Dietary protein has recently attracted considerable attention in appetite control. Several studies have shown that protein is more satiating than other macronutrients(Reference Latner and Schwartz1–Reference Astrup3), possibly due to increased postprandial thermic effects, altered gastrointestinal (GI) functions and postprandial metabolism, e.g. increased amino acid (AA) concentration and gluconeogenesis(Reference Veldhorst, Smeets and Soenen4). Previous evidence(Reference Karhunen, Juvonen and Huotari5) also indicates the increased release of GI hormones, such as cholecystokinin (CCK), glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) in response to protein intake.
As an analogy with dietary carbohydrates, Boirie et al. (Reference Boirie, Dangin and Gachon6) proposed the concept of ‘fast’ and ‘slow’ dairy proteins, which has been recently supported by other groups(Reference Hall, Millward and Long7, Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen8). Between the two main milk protein fractions, whey protein (Wh) has been considered as a ‘fast’ digestible protein with soluble characteristics and a high and rapid AA profile, whereas casein (Cas) has been characterised as a ‘slow’ protein that precipitates in the stomach, leading to delayed gastric emptying (GE) and slower and lower AA levels. In addition, Cas and Wh have been shown to differentially affect postprandial GI hormone secretion and sensations of appetite(Reference Hall, Millward and Long7).
Foods and their components possess complex structural and textural characteristics that have been shown to influence postprandial GI function and metabolism(Reference Juntunen, Niskanen and Liukkonen9–Reference Juvonen, Purhonen and Salmenkallio-Marttila12). In general, viscous and solid food matrix and food characteristics resistant to post-ingestive digestion have been shown to delay GE and the subsequent absorption of nutrients in the upper GI tract(Reference Kong and Singh11–Reference Kristensen and Jensen13). Previous studies have also shown that food texture and structure modulates the postprandial release of GI hormones(Reference Marciani, Wickham and Singh10, Reference Juvonen, Purhonen and Salmenkallio-Marttila12–Reference Tieken, Leidy and Stull15) and may concomitantly affect sensations of appetite(Reference Tieken, Leidy and Stull15–Reference Zijlstra, Mars and de Wijk19). Furthermore, recent data demonstrate the importance of orosensory stimulation and oral processing time regarding different food characteristics, which may subsequently influence appetite sensations and food intake(Reference Zijlstra, de Wijk and Mars20–Reference Karl, Young and Montain22). However, a controversy prevails over the effects of the physical form of foods and macronutrients on postprandial physiology, which warrants further clarification(Reference Almiron-Roig, Chen and Drewnowski23).
Cross-linking enzymes (e.g. transglutaminase, TG) provide an ideal tool for proteinaceous matrices to create food structures and textures with desirable mechanical and sensory properties(Reference Gerrard24, Reference Lantto25). Enzymatic cross-linking changes the complex food protein network, affecting the functional characteristics of proteins and protein-containing foods(Reference Gerrard24, Reference Feeney and Whitaker26–Reference Lille, Ercili-Cura and Partanen28). Based on current knowledge, cross-linking treatment does not, however, diminish the bioavailability and biological value of the cross-linked proteins since they can still be digested, absorbed and utilised in the body(Reference Seguro, Kumazawa and Kuraishi29). Despite the considerable amount of literature on the effects of cross-linking on food structure and texture, in vivo experiments to investigate their effects on postprandial physiology are very limited.
The objective of the present study was to investigate the effect of the type and structure of milk-protein based model foods on postprandial hormonal, metabolic and appetitive responses in healthy young males. The cross-linking enzyme TG was used as a tool to create two Cas-based test products of identical composition but with a difference in rheological properties (liquid v. gel).
Our hypothesis was that a more solid structure would promote satiety and attenuate postprandial GI peptide responses.
Methods
Subjects
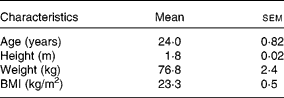
Healthy male volunteers (n 8; Table 1) participated in the study at the Department of Physiology at Oulu University, Finland. The volunteers were recruited via advertisements posted across the campuses of Oulu University. A total of twelve volunteers were recruited to the study, of which eight individuals finished the study protocol including three subsequent visits. All volunteers were interviewed about their medical history, dietary habits and physical activity before the beginning of the study. Individuals with food intolerances or allergies, who were smokers, who had modified their diet or exercise routines during the past year to lose weight, or who were on medication were excluded.
