Anxiety disorders not only interfere with a person’s daily life by adversely having an impact on functioning and quality of life, Reference Bijl and Ravelli1 but also by decreasing somatic health. Reference Roy-Byrne, Davidson, Kessler, Asmundson, Goodwin and Kubzansky2 Various longitudinal studies have confirmed that anxiety is a risk factor for several ageing-related somatic conditions including coronary heart disease, Reference Roest, Martens, de and Denollet3 diabetes Reference Atlantis, Vogelzangs, Cashman and Penninx4 and disability, Reference Brenes, Penninx, Judd, Rockwell, Sewell and Wetherell5 as well as for overall mortality. Reference Denollet, Maas, Knottnerus, Keyzer and Pop6 These increased risks remain even when controlling for lifestyle factors such as smoking, alcohol intake and physical activity, which suggests the involvement of underlying biological processes. Anxiety disorders are considered to be chronic and stressful conditions Reference Penninx, Nolen, Lamers, Zitman, Smit and Spinhoven7 known to be accompanied by dysregulations of the major bodily stress systems including the hypothalamic-pituitary-adrenal (HPA) axis, Reference Vreeburg, Zitman, van der Pelt, de Rijk, Verhagen and van Dyck8 the autonomic nervous system Reference Licht, de Geus, van and Penninx9 and the immune system. Reference Vogelzangs, Beekman, de Jonge and Penninx10 It has been hypothesised that these stress-induced dysregulations result in accelerated cell ageing, Reference Wolkowitz, Epel, Reus and Mellon11 which may partly account for the decreased somatic health in people with anxiety disorder.
Cellular ageing can be indexed by the length of telomeres, which are specialised nucleic acid-protein complexes that cap the ends of linear DNA and protect DNA from damage. Because of the ‘end-replication problem’, the final part of the telomere fails to be replicated during every cell division, causing telomeres to become progressively shorter. When telomeres reach a critically short length, cells become susceptible to senescence or apoptosis. Reference Blackburn12 Telomere shortening is counteracted by the cellular enzyme telomerase that adds telomeric DNA, thus preserving telomere length and healthy cell function. Accelerated shortening may be the consequence of disturbances of stress systems such as the HPA axis Reference Tomiyama, O'Donovan, Lin, Puterman, Lazaro and Chan13 and the immune system. Reference De la Fuente and Miquel14 These dysregulations possibly contribute to telomere shortening by increasing oxidative stress, which in turn damages telomeres. Reference De la Fuente and Miquel14 Shortened telomeres have been shown to be a prognostic marker of various ageing-related conditions such as cardiovascular disease, Reference Fitzpatrick, Kronmal, Gardner, Psaty, Jenny and Tracy15 obesity, Reference Valdes, Andrew, Gardner, Kimura, Oelsner and Cherkas16 diabetes, Reference Sampson, Winterbone, Hughes, Dozio and Hughes17 cancer, Reference Willeit, Willeit, Mayr, Weger, Oberhollenzer and Brandstatter18 cognitive decline Reference Martin-Ruiz, Dickinson, Keys, Rowan, Kenny and von Zglinicki19 and of earlier mortality. Reference Cawthon, Smith, O'Brien, Sivatchenko and Kerber20
Although some recent studies have suggested patterns of accelerated cellular ageing among patients with depression Reference Simon, Smoller, McNamara, Maser, Zalta and Pollack21–Reference Verhoeven, Révész, Epel, Lin, Wolkowitz and Penninx24 research into cellular ageing in patients with anxiety disorders has been very limited up until now. One study by Kananen et al Reference Kananen, Surakka, Pirkola, Suvisaari, Lonnqvist and Peltonen25 found shorter telomere length in patients with panic disorder, generalised anxiety disorder (GAD), social phobia or agoraphobia, but only in an older subgroup of the patients with anxiety disorder (>48 years old) compared with a similar-aged control group, and not in the whole sample of 321 patients compared with 653 controls. Hoen et al Reference Hoen, Rosmalen, Schoevers, Huzen, van der Harst and de Jonge26 found that 108 patients with an anxiety disorder had shorter telomere length than 970 non-anxious people at a 2-year follow-up period. Higher stress appraisals Reference O'Donovan, Tomiyama, Lin, Puterman, Adler and Kemeny27 and high phobic anxiety Reference Okereke, Prescott, Wong, Han, Rexrode and De Vivo28 were also found to be associated with shorter telomere length in non-psychiatric samples. In summary, there are only a few studies on the link between anxiety disorders and telomere length and results so far have been mixed. It thus remains unclear whether anxiety disorders are accompanied by accelerated cellular ageing. This paper examines the association between leukocyte telomere length (LTL) and the presence of the most common anxiety disorders: panic disorder with and without agoraphobia, GAD, social phobia and agoraphobia in a large adult sample (n = 2324) including people with current and remitted anxiety disorders and healthy controls. The large sample size allowed us to control for the most important confounding variables and to examine whether specific psychiatric characteristics, such as type of anxiety disorder, severity and symptom duration, have an impact on cellular ageing.
