating the proportion of records containing a discharge code for ‘deceased’ (signifying in-hospital death). Direct comparisons of case-fatality by HIV status, demographic characteristics, HIV-care period and year were made using Pearson's χ2 test and were expressed as odds ratios (OR). Trends in case-fatality by HIV status were assessed using seasonally adjusted, generalized log-linear models as outlined above.
To further assess the impact of HAART on P&I case-fatality in HIV-positive seniors, we constructed a generalized linear model adapted for time-series data. The independent variable of interest was a variable indicating the HIV-care period. A time-sensitive component reflecting trends in case-fatality in HIV-positive persons in the pre-HAART and HAART eras was included. To adjust for improvements in P&I management and virulence of influenza strain, we included a term for P&I case-fatality for HIV-negative persons. Finally, a variable indicating the proportion of HIV-positive persons among all patients hospitalized with P&I was included to account for instability in the dependent variable at the start of the time-series (due to small numbers of HIV-positive persons). Thus, the final model was as follows:
where Y i is the proportion of HIV-positive people who died during hospitalization in month i; TU i is an indicator variable for the HIV-care period in month i (0 for pre-HAART and 1 for HAART era); MB i and MA i are the time before and after the transitional period, expressed in months (the transitional period was excluded from the model); D i is the case-fatality in HIV-negative persons hospitalized with P&I in month i; and H i is the proportion of HIV-positive persons in the total elderly population hospitalized with P&I.
Data analysis was performed using SAS, v. 9.2 (SAS Institute, USA), SPSS, v. 17 (SPSS Inc., USA), S-Plus, v. 8.0 (TIBCO, USA) and R, v. 2.9.0 (R Development Core Team, Austria). Use of the CMS dataset was supported by a data use agreement with Tufts University and approved by the Tufts-TMC Institutional Review Board.
RESULTS
Between 1991 and 2004, there were 16 381 579 P&I hospitalizations among persons aged ⩾65 years. P&I was the primary admission diagnosis (first or second diagnostic field) in 75% of records. A concurrent HIV diagnosis was identified in 6518 records, equivalent to 39·8 P&I hospitalizations among HIV-positive older adults/100 000 P&I hospitalizations.
Demographic characteristics
Table 1 summarizes the demographic characteristics of persons hospitalized with P&I. While black persons comprised only 8·1% of P&I hospitalizations among HIV-negative persons, they comprised 48·0% of P&I hospitalizations among HIV-positive persons (P<0·001). HIV-positive seniors were on average younger than HIV-negative seniors hospitalized with P&I (70·3±5·1 vs. 79·9±8·2 years, P<0·001). The rate of P&I hospitalization increased exponentially with age in HIV-negative persons, a trend that was observed for both blacks and whites (P<0·014, for each group). In contrast, the fraction of admissions representing HIV-positive persons declined exponentially with age in both races (P<0·047, for each group).
Table 1. Age- and race-specific counts and estimated rates of P&I hospitalization, by HIV status
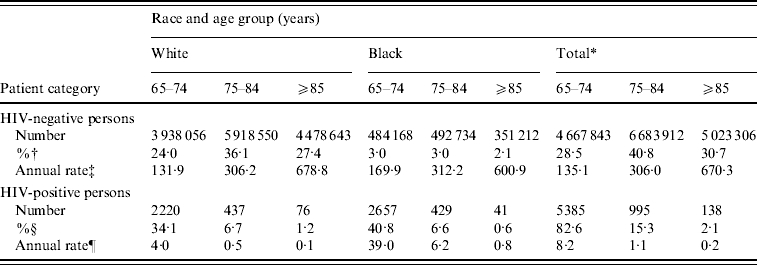
* Total includes all persons, including white and black and five other racial groups reported by CMS as follows: Hispanic (n=194 618 HIV-negative/374 HIV-positive), Asian (83 086/19), Native American (33 605/10), Other (227 921/200), and Unknown (172 468/55), representing 658 HIV-positive cases.
† Calculated as a percentage of all HIV-negative persons hospitalized with P&I (n=16 375 061).
‡ Per 100 000 Medicare beneficiaries.
§ Calculated as a percentage of all HIV-positive persons hospitalized with P&I (n=6518).