Table 1 Participants' characteristics
(Mean values with their standard errors, n 8)
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of the University of Kuopio and Kuopio University Hospital. Written informed consent was obtained from all subjects.
Study design
The study had a randomised, repeated-measures, cross-over design. All participants tested each test product, with a minimum of 2 d separating the individual test days. The participants were requested to maintain their habitual diet and exercise routines throughout the study period.
After a 12 h fast, the participants ingested one of the following milk protein-based test products along with 400 ml of water in a randomised order: (1) high-viscous Cas solution (Cas), (2) rigid Cas gel (Cas cross-linked by TG; Cas-TG), (3) low-viscous Wh solution (Wh). The test products were to be consumed within 30 min.
Blood samples for the determination of plasma glucose, insulin, CCK, GLP-1 and PYY concentrations were taken via an antecubital cannula inserted before the start of the experiment. The blood samples were drawn before and at 15, 30, 60, 120, 180 and 240 min following the ingestion of the test products.
Test products
A total of three isoenergetic and isovolumic milk protein-based pH-neutral model foods were served as test products. The detailed composition of the products and ingredients used are shown in Tables 2 and 3, respectively.
Table 2 Composition of the test products*

Cas, casein; Cas-TG, transglutaminase-treated Cas; Wh, whey protein.
* Ingested with 400 ml of water.
Table 3 Ingredients used in the test products (g/portion)

Cas, casein; Cas-TG, transglutaminase-treated Cas; Wh, whey protein.
* Sodium caseinate EM7, (mainly Cas, proportion of individual caseins similar to that in milk), composition: 90 % protein, 5 % moisture, 4 % ash, 0·8 % fat and 0·2 % lactose (DMV International, Veghel, The Netherlands).
† Wh isolate (mainly β-lactoglobulin), BiPRO®, composition: 92·9 % protein, 5 % moisture, 1·9 % ash, 0·4 % fat and 0·5 % lactose (Davisco Foods International, Incorporation, MN, USA).
‡ Transglutaminase powder, Activa MP, Ajinomoto Foods Deutschland GmbH, Hamburg, Germany, activity 78–126 EU according to the manufacturer, composition: 94 % carbohydrates, 5 % moisture and 1 % protein.
§ Vanilla aroma, Iberchem, Murcia, Spain.
∥ Hermesetas Liquid, Hermes Sweeteners Limited, Zurich, Switzerland.
The preparation of the Cas-based test products was performed as follows: protein powder was mixed in a bowl with boiling water under continuous stirring until a smooth solution was formed. The solution was allowed to cool down to room temperature before the addition of TG, which was suspended in a small amount of water. For the Cas test product, TG was inactivated by boiling the suspension for 5 min before adding it to the Cas solution. After the addition of the TG suspension, sweetener, aroma and water were added to each product to a final weight of 400 g. The mixture was stirred for 1 h at room temperature and thereafter stored at 5°C for 14 h, during which gelation took place in the product where TG was active (Cas-TG). The test products were served immediately after the 14 h storage time.
The preparation of the Wh-based product followed the procedure used with Cas, except that the added water was boiled and cooled down before mixing with the whey powder to avoid heat-induced denaturation of Wh. Inactivated TG was added to the Wh product as well.
Texture of the test products
The texture of the test products was measured instrumentally with a Texture Analyser device (TA-HDi; Stable Micro Systems Limited, Godalming, UK) equipped with a 5 kg load cell. After all the ingredients were mixed with each other as described earlier, small plastic containers (inner diameter 42 mm) were filled up to 50 ml with test liquid (sample height approximately 35 mm). The containers were closed with caps and 1 h after the TG addition transferred to a refrigerator (5°C). The texture of the test products was measured after 14 h cold storage by recording the force required to push a 12·7 mm diameter hemispherical Delrin cylinder probe into the test product with a speed of 1 mm/s. The force at a depth of 15 mm was taken as a measure of sample firmness. At least five samples were measured for each test product. Wh was a low-viscous fluid (comparable to, for example, water or skimmed milk), Cas a high-viscous fluid with some elasticity (comparable to, for example, regular yogurt) and Cas-TG a very strong elastic gel (comparable to, for example, thick marmalade). The puncture test showed that the firmness of the Cas-TG product was very much higher than that of Cas or Wh (Fig. 1(A)).