Method
Study sample
Data are from the baseline assessment of the Netherlands Study of Depression and Anxiety (NESDA), an ongoing longitudinal cohort study examining the course and consequences of depressive and anxiety disorders. The NESDA sample consists of 2981 participants between 18 and 65 years including people with a current or remitted diagnosis of a depressive and/or anxiety disorder (74%) and healthy controls (26%). To represent various settings and stages of psychopathology, participants with depression and anxiety disorders were recruited at three different locations in The Netherlands in different settings: the community, primary care and specialised mental healthcare settings. People with insufficient command of the Dutch language or a primary clinical diagnosis of other severe psychiatric conditions, such as bipolar disorder, obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), severe substance use disorder or a psychotic disorder, as reported by themselves or their mental health practitioner, were excluded. Participants were recruited between September 2004 and February 2007. The study was approved by the ethical review board of participating centres, and all participants signed informed consent. Participants in NESDA were assessed during a 4 h clinic visit. The population and methods of the NESDA study have been described in more detail elsewhere. Reference Penninx, Beekman, Smit, Zitman, Nolen and Spinhoven29
For the current study, three groups were created: a control group, a group of participants with remitted anxiety disorders and a group of participants with current anxiety disorders including panic disorder with and without agoraphobia, social phobia, GAD and agoraphobia, thereby excluding 657 participants who did not meet the criteria of one of the three groups. A total of 37 participants were subsequently excluded from analyses because of missing telomere length data, leaving 2324 individuals. Individuals in the control group (n = 582) were defined as having no lifetime history of anxiety or depressive disorders as assessed by the DSM-IV Composite International Diagnostic Interview (CIDI, version 2.1), 30 and an anxiety severity score below 11 on Beck’s Anxiety Inventory (BAI). Reference Beck, Epstein, Brown and Steer31 Participants in the current anxiety disorder group (n = 1283) had one or more CIDI-diagnosed anxiety disorders (747 had one anxiety diagnosis, 404 had two diagnoses and 132 had three comorbid anxiety disorders) in the past 6 months. The participants in the remitted anxiety disorder group (n = 459) had an anxiety disorder previously but not in the past 6 months according to the CIDI.
Measurements
Telomere length
Fasting blood was drawn from participants in the morning between 08.30 h and 09.30 h and blood samples were stored in a –80°C freezer afterwards. Leukocyte telomere length (LTL) was determined at the laboratory of Telomere Diagnostics, Inc. (Menlo Park, California, USA), using quantitative polymerase chain reaction (qPCR), adapted from the published original method by Cawthon et al. Reference Cawthon32 Telomere sequence copy number in each patient’s sample (T) was compared with a single-copy gene copy number (S), relative to a reference sample. The resulting T/S ratio is proportional to mean LTL. Reference Cawthon32,Reference Aviv, Hunt, Lin, Cao, Kimura and Blackburn33 The detailed method is described elsewhere. Reference Verhoeven, Révész, Epel, Lin, Wolkowitz and Penninx24
To compare T/S ratios to telomere restriction fragments (TRF) reported by studies using Southern blot analysis, we used the following steps to derive a conversion formula. Lin et al Reference Lin, Epel, Cheon, Kroenke, Sinclair and Bigos34 used a formula of base pairs (bp) = 3274 + 2413 × T/S based on comparison of T/S ratios and TRF analysis of a series of genomic DNA samples from the human fibroblast cell line IMR90. Comparison of the T/S ratios of eight quality control DNA samples from the Telome Health Inc. laboratory that were included on each PCR run, generated the following formula: T/S(Lin et al Reference Lin, Epel, Cheon, Kroenke, Sinclair and Bigos34 ) = (T/S(TelomereDiagnostics)–0.0545)/1.16. Therefore, the final formula we used to convert T/S ratios to bp is: bp = 3274 + 2413 ((T/S-0.0545)/1.16). The reliability of the assay was adequate: eight included quality control DNA samples on each PCR run illustrated a small intra-assay coefficient of variation of 5.1% and the interassay coefficient of variation was also sufficiently low (4.6%).
Psychiatric characteristics
Four severity measures were included, corresponding with the anxiety disorders in this study: (a) panic disorder arousal symptoms were assessed by the 21-item BAI; Reference Beck, Epstein, Brown and Steer31 (b) social phobic symptoms were measured with the five-item social phobia subscale of the Fear Questionnaire; Reference Marks and Mathews35 (c) the five-item agoraphobia subscale of the Fear Questionnaire assessed agoraphobic symptoms; and (d) GAD’s worrying and rumination were assessed with the Penn State Worry Questionnaire (PSWQ). Reference Meyer, Miller, Metzger and Borkovec36 Anxiety duration in recent years was assessed by the Life Chart interview (LCI), Reference Lyketsos, Nestadt, Cwi and Heithoff37 which uses a calendar method to assess the number of months in which anxious symptoms and avoidance were present during the past 4 years. The CIDI also assessed comorbid major depressive disorder (MDD), which was considered present when diagnosed in the past 6 months. Current use of psychoactive medication was measured by inspection of the active component or medication brand, the dose and frequency of intake from the label on the containers brought in and was categorised using the World Health Organization Anatomical Therapeutic Chemical (ATC) classification 38 into antidepressants (tricyclic antidepressants (N06AA), selective serotonin reuptake inhibitors (N06AB) and other antidepressants (N06AF, N06AG, N06AX)) and benzodiazepines (N03Ae, N05BA, N05CD, N05CF).