¶ Per 100 000 P&I hospitalizations.
The age distribution of HIV-positive seniors is shown in Figure 1. Males comprised the majority (76·4%) of HIV-positive persons hospitalized with P&I (vs. 46·0% of HIV-negative persons, P<0·001). In white males, black males and black females, the ratio between the three age groups (65–74, 75–84, ⩾85 years) was approximately 84:14:2. In contrast, in white females this ratio was 73:19:8, indicating that a substantially greater proportion (26·9%) of HIV-positive, white females were aged ⩾75 years.
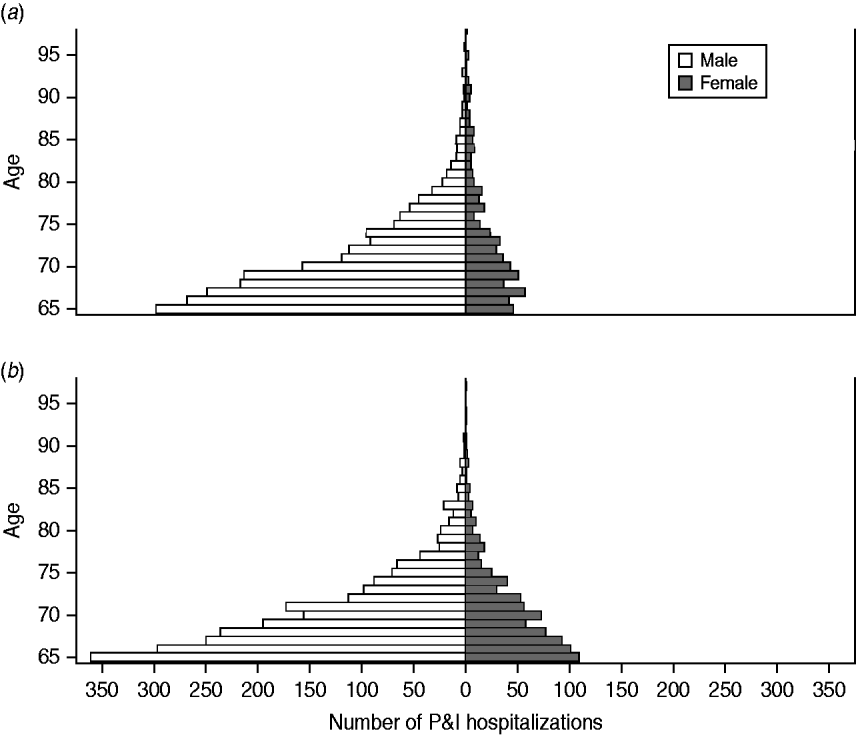
Fig. 1. Age distribution of HIV-positive older adults hospitalized with pneumonia and influenza (P&I), by race and gender. (a) Whites; (b) Blacks.
Temporo-demographic trends
Temporal trends in P&I hospitalization are shown in Figure 2. Adjusting for seasonality, we observed a 36% increase in P&I hospitalization in HIV-negative persons over the 14-year period [RR 1·36, 95% confidence interval (CI) 1·32–1·41, P<0·001]. During this period, the fraction of admissions representing HIV-positive persons almost doubled (RR 1·95, 95% CI 1·80–2·13, P<0·001).
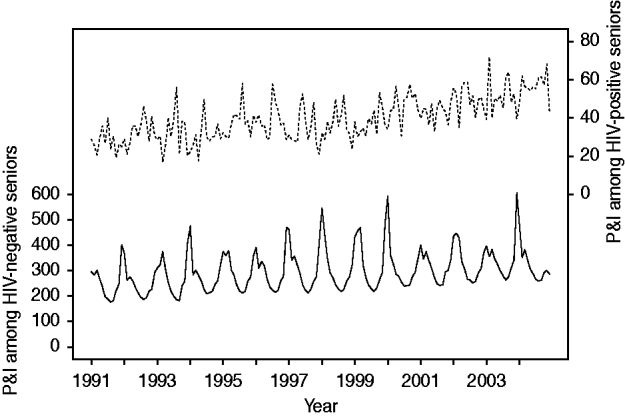
Fig. 2. Temporal trend in pneumonia and influenza (P&I) hospitalization in older adults, by HIV status. Solid line indicates the P&I hospitalization rate among HIV-negative persons (per 100 000 Medicare beneficiaries). Dotted line indicates the fraction of P&I admissions representing HIV-positive persons (per 100 000 P&I hospitalizations).