Fig. 1 (A) Firmness of the test products as measured by a puncture test using the Texture Analyser: casein (Cas, –△–), transglutaminase-treated Cas (Cas-TG, –■–) and whey protein (Wh, ··⋄··). Values are means, with standard errors represented by vertical bars. (B) Comparison of the viscosity of Cas (–△–) and Wh (··⋄··).
Viscosity of whey protein and casein
The viscosity of the liquid-like test products Wh and Cas was measured at 29°C with a stress-controlled rotational rheometer (AR-G2; TA Instruments, Crawley, West Sussex, UK) equipped with a four-bladed vane geometry. The diameter of the cylindrical sample cup was 30 mm and that of the vane 28 mm. The length of the vane was 42 mm. About 40 ml of the sample were poured into the measuring cup and the vane was lowered into it so that the blades were just immersed. After positioning the vane, the samples were allowed to rest for 5 min before the measurement was started. The steady-state viscosity was measured with a gradually increasing shear stress with values resulting in shear rates in the range 0·1–150/s. The viscosity was presented as a function of shear rate (Fig. 1(B)). The viscosity of the syrup resembling Cas was very much higher than the viscosity of Wh (Fig. 1(B)), which was almost the same as water.
Appetite measurements
Participants rated their appetite immediately after each blood sample was drawn. In addition, pleasantness of the test products was rated before and immediately after the consumption of the test products. Profiles of appetite (hunger, satiety, desire to eat, fullness and thirst) and pleasantness of the test products were measured using visual analogue scales(Reference Stubbs, Hughes and Johnstone30). Each scale consisted of a 100 mm horizontal line with verbal anchors expressing the weakest or strongest statement (i.e. ‘I am not hungry at all’ or ‘I have never been hungrier’). The participants were requested to mark a vertical line on the horizontal axis corresponding to their sensations that were most appropriate at the time. Visual analogue scale ratings were measured in mm, resulting in scores between 0 and 100.
Biochemical measurements
Immediately after the blood samples were taken, 50 μl of the protease dipeptidyl peptidase IV inhibitor (DPP IV Inhibitor; Millipore, West Sussex, UK) were added to the sample tubes to prevent the degradation of native GLP-1 by inhibiting the activity of the dipeptidyl peptidase IV enzyme.
Prechilled EDTA and trasylol (250 KIU/5 ml) containing tubes were used for plasma insulin, CCK, GLP-1 and PYY, and fluoride citrate-containing tubes for plasma glucose. Plasma glucose, insulin, CCK, GLP-1 and PYY samples were centrifuged for 10 min at 1700 g at 4°C. All samples were immediately frozen and stored at − 70°C until analysed. All the samples of each individual were analysed in duplicate and within the same assay. Results were obtained from all the study participants except for plasma GLP-1 measurements where six participants were included.
Plasma glucose was analysed using an enzymatic photometric assay (Thermo Electron Corporation, Vantaa, Finland) and plasma insulin with a luminometric immunoassay (Siemens Medical Solutions Diagnostics, Tarrytown, NY, USA). The intra-assay CV for plasma glucose was 2·7 % at 10·2 mmol/l and the inter-assay CV was 4·1 % at 2·05 mmol/l and 1·8 % at 8·2 mmol/l. For plasma insulin, the intra-assay CV was 2·7 % at 667 pmol/l and the inter-assay CV was 6·6 % at 41 pmol/l and 5·1 % at 444 pmol/l. Total plasma PYY, i.e. both PYY1–36 and PYY3–36, and active plasma GLP-1, i.e. GLP-17–36 amide and GLP-17–37, concentrations were quantified in a direct assay with the use of a Human Gut Hormone Panel Milliplex kit (Millipore, St Charles, MO, USA) utilising a Bio-Plex instrument based on Luminex xMAP technology (Bio-Rad Laboratories, Incorporation, CA, USA). The intra-assay CV for the total GLP-1 was 10·8 % at 40·1 pg/ml and 10·6 % at 84·9 pg/ml and the inter-assay CV was 28·9 % at 26 pg/ml and 15·8 % at 202 pg/ml. For the total PYY, the intra-assay CV was 4·1 % at 78 pg/ml and 2·3 % at 141·3 pg/ml and the inter-assay CV was 13·1 % at 74·5 pg/ml and 12·2 % at 164·1 pg/ml.