Covariates
Gender, age and years of education were assessed during the interview. Body mass index (BMI) was calculated as measured weight divided by height-squared and divided into underweight (<18.5), normal (18.5–24.9), overweight (25.0–30.0) and obese (>30.0). Alcohol consumption was categorised as non-drinker, moderate drinker (female <14 and male <21 drinks/week) or heavy drinker (female ≥14 and male ≥21 drinks/week). Smoking status was categorised into current, former or never smoker. Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ) Reference Craig, Marshall, Sjostrom, Bauman, Booth and Ainsworth39 and expressed as overall energy expenditure in metabolic equivalent total (MET)-minutes per week (MET level × minutes activity × events per week, see Ainsworth et al Reference Ainsworth, Haskell, Herrmann, Meckes, Bassett and Tudor-Locke40 ). The number of current somatic diseases for which participants received medical treatment (i.e. heart disease, epilepsy, diabetes, osteoarthritis, stroke, cancer, chronic lung disease, thyroid disease, liver disease, intestinal disorders and ulcers) was counted.
Statistical analyses
Baseline characteristics were compared across anxiety disorder status (control, remitted and current anxiety disorder groups) using chi-squared statistics and analyses of variance (ANOVA). We used analyses of covariance (ANCOVA) to determine differences in LTL in the remitted and current anxiety disorder groups compared with each other and with the control group, controlling for all covariates. Within the remitted anxiety group we tested whether the time since remission (in years) was associated with LTL to test the hypothesis that LTL restores with a longer time since remission. Also, sensitivity analyses were performed in which participants with current and lifetime MDD were excluded to check whether a possible association is present in people with anxiety disorder only and not caused by comorbid MDD. The extra analyses were all fully adjusted. To examine the role of type of anxiety disorder, adjusted mean LTL of the control group was compared with each of the five disorder subgroups: panic disorder with agoraphobia, panic disorder without agoraphobia, GAD, social phobia and agoraphobia. Cohen’s d was determined as an effect size estimation for significant results. Finally, covariate-adjusted multiple linear regression analyses were used to analyse the association of anxiety characteristics with LTL both within the total sample and within the current anxiety sample. All analyses were conducted using SPSS version 20 for Windows.
Results
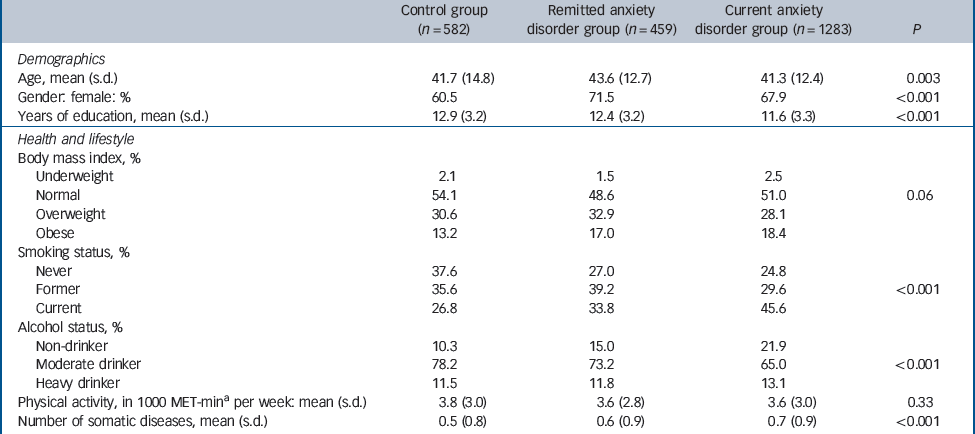
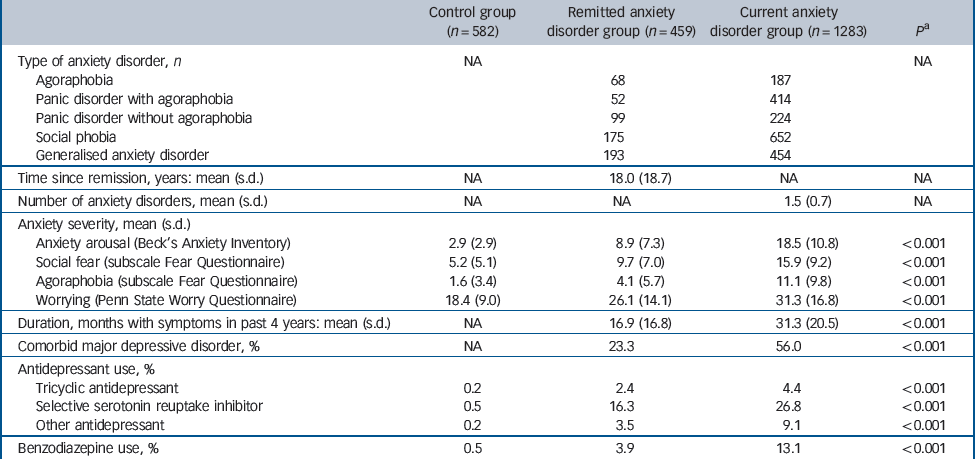
Table 1 shows baseline characteristics across anxiety status. The mean age of the total study sample (n = 2324) was 41.7 years (s.d. = 13.1, range 18–65) and 66.7% were female. The remitted anxiety disorder group was slightly older than the two other groups, while the control group contained fewer women than both anxiety groups. The current anxiety disorder group were more often less educated, current smokers and non-drinkers or heavy drinkers, and both remitted and current anxiety disorder groups had more somatic diseases than controls. Table 2 shows that the three groups differed on all psychiatric characteristics with the current anxiety disorder group showing the most severe characteristics. These data demonstrate that people with anxiety disorders are in several ways different from non-psychiatric controls and suggest that this epidemiological study sample consists of people with anxiety disorder as actually seen in the community, but that controlling for these differences is insuperable.