Figure 3 shows the temporal trend with respect to age distribution of HIV-positive elderly admitted for P&I. The smoothed surface demonstrates a pronounced, age-specific increase in the number of P&I hospitalizations in patients with HIV infection. For example, the number of patients aged 65–67 years doubled over the 14-year study period, while the number aged 73–74 years tripled during this period, indicating improved survival in HIV-positive persons.
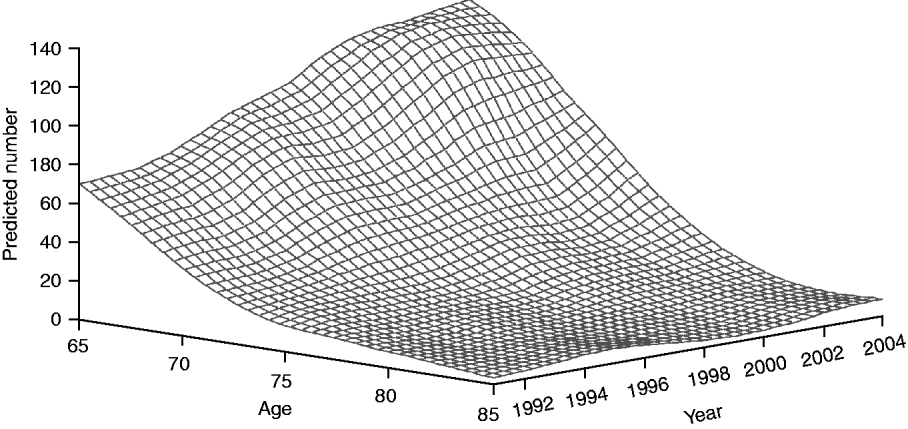
Fig. 3. Predicted temporal trend in age distribution of HIV-positive seniors hospitalized with pneumonia and influenza.
In-patient case-fatality
We identified 2 061 246 records containing a deceased discharge code, signifying an overall in-patient case-fatality of 12·6%. Death was more common in HIV-positive seniors (18·0% vs. 12·6% in HIV-negative seniors, P<0·001). This difference in case-fatality persisted when stratified by age category (data not shown). For HIV-positive seniors, case-fatality was not significantly different between blacks and whites.
Figure 4 depicts the temporal changes in case-fatality. During the 14-year time-series, case-fatality decreased by 36% in HIV-negative persons (RR 0·64, 95% CI 0·56–0·75, P<0·001) and by 63% in HIV-positive seniors (RR 0·37, 95% CI 0·33–0·42, P<0·001). In direct comparisons, HIV-positive seniors hospitalized in the HAART era were 55% less likely to die in hospital compared to those hospitalized in the pre-HAART era (OR 0·45, 95% CI 0·38–0·53, P<0·001). Adjusting for other variables, we estimated that for every 100 HIV-positive persons hospitalized with P&I, about six deaths were averted as a result of HAART (P=0·032). Despite these improvements, direct comparisons indicated that HIV-positive seniors were still 51% more likely to die following hospitalization with P&I than HIV-negative seniors in 2004 (OR 1·51, 95% CI 1·23–1·85, P<0·001).
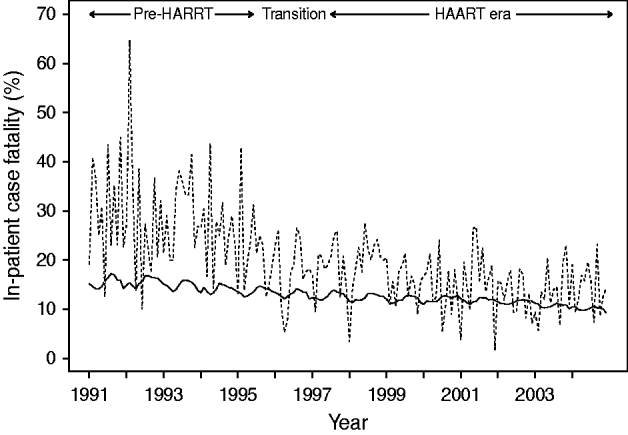
Fig. 4. Temporal trend in in-patient case-fatality in older adults hospitalized with pneumonia and influenza, by HIV status. Case-fatality among HIV-negative (–––) and HIV-positive (……) persons.