Plasma CCK concentrations were analysed after extraction using a RIA kit (Euro-Diagnostica AB, Malmö, Sweden). The plasma samples were extracted with a SepPac C18 cartridge preconditioned with 1·5 ml of 2-propanol and 1·5 ml of 0·1 % trifluoroacetic acid (Waters, Milford, MA, USA) in an automated Gilson Aspec XL system (Gilson, Middleton, WI, USA). Then, 1 ml of the plasma samples was acidified with 0·2 ml of 1 m-HCl containing 1·6 % glycine. After loading the sample, the cartridge was washed with 2 ml of 0·1 % trifluoroacetic acid, and the samples were eluted with 2 ml of 80 % acetonitrile in 0·1 % trifluoroacetic acid. The samples were evaporated into dryness overnight. The dry residue was dissolved in 500 μl of RIA buffer and RIA was conducted according to the manufacturer's instructions. Briefly, the samples were incubated with the primary antibody for 48 h at 4°C and I-125-labelled CCK-8 was added before 96 h incubation at 4°C. A double-antibody solid phase was added and the samples were incubated for 60 min at 4°C. Before the measurement, the samples were centrifuged for 15 min at 1700 g at 4°C. The supernatant was decanted and the activity of aliquot was measured with a gammacounter (Wallac 1272 Clinigamma; Perkin-Elmer, Waltham, MA, USA). The intra-assay CV for CCK was 5·5 % at 4·4 pmol/l and 2·0 % at 20·6 pmol/l and the inter-assay CV was 13·7 % at 4·2 pmol/l and 4·1 % at 20·6 pmol/l.
Statistical analysis
Data analyses were performed with SPSS for Windows software (SPSS for Windows, version 17.0; SPSS Inc., Chicago, IL, USA). Results are expressed as means and standard errors of the mean with a value P ≤ 0·05 (two-sided) as the criterion for statistical significance.
Linear mixed-effects modelling was used to compare the effects of the test products on the postprandial metabolic and hormonal responses and appetite sensations. The results have been analysed and are expressed based on the raw outcome values. The baseline value of each parameter was used as a covariate to take into account the possible effect of baseline differences on the analysis. The method takes into account the sources of variation, where the participant is used as a random factor and product, time and product × time as fixed factors. Where a significant main effect of a product, time or product × time interaction was observed (P < 0·05), post hoc analyses were performed using the Bonferroni correction for multiple comparisons to identify the significant differences among the test products at each time point of measurement.
Results
Mean time of test product consumption
The mean time for the consumption of the test products was 20·3 (sem 2·9) min for Cas-TG, 6·0 (sem 1·2) min for Cas and 2·5 (sem 0·7) min for Wh.
Plasma glucose and insulin responses
Postprandial plasma glucose and insulin concentrations were significantly different following ingestion of the test products (P < 0·05; Fig. 2(A) and (B)). After the ingestion of all test products, the concentration of plasma glucose slightly increased for 15 min, declining thereafter and returning towards the preprandial concentrations after 60 min. The postprandial insulin responses increased after the consumption of all test products and peaked at 30 min after which the concentration returned towards the baseline. Plasma insulin was higher at 15 min after the consumption of Wh and at 30 min after the consumption of Cas and Wh than after the consumption of Cas-TG (P < 0·05). On the contrary, the decrease in plasma glucose at 30 and 60 min was greater after the consumption of Cas and Wh than after the consumption of Cas-TG (P < 0·05).