Table 1 Sample characteristics by anxiety status
| Control group (n = 582) |
Remitted anxiety disorder group (n = 459) |
Current anxiety disorder group (n = 1283) |
P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (s.d.) | 41.7 (14.8) | 43.6 (12.7) | 41.3 (12.4) | 0.003 |
| Gender: female: % | 60.5 | 71.5 | 67.9 | <0.001 |
| Years of education, mean (s.d.) | 12.9 (3.2) | 12.4 (3.2) | 11.6 (3.3) | <0.001 |
| Health and lifestyle | ||||
| Body mass index, % | ||||
| Underweight | 2.1 | 1.5 | 2.5 | |
| Normal | 54.1 | 48.6 | 51.0 | 0.06 |
| Overweight | 30.6 | 32.9 | 28.1 | |
| Obese | 13.2 | 17.0 | 18.4 | |
| Smoking status, % | ||||
| Never | 37.6 | 27.0 | 24.8 | |
| Former | 35.6 | 39.2 | 29.6 | <0.001 |
| Current | 26.8 | 33.8 | 45.6 | |
| Alcohol status, % | ||||
| Non-drinker | 10.3 | 15.0 | 21.9 | |
| Moderate drinker | 78.2 | 73.2 | 65.0 | <0.001 |
| Heavy drinker | 11.5 | 11.8 | 13.1 | |
| Physical activity, in 1000 MET-minFootnote a per week: mean (s.d.) | 3.8 (3.0) | 3.6 (2.8) | 3.6 (3.0) | 0.33 |
| Number of somatic diseases, mean (s.d.) | 0.5 (0.8) | 0.6 (0.9) | 0.7 (0.9) | <0.001 |
a. MET-min, metabolic equivalent of number of calories spent per minute.
Table 2 Psychiatric characteristics by anxiety status
| Control group (n = 582) |
Remitted anxiety disorder group (n = 459) |
Current anxiety disorder group (n = 1283) |
P Footnote a | |
|---|---|---|---|---|
| Type of anxiety disorder, n | NA | NA | ||
| Agoraphobia | 68 | 187 | ||
| Panic disorder with agoraphobia | 52 | 414 | ||
| Panic disorder without agoraphobia | 99 | 224 | ||
| Social phobia | 175 | 652 | ||
| Generalised anxiety disorder | 193 | 454 | ||
| Time since remission, years: mean (s.d.) | NA | 18.0 (18.7) | NA | NA |
| Number of anxiety disorders, mean (s.d.) | NA | NA | 1.5 (0.7) | NA |
| Anxiety severity, mean (s.d.) | ||||
| Anxiety arousal (Beck’s Anxiety Inventory) | 2.9 (2.9) | 8.9 (7.3) | 18.5 (10.8) | <0.001 |
| Social fear (subscale Fear Questionnaire) | 5.2 (5.1) | 9.7 (7.0) | 15.9 (9.2) | <0.001 |
| Agoraphobia (subscale Fear Questionnaire) | 1.6 (3.4) | 4.1 (5.7) | 11.1 (9.8) | <0.001 |
| Worrying (Penn State Worry Questionnaire) | 18.4 (9.0) | 26.1 (14.1) | 31.3 (16.8) | <0.001 |
| Duration, months with symptoms in past 4 years: mean (s.d.) | NA | 16.9 (16.8) | 31.3 (20.5) | <0.001 |
| Comorbid major depressive disorder, % | NA | 23.3 | 56.0 | <0.001 |
| Antidepressant use, % | ||||
| Tricyclic antidepressant | 0.2 | 2.4 | 4.4 | <0.001 |
| Selective serotonin reuptake inhibitor | 0.5 | 16.3 | 26.8 | <0.001 |
| Other antidepressant | 0.2 | 3.5 | 9.1 | <0.001 |
| Benzodiazepine use, % | 0.5 | 3.9 | 13.1 | <0.001 |
a. P-value based on one-way ANOVA for continuous variables and chi-squared test for categorical variables.
LTL was normally distributed and on average 5463 bp (s.d. = 614). It exhibited a significant negative correlation with age (r = –0.326, P<0.001) that corresponded to a mean shortening rate of 14 bp/year. This shortening rate is comparable with previously reported rates based on cross-sectional data (14 bp/year by Cawthon et al, Reference Cawthon, Smith, O'Brien, Sivatchenko and Kerber20 20 bp/year by Hartmann et al Reference Hartmann, Boehner, Groenen and Kalb22 and 15–17 bp/year by Wikgren et al Reference Wikgren, Maripuu, Karlsson, Nordfjall, Bergdahl and Hultdin23 ). Female participants had longer LTL than male participants (F = 14.23; P<0.001, corrected for age). LTL was, besides age and gender, associated with weight (shorter LTL for being underweight, overweight or obese), smoking and drinking status (former and current smokers as well as heavy drinkers have shorter LTL) and number of somatic diseases (more diseases, shorter LTL), but not with education and physical activity.