DISCUSSION
We found that the demographic profile of HIV-infected patients with P&I dramatically shifted during the period spanning the introduction of HAART. Between 1991 and 2004, the fraction of P&I admissions representing HIV-positive persons almost doubled. Within this patient pool, the number of older seniors steadily increased. Our results demonstrate that HAART has had a beneficial effect on in-patient case-fatality associated with P&I. Projected to the total number of P&I hospitalizations in HIV-positive seniors during the HAART era (up to 2004, n=4271), we estimate that 248 lives were saved due to the widespread use of HAART.
It is widely recognized that the US population is becoming older. Between 1975 and 2008, the number of Medicare enrollees aged ⩾65 years increased from 22·4 to 37·6 million [10]. Concurrent with this increase has been a shift towards coverage of older adults; persons aged ⩾85 years comprised only 8·4% of Medicare enrollees in 1975 compared to 13·9% in 2008 [10]. Expansion of this population is largely due to improvements in health. Nevertheless, this population bears the burden of disease and consumes the most patient resources [Reference Alemayehu and Warner24]. Pneumonia was the sixth most common reason for hospitalization among Medicare enrollees in 2008, with an average cost per discharge of $21 378 [10]. We document that P&I hospitalization increased significantly between 1991 and 2004, a finding that is corroborated by other studies [Reference Thompson19, Reference Hebert25]. Reasons for this increase may relate to demographic changes occurring within the Medicare population; older adults are particularly vulnerable to P&I, as evidenced by the higher hospitalization rates and case-fatality noted in this and other studies [Reference Hebert25, Reference Thompson26].
We observed that the number of P&I hospitalizations among older adults infected with HIV has similarly increased. This pattern is consistent with the changing epidemiology of HIV in the USA. In 2007, CDC reported that 16 982 persons aged ⩾65 years were living with HIV/AIDS in the USA, compared to just 2985 in 1999 [27, 28]. These changes in demographics have led to projections that by 2015, more than one half of all HIV-infected persons in the USA will be aged >50 years [29]. This dramatic increase has resulted both from advancements in HIV treatment (with associated gains in life expectancy), as well as from new HIV infections in this age group [Reference Ledergerber30]. In Figure 3 we demonstrate that this has contributed to an age-related demographic shift in the HIV-positive population that is hospitalized with P&I. Surviving to an older age exposes such individuals to age-related causes of morbidity and mortality, as well as to seasonal re-exposure to respiratory pathogens. We note, however, that the fraction of all P&I hospitalizations representing HIV-positive seniors declined with age, reflecting depletion of HIV-positive persons in older age categories.
Our findings show that P&I case-fatality has declined significantly since 1991 in HIV-positive seniors. A study conducted during the early HAART era found that cardiopulmonary death rates among HIV-infected persons aged 15–50 years declined by 77% between 1995 and 1999 [Reference Neuzil17]. We observed a similar decline (74%) when we limited our data to this time period, and a slightly more modest decline of 63% over the entire time-series. According to our data, 27·4% and 14·8% of HIV-positive Medicare enrollees died during the course of hospitalization in the pre-HAART and HAART eras, respectively. These point estimates are higher than those reported for bacterial pneumonia in the general (non-elderly), HIV-infected population [Reference Sureka13, Reference Hirschtick31, Reference Kohli32], and may reflect a particular vulnerability in older persons [Reference Sureka13].
Decreasing mortality in HIV-infected persons in the HAART era has been widely documented [Reference Walensky18, Reference Karon33, Reference Hariri and McKenna34], although few studies have examined the population-level effect of HAART on mortality due to non-HIV/AIDS-related conditions. We have demonstrated that HAART contributed independently to the decline in P&I case-fatality. Nevertheless, our data attest that a gap in case-fatality still existed between HIV-positive and -negative persons in 2004. The reason(s) behind the significantly different case-fatality rates is unclear, and may relate to differences in the immune response to infection, the spectrum of comorbidities or healthcare service utilization in HIV-positive and -negative persons.