Fig. 2 Changes in the concentrations of plasma (A) insulin, (B) glucose, (C) glucagon-like peptide 1 (GLP-1) and (D) cholecystokinin (CCK) during the 240 min postprandial period in young men consuming casein (Cas, –△–), transglutaminase-treated Cas (Cas-TG, –■–) or whey protein (Wh, ··⋄··) test products. Values are means, with their standard errors represented by vertical bars (n 8, except for GLP-1 n 6; linear mixed-effects modelling with Bonferroni correction). a,b,c Mean values with unlike letters were significantly different between Wh and Cas-TG, between Wh/Cas and Cas-TG and between Cas and Wh/Cas-TG (P < 0·05).
Plasma cholecystokinin, glucagon-like peptide 1 and peptide YY responses
Postprandial concentrations of plasma CCK peaked at 15 min and GLP-1 at 30 min after the consumption of all test products, after which the concentrations returned towards the baseline values (Fig. 2(C) and (D)). At the same time, postprandial PYY concentrations varied only slightly from the baseline values (about 90 pg/ml, data not shown) during the experimental period. The test products resulted in significant differences in plasma CCK (P < 0·05), but not in plasma PYY (P>0·05) concentration. CCK concentrations were increased after the consumption of Cas and Wh at 15 min compared with Cas-TG (P < 0·001). Furthermore, at 30 min, the CCK concentration was higher after the consumption of Cas than after the consumption of Cas-TG or whey products (P < 0·05). A trend (P = 0·074) was observed in GLP-1 responses among the test products such that GLP-1 concentration was increased after the consumption of Wh at 15 min and after the consumption of Cas at 30 min when compared with Cas-TG.
Appetite ratings
Ratings on estimated pleasantness differed before and after the consumption of the products (P < 0·05). Mean pleasantness ratings immediately before (Cas-TG 21, Cas 33 and Wh 41) and after (Cas-TG 3, Cas 45 and Wh 67) the ingestion of the test products indicated that Cas-TG was rated least pleasant followed by Cas and Wh, respectively.
Ratings for hunger, satiety, desire to eat, fullness and thirst varied significantly with time (P < 0·001; Fig. 3). Ratings of hunger, desire to eat and thirst initially decreased after the consumption of all test products, after which ratings returned gradually towards the baseline after 60 min at the latest. Measures of satiety and fullness increased after the ingestion of each product, reached peak values by 60 min and declined thereafter. Moreover, significant differences in fullness ratings were detected among the test products: fullness was markedly increased after the consumption of Cas-TG at 15, 30 and 120 min when compared with Wh and at 30 min when compared with Cas (Fig. 3(D)).

Fig. 3 Changes in the feeling of (A) hunger, (B) desire to eat, (C) satiety and (D) fullness during the 240 min postprandial period in young men consuming casein (Cas, –△–), transglutaminase-treated Cas (Cas-TG, –■–) or whey protein (Wh, ··⋄··) test products. Values are means, with their standard errors (n 8; linear mixed-effects modelling with Bonferroni correction). a,b Mean values with unlike letters were significantly different between Cas-TG and Wh and between Cas-TG and Cas/Wh (P < 0·05). VAS, visual analogue scale.
Discussion
In the present study, we found that the structure of three milk protein-based test foods affected postprandial glucose and insulin responses, GI hormone release and appetite ratings. Gelation of the test product by cross-linking Cas with TG resulted in attenuated postprandial glucose and insulin responses and CCK release and accentuated ratings of fullness.
Both the macro- and microstructure of food are potent modulators of postprandial physiology, affecting GE, digestion and absorption of nutrients with subsequent reflections in glucose and insulin responses, concomitant GI peptide secretion and appetite(Reference Juntunen, Niskanen and Liukkonen9–Reference Juvonen, Purhonen and Salmenkallio-Marttila12, Reference Martens, Lemmens and Born18).
An increase in the viscosity or firmness of a food delays GE rate, and GE rate as well as post-ingestive hydrolysis affects postprandial glucose, insulin and GI peptide responses(Reference Kong and Singh11, Reference Juvonen, Purhonen and Salmenkallio-Marttila12, Reference Marciani, Gowland and Spiller31, Reference Karhunen, Juvonen and Flander32). The present results did thus support our hypothesis that firm food structure enhances satiety and attenuates postprandial metabolic responses. To our knowledge, the present study is the first one to demonstrate the effects of food texture and structure modification by enzymatic cross-linking on postprandial responses.