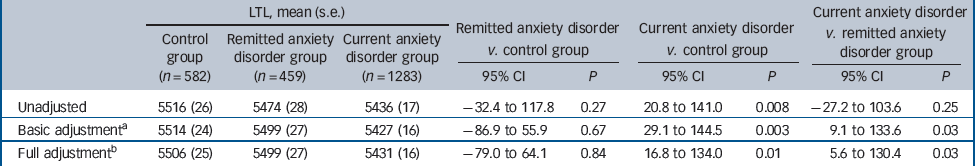
Between-group analyses showed that the LTL of the control, remitted and current anxiety groups differed in unadjusted (F(2,2321) = 3.57, P = 0.028), sociodemographic-adjusted (F(2,2318 = 5.40, P = 0.005) and fully adjusted (sociodemographic, health and lifestyle) analyses (F(2,2313 = 4.22, P = 0.015). Post hoc analyses showed that those in the current anxiety group had shorter LTL than healthy controls in unadjusted analyses (Table 3). Further, when adjusted for age, gender and education, LTL was significantly shorter for the current anxiety group (bp = 5427) compared with the control (mean bp = 5514, P = 0.003) and the remitted anxiety group (bp = 5499; P = 0.025). Differences remained significant in analyses fully adjusted for sociodemographic, health and lifestyle variables: the current anxiety group had on average 75 bp shorter LTL compared with the control group (P = 0.01, Cohen’s d = 0.13) and 68 bp shorter LTL (P = 0.03, Cohen’s d = 0.12) compared with the remitted group (see Table 3 for the 95% confidence intervals). The remitted anxiety group did not significantly differ from the control group (P = 0.84). However, within the remitted anxiety group, the time since remission was positively associated with LTL (β = 0.107, P = 0.024, adjusted for all covariates). In further analysis we performed a median-split, resulting in a subgroup that remitted between 6 months to 9 years ago (n = 225) and a group that were remitted for 10 years or longer (n = 220). The first subgroup (bp = 5372) had significantly shorter LTL than the 10+ years subgroup (bp = 5529; P = 0.022; Cohen’s d = 0.22), suggesting that the cellular ageing process is in part reversible.
Table 3 Mean leukocyte telomere length (LTL) in base pairs by anxiety disorder status in unadjusted, basic and fully adjusted analyses
| LTL, mean(s.e.) | Remitted anxiety disorder v. control group |
Current anxiety disorder v. control group |
Current anxiety disorder v. remitted anxiety disorder group |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control group (n = 582) |
Remitted anxiety disorder group (n = 459) |
Current anxiety disorder group (n = 1283) |
|||||||
| 95% CI | P | 95% CI | P | 95% CI | P | ||||
| Unadjusted | 5516 (26) | 5474 (28) | 5436 (17) | –32.4 to 117.8 | 0.27 | 20.8 to 141.0 | 0.008 | –27.2 to 103.6 | 0.25 |
| Basic adjustmentFootnote a | 5514 (24) | 5499 (27) | 5427 (16) | –86.9 to 55.9 | 0.67 | 29.1 to 144.5 | 0.003 | 9.1 to 133.6 | 0.03 |
| Full adjustmentFootnote b | 5506 (25) | 5499 (27) | 5431 (16) | –79.0 to 64.1 | 0.84 | 16.8 to 134.0 | 0.01 | 5.6 to 130.4 | 0.03 |
a. Adjusted for age, gender, education.
b. Adjusted for age, gender, education, body mass index, smoking, alcohol, physical activity and somatic diseases.
In contrast, in an earlier study in the same cohort we found that individuals who had had depression earlier in life but did not currently meet the MDD diagnosis had shorter telomeres than controls. Reference Verhoeven, Révész, Epel, Lin, Wolkowitz and Penninx24 Both the remitted patient groups differed from each other in terms of subthreshold symptomatology and length of remission: remitted patients with MDD scored 18.0 (s.d. = 10.2) on the Inventory of Depressive Symptoms, Reference Verhoeven, Révész, Epel, Lin, Wolkowitz and Penninx24 which is still indicative of ‘mild symptomatology’; Reference Rush, Trivedi, Ibrahim, Carmody, Arnow and Klein41 whereas the remitted patients with anxiety disorder scored 8.9 (s.d. = 7.3) on BAI, which is below the clinically relevant cut-off score of 11. Reference Karsten, Nolen, Penninx and Hartman42 Moreover, the average time to remission in the remitted patients with MDD was 5.2 years (s.d. = 6.2), whereas it was much longer in the remitted anxiety group: 18.0 years (s.d. = 18.7) years.
To test whether the associations found in the present study were not caused by comorbid depressive conditions, sensitivity analyses were performed in which participants with current MDD were excluded. These analyses showed that the 564 participants with current anxiety disorders (but no current MDD) still had significantly shorter LTL than controls (P = 0.021; Cohen’s d = 0.14) and marginally significantly shorter telomeres than the participants with remitted anxiety disorders (P = 0.078; both adjusted for all covariates), indicating that the association is truly present in those with anxiety disorders and not just caused by comorbid depressive conditions. Even when excluding lifetime MDD diagnoses, 268 participants with current anxiety disorders had shorter LTL compared with the controls (P = 0.024), but not compared with those participants in the remitted anxiety group (P = 0.273).
To examine the role of type of disorder, mean LTLs for the different anxiety disorder subgroups were each compared with the control group (Fig. 1). To reflect high comorbidity rates, patients with comorbid disorders were included in each anxiety disorder subgroup for which they met the diagnosis. Patients with panic disorder with agoraphobia (bp = 5428, P = 0.03, Cohen’s d = 0.14), social phobia (bp = 5443, P = 0.04, Cohen’s d = 0.12) and GAD (bp = 5404, P = 0.002, Cohen’s d = 0.18) had significantly shorter LTL than the control group. Patients with agoraphobia (bp = 5497, P = 0.91) and panic disorder without agoraphobia (bp = 5458, P = 0.19), however, did not differ from controls.