The discrepancy in case-fatality between HIV-positive and -negative persons in an era when HAART was widely available, suggests that HAART alone may not be sufficient to reduce the risk of death from P&I in HIV-infected elderly persons. This emphasizes the need for further evaluation of specific preventative measures – such as influenza and pneumococcus vaccination – in this population. Currently there is limited evidence for a benefit of vaccination in HIV-positive persons using existing influenza vaccines [Reference Kunisaki and Janoff35, Reference Anema36]. New vaccines specifically formulated for HIV-infected persons are, however, showing some promise for other viral diseases [Reference Geretti and Doyle37]. Alternative strategies to reduce overall exposure to influenza in the elderly population, such as vaccination of children [Reference Cohen20], may also prove particularly useful in preventing infection in this dually susceptible population.
Our study is one of the first to examine the Medicare-covered, HIV-infected population. CMS databases contain medical claims for about 98% of persons aged ⩾65 years in the USA [Reference Cohen and Naumova38], and offer the most complete record of hospitalizations among elderly Americans. Nevertheless, there are several limitations that warrant mention. Because there is no data on HIV prevalence among the Medicare beneficiary population, it was not possible to estimate the true incidence of P&I hospitalization among older adults with HIV. Comparison with CDC surveillance data for 2004 (which includes reporting from only 33 states and five dependent territories) yields an estimate of 7·0 P&I hospitalizations/100 HIV-positive persons aged ⩾65 years. Two prospective studies in the general (non-elderly), HIV-positive population report similar hospitalization rates for bacterial pneumonia in the HAART era [Reference Kohli32, Reference Tumbarello39]. We note that these estimates are about tenfold higher than in the general, Medicare population.
We cannot reliably comment on the aetiology of pneumonia or the contribution and trends of vaccine-preventable respiratory infections (influenza and pneumococcus) in this population since the majority (70%) of records did not identify a specific causative organism. Although the diagnostic armamentarium to determine the underlying aetiology for both pneumonia and viral respiratory tract infections improved significantly during the study period, laboratory diagnosis of influenza infection was not universally confirmed in patients presenting with an influenza-like illness. We note that the P&I definition used in our study (ICD 480-487) did not include Pneumocystis pneumonia (ICD 136·3), although 3·7% of HIV-positive persons hospitalized with P&I had this concurrent diagnoses. Declines in Pneumocystis pneumonia have been widely documented in the HAART era [Reference Mocroft15, Reference Krentz40, Reference Wolff and O'Donnell41], therefore we did not specifically examine this trend.
Our study is based on all Medicare-covered P&I hospitalizations occurring between 1991 and 2004. While this time period allowed assessment of the population-level impact of HAART on in-patient case-fatality, we are not able to comment directly on the influence of immune reconstitution since data on CD4 counts were not available in the CMS database. Further, no information on vaccination or pneumonia prophylaxis status was available in our dataset, limiting our ability to assess the population-level impact of these interventions. In-hospital death does not include all deaths resulting from P&I, since not all persons may be hospitalized preceding death, and some persons may be discharged to home or other health facilities where they later die from the same episode of illness. Since deaths occurring due to this latter circumstance are not reflected in our dataset, our estimates may underestimate the true case-fatality associated with P&I hospitalization. Moreover, it was not possible to link Medicare records with death records for verification purposes, as the dataset was provided with limitations required under the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Finally, it is possible that a small number of persons experienced repeated hospitalizations, due to premature discharge, or vulnerability to influenza-associated pneumonia in subsequent seasons.
In summary, our results indicate that HIV-positive persons comprise an increasing fraction of the Medicare-covered population that is hospitalized with P&I. While HAART has contributed to substantial reductions in in-patient case-fatality, further measures are required to facilitate prevention of death related to P&I in HIV-infected seniors. Consistent monitoring for observed trends in hospitalization among HIV-infected individuals in the HAART era offers insights into effectiveness of preventative measures and treatment on a national scale.
ACKNOWLEDGEMENTS
This study was supported by grant N01 AI-50032 from the National Institute of Allergy and Infectious Diseases. We thank the Centers for Medicare and Medicaid Services for providing the data.
DECLARATION OF INTEREST
None.