The release in insulin showed a marked increase after the ingestion of all protein products, but was attenuated after the consumption of Cas-TG. The fact that dietary protein stimulates postprandial insulin secretion is well documented(Reference Karamanlis, Chaikomin and Doran33, Reference Nilsson, Stenberg and Frid34). The type and form (intact v. hydrolysate) of protein affects insulin release(Reference Karamanlis, Chaikomin and Doran33–Reference Nilsson, Holst and Björck36). The insulin response did not differ between Cas and Wh in the present study, which is in contrast to some previous findings conducted with protein-rich test products suggesting that Wh would be more insulinotropic than intact Cas or glucose(Reference Nilsson, Stenberg and Frid34–Reference Nilsson, Holst and Björck36). The reason for the comparable insulin responses between Wh and Cas in the present study is unknown and needs further clarification. Instead, an explanation for the different insulinotropic responses between Cas-TG and Cas/Wh in the present study might be the physical form of the test products. The liquid Wh and the viscous Cas were digested and absorbed more rapidly than the solid cross-linked Cas gel, which resulted most probably in a more pronounced AA response and, concomitantly, more enhanced insulin responses.
In the present study, the smaller decrease in postprandial glucose after the consumption of Cas-TG than after the consumption of Cas or Wh probably reflects the attenuated release of insulin following Cas-TG. The present glucose results are comparable with those of previous studies, also showing a slight decrease in postprandial glucose responses after protein drinks(Reference Karamanlis, Chaikomin and Doran33, Reference Claessens, Saris and van Baak37, Reference Pal and Ellis38). Several previous studies have investigated the effects of various dietary proteins combined with carbohydrates or in mixed meals on postprandial glucose and insulin responses(Reference Karamanlis, Chaikomin and Doran33, Reference Nilsson, Stenberg and Frid34, Reference Nilsson, Holst and Björck36, Reference Nuttall and Gannon39–Reference Lang, Bellisle and Oppert41), but studies comparing the postprandial effects of different protein types as such are sparse. The results suggest that protein ingestion alone may result in a small decrease in postprandial glucose both in healthy individuals(Reference Karamanlis, Chaikomin and Doran33, Reference Claessens, Saris and van Baak37, Reference Pal and Ellis38) and in patients with type 2 diabetes(Reference Gannon, Nuttall and Neil42). Postprandial blood glucose and subsequent insulin concentrations are largely determined by the rate at which nutrients are delivered to the proximal small intestine(Reference Rayner, Samsom and Jones43, Reference Ma, Rayner and Jones44). Since glucose and insulin responses were attenuated after the consumption of the Cas-TG test product, the present results suggest that GE may have been delayed after the consumption of Cas-TG. This is supported by previous studies showing that GE rate affects the magnitude and timing of postprandial blood glucose and insulin concentrations(Reference Rayner, Samsom and Jones43). Moreover, protein type and food texture and structure have been shown to influence GE rate so that GE is slowed down after the consumption of Cas protein (v. Wh) and foods with solid texture(Reference Kong and Singh11, Reference Mahé, Roos and Benamouzig45). We did not measure GE via our stable isotope technique available, because of the obvious differences in structure and thus the need for different tracers, which would not have been comparable.
Although data on milk proteins are still rather limited, dietary protein also affects the release of the GI-originating peptides, especially CCK, but also GLP-1 and PYY(Reference Karhunen, Juvonen and Huotari5). We observed a trend in postprandial GLP-1 responses, indicating that the test product with Wh and Cas stimulated GLP-1 secretion more than Cas-TG in the beginning of the study period. As would be expected, the trend was in the same direction as the postprandial responses of insulin. Because the difference in GLP-1 release between Cas-TG and CAS/Wh was not quite statistically significant, GLP-1 does not solely explain the difference in postprandial insulin concentrations. The postprandial release of GLP-1 has been shown to be directly related both to the quality and quantity of dietary protein(Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen8). Previously, Wh has been reported to be a more potent stimulator for GLP-1 secretion than Cas, possibly because Wh may inhibit dipeptidyl peptidase IV, the enzyme responsible for the degradation of GLP-1(Reference Gunnarsson, Winzell and Deacon46). We found no significant difference in GLP-1 release between Wh and Cas, but, overall, the present results suggest that meal structure is more important than the type of protein on these GI hormone responses.