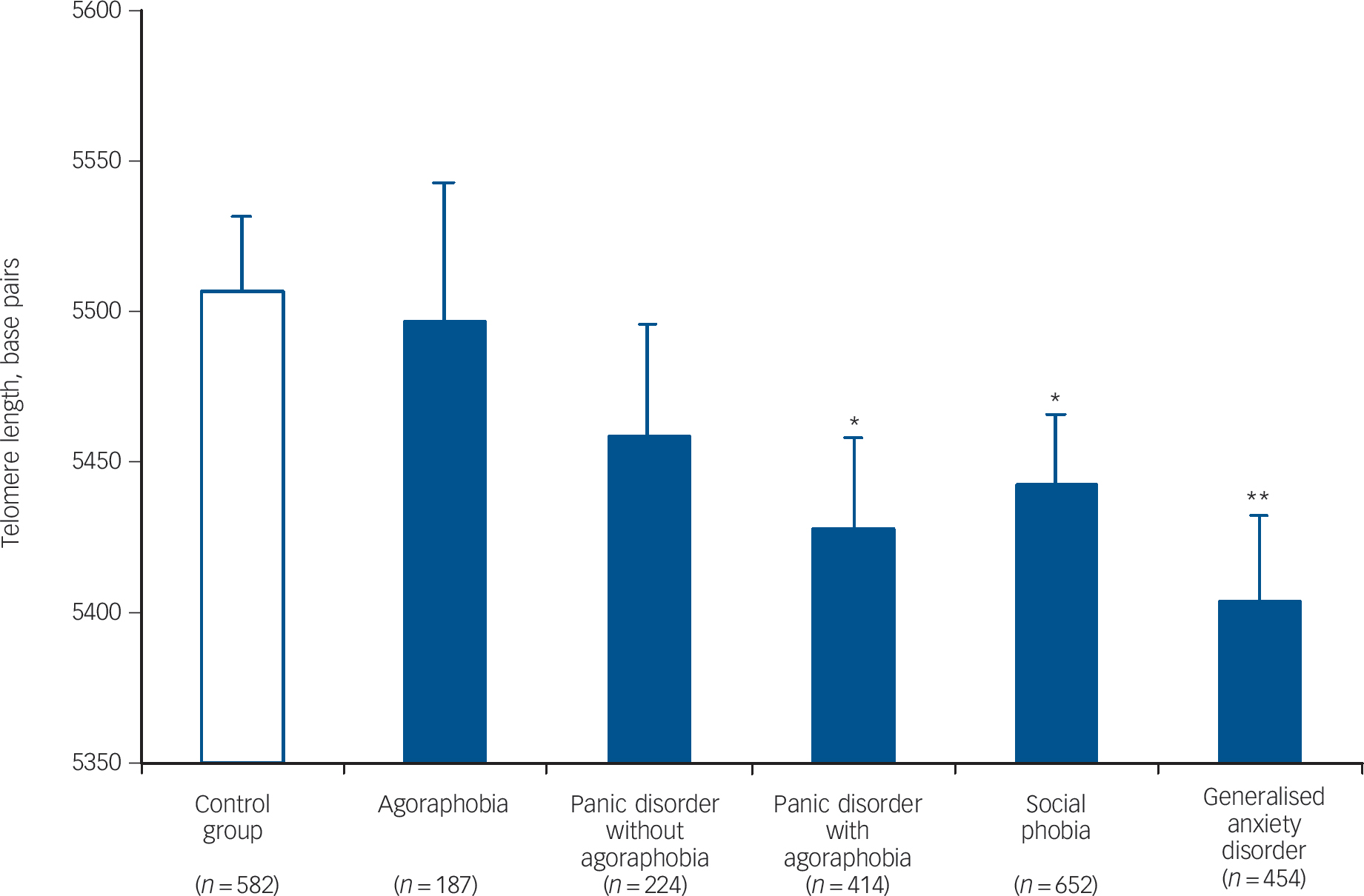
Fig. 1 Mean leukocyte telomere length (with s.e.) across various anxiety disorder subgroups v. the control group.
Patients with comorbid anxiety disorders appear in more than one disorder subgroup. All analyses are adjusted for age, gender, education, body mass index, smoking, alcohol, physical activity and somatic diseases.
*P<0.05, **P<0.01 v. control group.
Subsequently, the association between several anxiety characteristics and LTL was examined in the total sample as well as within the 1283 participants in the current anxiety disorder group (Table 4). Within the total sample, severity of anxiety arousal symptoms (β = –0.056, P= 0.007), social phobic symptoms (β = –0.043, P = 0.03) and worrying (β = –0.041, P = 0.04) were associated with shorter LTL. A higher number of current anxiety disorder diagnoses was also significantly associated with shorter LTL in the total sample (β = –0.047, P = 0.020), confirming the difference between people with and without a current anxiety disorder. No LTL-associations were found for symptom duration in the past 4 years (P = 0.14), comorbid MDD (P = 0.54) or the use of psychopharmacological medications (Table 4). Also, no anxiety characteristics were associated with LTL within the current anxiety disorder group by itself, suggesting that differences in LTL were only apparent when using the entire spectrum of participants in the healthy control and anxiety groups, but did not further differentiate within the current anxiety group.
Table 4 Associations between leukocyte telomere length and anxiety characteristics in different samplesFootnote a

| Total sample (n = 2324) | Current anxiety disorder group (n = 1283) | |||
|---|---|---|---|---|
| β | P | β | P | |
| Anxiety severity | ||||
| Anxiety arousal (Beck’s Anxiety Inventory) | –0.056 | <0.01 | –0.029 | 0.30 |
| Social fear (subscale Fear Questionnaire) | –0.043 | 0.03 | –0.029 | 0.28 |
| Agoraphobia (subscale Fear Questionnaire) | –0.021 | 0.31 | 0.011 | 0.70 |
| Worrying (Penn State Worry Questionnaire) | –0.041 | 0.04 | –0.002 | 0.95 |
| Duration (months with anxiety symptoms in past 4 years) | –0.029 | 0.14 | –0.010 | 0.73 |
| Number of current anxiety disorders | –0.047 | 0.02 | –0.001 | 0.98 |
| Comorbid major depressive disorder | –0.012 | 0.54 | 0.016 | 0.54 |
| Antidepressant use | ||||
| Tricyclic antidepressant | –0.006 | 0.76 | 0.011 | 0.67 |
| Selective serotonin reupake inhibitor | –0.033 | 0.10 | –0.009 | 0.73 |
| Other antidepressant | –0.036 | 0.07 | –0.013 | 0.64 |
| Benzodiazepine use | –0.007 | 0.73 | –0.002 | 0.94 |
a. All adjusted for age, gender, education, body mass index, smoking, alcohol, physical activity and somatic diseases.
Discussion
Main findings
This large-scale study demonstrated that patients with a current anxiety disorder on average had shorter LTL than non-psychiatric controls and patients with remitted anxiety disorder. Although effect sizes were modest, the difference may indicate 3–5 years of accelerated ageing for the current anxiety group, based on the estimated mean telomere shortening rate of 14–20 bp/year as found in our and other studies. Reference Cawthon, Smith, O'Brien, Sivatchenko and Kerber20,Reference Hartmann, Boehner, Groenen and Kalb22,Reference Wikgren, Maripuu, Karlsson, Nordfjall, Bergdahl and Hultdin23 The observed associations remained significant after controlling for lifestyle and health variables suggesting that the shorter telomeres were not simply because of a unhealthy lifestyle or physical illnesses within the anxiety group.
Within the current anxiety disorder group, we showed that type of anxiety disorder further differentiated across disorders: patients with panic disorder with agoraphobia, social phobia and GAD, but not panic disorder without agoraphobia and agoraphobia without panic disorder, had significantly shorter LTL than the control group. The differences in LTL for the anxiety subgroups corresponded with an estimated 3.5–5 years (social phobia), 4–6 years (panic disorder with agoraphobia) and 5.5–8 years (GAD) of accelerated ageing compared with the control group. Based on descriptive information (data not shown) it appeared that the two anxiety disorders that did not differ from controls in terms of LTL, showed less severe characteristics compared with other anxiety disorder subgroups. For instance, participants with agoraphobia and panic disorder without agoraphobia tended to have a smaller number of comorbid anxiety disorders, less often comorbid depression and/or slightly lower anxiety severity scores than the other three anxiety subgroups. Consequently, the fact that participants with agoraphobia and panic disorder without agoraphobia reported generally milder symptomatology might explain why LTL was less significantly decreased in these subgroups than in, for example. patients with panic disorder with agoraphobia, social phobia and GAD.
Our results suggest that telomere shortening might in part be reversible since the LTL difference with controls was not present in the group with remitted anxiety disorder. Furthermore, the time since remission was positively associated with LTL within the remitted anxiety group and participants with a remission time under 10 years had shorter telomeres than those who were remitted for 10 years or longer. An earlier study in the same cohort showed that patients with remitted MDD had shorter LTL than controls, suggesting that depression does leave a ‘biological scar’. Reference Verhoeven, Révész, Epel, Lin, Wolkowitz and Penninx24 But alternatively, the fact that there was a difference in LTL between the patients with remitted MDD compared with controls, whereas it did not differ in the patients with remitted anxiety disorder, might have been the result of the relatively higher levels of subthreshold symptoms and shorter remission time found in the remitted MDD group.
We further found that anxiety severity scores (anxiety arousal, social fear and worrying) were negatively associated with LTL in the total study sample. However, these variables were not associated with LTL within the current anxiety group, suggesting a dose-response effect across the entire spectrum of healthy controls and participants with anxiety but no further differentiations within the current anxiety group. In line with this, the number of anxiety disorders was also associated only in the total study sample. An explanation for this is that the overall effect of diagnosis status is stronger than that of a dose-response relationship within the participants with anxiety. Alternatively, the severity and duration measures used in our study may not fully represent the ‘anxiety dose’ component in patients. For instance, the anxiety severity measurements used were reflectors of symptom severity over the past week, but not over a much longer period and may therefore not be the best representation of general anxiety severity in patients. Duration of anxiety symptoms was not associated with LTL in this study. This might be as a result of the fact that generally most patients with anxiety disorder reported rather long symptom duration and therefore the range in this variable may not have been enough to illustrate associations with LTL. Comorbid MDD was not associated with LTL either, suggesting that the effects of anxiety disorder status on LTL were independent of MDD diagnosis, which was moreover confirmed by sensitivity analyses that showed that LTL of participants with anxiety disorder still differed from controls when we excluded patients with current and lifetime MDD. We did not find an association between psychopharmological medication and LTL, but more specific relationships between duration, type and dose of medication remain to be investigated.