Dietary fat and protein have been shown to be the main stimulants for the CCK release(Reference Liddle, Goldfine and Rosen47). In addition, the effects of Cas and Wh on the postprandial CCK release might also differ(Reference Hall, Millward and Long7), or remain unchanged(Reference Bowen, Noakes and Trenerry48), with comparable CCK responses after the consumption of liquid Cas and Wh test products. In the present study, the CCK response was more enhanced after the consumption of Cas and Wh than after the consumption of Cas-TG, indicating more rapid and pronounced digestion and subsequent stimulation of CCK producing I-cells after the consumption of Cas and Wh than after the consumption of Cas-TG.
We did not detect any marked postprandial PYY increase after the consumption of any of the protein-based test products nor were significant differences observed in PYY responses among the products. Previously, dietary protein in mixed meals has been shown to stimulate the postprandial secretion of PYY(Reference Batterham, Heffron and Kapoor49), but results are still conflicting(Reference Helou, Obeid and Azar50, Reference Essah, Levy and Sistrun51) and studies utilising test products based exclusively on protein are very limited. Earlier studies have suggested that PYY is released in proportion to the energy content and macronutrient composition of the test meals(Reference Adrian, Ferri and Bacarese-Hamilton52, Reference Degen, Oesch and Casanova53), indicating that the larger the energy and dietary fat content of the meal is, the more PYY is released. Thus, test products containing only protein, together with low and equal energy content, may explain partly the PYY results observed in the present study.
Fullness was significantly greater after the consumption of Cas-TG than after the consumption of Cas and Wh, which is in line with previous findings of greater satiety or fullness after the consumption of solid v. liquid foods(Reference Mattes and Rothacker16, Reference Akhavan, Luhovyy and Anderson17, Reference Hulshof, De Graaf and Weststrate54). In contrast, we did not observe any difference in postprandial satiety between liquid Cas and Wh. Some previous studies have suggested that Wh promotes greater satiety than Cas. The authors speculated that Wh is more soluble in the stomach, with faster GE and digestion rate and a more rapid post-absorptive AA response and more pronounced hormone secretion than after the consumption of Cas(Reference Boirie, Dangin and Gachon6–Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen8). The reason for this discrepancy might be due to the high protein content of the test products of the present study, which probably induced postprandial AA concentrations high enough to abolish differences between Wh and Cas in postprandial hormone responses and feelings of satiety(Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen8).
Modification of the physico-chemical properties of foods can be used to affect postprandial GI function, metabolism and appetite. The difficulty in investigating the effects of food structure and texture on postprandial responses lies often in preparing of the test products, which would differ in these properties without changing the nutritional composition. In the present study, this was enabled by the utilisation of the cross-linking enzyme TG. As far as we are aware, this is the first human study in healthy individuals to compare the postprandial effects of two Cas-based model foods of similar composition and pH, but of clearly differing texture and structure.
In conclusion, the present results suggest that increasing the firmness of dairy proteinaceous food is more effective in modulating the postprandial responses than the type of protein. Modification of protein-based food structure could thus offer a tool for optimising the postprandial glucose and insulin concentrations and influencing postprandial fullness.
Acknowledgements
We would like to express our sincere acknowledgments to Tuula Taskinen and Meeri Kröger for their excellent technical and laboratory assistance throughout the study. We gratefully acknowledge Maritta Siloaho, who provided the expert assistance with biochemical assays and analysis. The present study was supported by the Academy of Finland, The Finnish Graduate School on Applied Bioscience: Bioengineering, Food & Nutrition, Environment and Kuopio University Hospital. K. R. J., L. J. K., M. E. L., K. S. P., D. E. L., H. M. M., L. K. N. and K.-H. H designed the research; K. R. J., E. V., T. K., A. J.-A. and K.-H. H. conducted the research; M. E. L. provided the protocol for preparing the test products; all authors contributed to the writing of the manuscript. All authors read and approved the final manuscript. None of the authors has conflicts of interest.