Possible mechanisms
Our results align with the general tendency of the limited research into telomere length and anxiety disorders up until now: previous studies found anxiety disorders to be prospectively related to reduced telomere length, Reference Hoen, Rosmalen, Schoevers, Huzen, van der Harst and de Jonge26 and telomere length to be shorter in an older subgroup of participants with anxiety disorder compared with a similar-aged control group. Reference Kananen, Surakka, Pirkola, Suvisaari, Lonnqvist and Peltonen25 The observed shorter LTL in this study might be the consequence of several disturbances found in people with anxiety disorders, such as dysregulations in the major bodily stress systems such as the HPA axis Reference Vreeburg, Zitman, van der Pelt, de Rijk, Verhagen and van Dyck8 and the immune system. Reference Vogelzangs, Beekman, de Jonge and Penninx10 Dysregulations of these stress systems could contribute to telomere shortening in patients with anxiety disorders. Reference Wolkowitz, Epel, Reus and Mellon11,Reference Epel43 In line with this, several in vitro and in vivo studies found increased cortisol, Reference Choi, Fauce and Effros44 oxidative stress Reference von Zglinicki45 and proinflammatory cytokines Reference Damjanovic, Yang, Glaser, Kiecolt-Glaser, Nguyen and Laskowski46 to be associated with shorter telomere length. However, the exact biological mechanisms that mediate the relationship between certain anxiety disorders and telomere shortening, as well as the direction of the link, remain to be further explored.
Strengths and limitations
The major strengths of the present study are the large sample size, the inclusion of participants with well-characterised current and remitted anxiety disorder diagnoses as well as healthy controls, the wide age range and the assessment of important covariates such as somatic health and lifestyle variables. However, some limitations of the present study should also be noted. First, the associations between anxiety disorder status and LTL were of rather small effect size. However, our effect sizes (Cohen’s d = 0.12–0.22) are not very different from those described in recent studies on pathophysiological mechanisms, for example, increased inflammatory markers in patients with anxiety disorder (Cohen’s d = 0.15–0.19), Reference Vogelzangs, Beekman, de Jonge and Penninx10 and decreased brain-derived neurotrophic factor (Cohen’s d = 0.15–0.23) Reference Molendijk, Bus, Oude Voshaar, Spinhoven, Penninx and Elzinga47 and increased cortisol (Cohen’s d = 0.15–0.25) Reference Vreeburg, Hoogendijk, van, Derijk, Verhagen and van48 across MDD status. Second, as stress arousal is inherent in people with anxiety disorder, it is possible that the association between anxiety disorder status and telomere length is – partly – mediated by exposure to stressful events and experienced stress. By controlling for stress, the sample might no longer reflect ecologically valid anxiety disorders. It is, however, important to note that the observed telomere shortening might not be specific to anxiety disorders. Third, the sample studied may not fully represent the population with anxiety disorders at large, since for example PTSD and OCD, which can be comorbid with the anxiety disorders in our sample, were exclusion criteria. Fourth, our study design was cross-sectional, which might undermine the complexity of telomere length regulating mechanisms, and which does not allow one to draw conclusions regarding causality. Even though we looked at patients with current and remitted anxiety disorders, suggestive of a temporal effect, this study is solely a reflection of the current state of different participants. Since longitudinal studies show complex telomere length dynamics with both shortening as well as lengthening of telomeres over time, Reference Aviv, Chen, Gardner, Kimura, Brimacombe and Cao49–Reference Shalev, Moffitt, Sugden, Williams, Houts and Danese52 future studies should explore the relationship longitudinally. It should also be noted that our variable base pairs is an estimate rather than a directly measured variable. Also, as in nearly all studies, we used leukocytes for telomere length measurement, which is a validated, non-invasive and often-used indicator for cellular ageing. It would, however, be worthwhile to examine cellular ageing processes in other tissues. Finally, telomerase activity was not measured in the current study, but information regarding telomere repair and maintenance would be of great value in future research, since this would give us a more dynamic picture of the telomere/telomerase system in affective disorders.
Clinical implications and further research
This large cohort study provides suggestive evidence that patients with anxiety disorders have shorter telomere length compared with healthy controls, suggesting a pathway of accelerated cellular ageing within the most common anxiety disorders. This might in turn explain the increased somatic health problems found in this population. As participants with remitted anxiety disorder resembled the healthy controls, in terms of telomere length, it is possible that this telomere shortening can be reversed, although the present data did not allow assessment of within-participant changes in telomere length. Lifestyle interventions such as increased physical activity have been shown to have a favourable impact on cellular ageing in a healthy population. Reference Puterman, Lin, Blackburn, O'Donovan, Adler and Epel53–Reference Osthus, Sgura, Berardinelli, Alsnes, Bronstad and Rehn55 Therefore, further studies should test whether such interventions could result not only in a reversal of psychiatric symptomatology but also in restoring cellular ageing and consequent somatic health.
Funding
The infrastructure for the NESDA study (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organization for Health Research and Development (Zon-Mw, grant number 10-000-1002) and is supported by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Institute for Quality of Health Care (IQ Healthcare), Netherlands Institute for Health Services Research (NIVEL) and Netherlands Institute of Mental Health and Addiction (Trimbos). B.W.J.H.P., J.E.V., D.R. and telomere length assaying were supported through a NWO-VICI grant (number 91811602).








eLetters
No eLetters have been published for this article.