In recent years, one of the main research topics in aquaculture nutrition has been the replacement of fishmeals (FM) and fish oils (FO) in the diets for fish exploiting alternative sources for protein and lipid, respectively. The use of FM and FO in fish nutrition, especially in the intensive culture of carnivorous species such as Atlantic salmon (Salmo salar), has been a common practice for years and, to a great extent, still is today. This is because they constitute excellent sources of essential amino acids and fatty acids (FA), and especially of highly unsaturated FA(Reference Sargent and Tacon1–3). However, the need for reduction in the consumption of these commodities in aqua feeds is required for a number of reasons, including environmental and economic concerns, including sustainability issues, price increases, etc.(Reference Pike and Barlow4–Reference Trushenski, Kasper and Kohler8). In addition, there are issues regarding the quality of the final product, as there is a potential risk of contamination of FM and FO with organic pollutants(Reference Bell, McGhee and Dick9–Reference Bell, Waagbø, Holmer, Black, Duarte, Marbà and Karakassis13).
Numerous commodities have been successfully tested as alternatives to FM and FO. In the case of FO replacement, vegetable oils (VO), including rapeseed oil (RO), have been included, either as single replacements or as part of VO blends. The effects of such dietary alterations have been recently reviewed(Reference Bell, Waagbø, Holmer, Black, Duarte, Marbà and Karakassis13–Reference Glencross15) and can be summarised as causing no detrimental effects on growth and feed utilisation(Reference Bell, McEvoy and Tocher16–Reference Caballero, Obach and Rosenlund22) or notably, in some cases, enhancing growth(Reference Torstensen, Bell and Rosenlund21, Reference Bendiksen, Berg and Jobling23, Reference Karalazos, Bendiksen and Dick24). However, the dietary inclusion of RO at the expense of FO leads to significant changes in tissue FA compositions, which reflect the FA compositions of the diets, and FA metabolism, including β-oxidation. It should be noted that RO's content of 18 : 2n-6 and 18 : 3n-3 is moderate and at a ratio of 2:1, and thus should result only in modest deposition of these FA in fish tissues and, perhaps, enhance the endogenous conversion of 18 : 3n-3 to 20 : 5n-3 and 22 : 6n-3. Also, RO contains high levels of MUFA, especially 18 : 1n-9, which are preferred substrates for energy production by Atlantic salmon, and hence growth rates of the fish should not be compromised(Reference Bell, McEvoy and Tocher16, Reference Henderson and Sargent25–Reference Kiessling and Kiessling27). Alterations of the fish tissue FA profile are of importance given the role of highly unsaturated FA, and especially EPA and DHA, to human health and the increasing demand of consumers for nutritious and health-promoting products(Reference Bell, Waagbø, Holmer, Black, Duarte, Marbà and Karakassis13). Fish are unique sources of these FA, and hence their nutritional characteristics should not be compromised.
However, there are issues regarding FM and FO replacement in fish diets, which remain unclear. For instance, the authors of the aforementioned reviews have pointed out that the simultaneous reduction of both FM and FO could be challenging due to the expected reduction in essential FA and amino acids.
In those terms, the investigation of the replacement of FO with a VO such as RO with a concurrent reduction of FM is of considerable interest. The use of energy-dense diets can be a potentially useful approach towards the reduction of FM, as such diet formulations require less rotein by exploiting lipids for energy production, and hence can be potentially advantageous for the nutrition of carnivorous species, such as Atlantic salmon(Reference Cho and Bureau28, Reference Halver, Hardy, Halver and Hardy29). Research findings agree that these diets are performing well in terms of fish growth and feed utilisation, while a sparing effect on protein by increased dietary oil has also been shown(Reference Bendiksen, Berg and Jobling23, Reference Karalazos, Bendiksen and Dick24, Reference Azevedo, Leeson and Cho30–Reference Solberg34). However, in most of these studies, relatively high dietary protein:lipid levels were used and, hence, how much protein reduction can occur, without performance loss, still needs to be elucidated. Moreover, the dietary protein:lipid content and the FO replacement with RO affect tissue FA composition and FA metabolism, including catabolism via β-oxidation which influences fish growth, while other interactions may also occur(Reference Turchini, Torstensen and Ng14, Reference Bendiksen, Berg and Jobling23, Reference Karalazos, Bendiksen and Dick24, Reference Bendiksen, Arnesen and Jobling35).
In an earlier trial, we investigated the interactive effects of the dietary protein:lipid ratio and the inclusion of RO at the expense of FO in the diets of salmon in cold (winter) water temperatures(Reference Karalazos, Bendiksen and Dick24). The results of that study showed a positive effect on fish growth, as well as a protein-sparing effect but also changes in tissue FA composition. Given that water temperature is a key factor in fish nutrition and FA retention and metabolism(Reference Bendiksen and Jobling36), the impact of even lower dietary protein:lipid ratios and different lipid sources in Atlantic salmon reared at high (sub-optimal, summer temperatures) water temperatures is of considerable interest. Moreover, the positive effects of RO inclusion on growth have been associated with changes in the digestibility of FA. The uptake and digestibility of lipids and ultimately the utilisation of diets could be affected by the intestinal phospholipid (PL) FA composition and the consequent alterations occurring due to dietary FA changes, especially when high-lipid diets are used. It should be noted that the interactive effects of the dietary protein:lipid level and RO inclusion on growth, feed utilisation, nutrient and FA digestibility and whole-body chemical composition of large Atlantic salmon (S. salar L.) that are shown in the present paper were presented and discussed in detail in Karalazos et al. (Reference Karalazos, Bendiksen and Bell37).
Hence, the aim of the present study was to elucidate the interactive effects of FO replacement with RO at various dietary protein:lipid ratios on tissue FA compositions and metabolism, including FA β-oxidation and PL FA compositions of the pyloric caeca, of large Atlantic salmon at high water temperatures.
Materials and methods
Fish and facilities
The 10-week feeding trial was carried out at the Fjord Research Station AS (Helgeland, Dønna, Norway, 66°N) using Atlantic salmon (S. salar) of an initial mean weight of 2053 g. Eighteen sea cages of 125 m3 (5 × 5 × 5 m) were used with approximately ninety-three fish randomly distributed in each cage. Before the experiment, the fish were acclimatised to the trial cages for 63 d at 12°C, being fed a commercial diet from BioMar AS (9 mm; 360 g/kg protein and 350 g/kg fat). During the experimental period, the average water temperature was 11·6 ± 1·1°C and the salinity was 32·5 (sd 0·4) g/l, while the fish were subjected to a natural photoperiod. Mortalities were recorded and dead fish were removed daily. Experimental procedures complied with the Norwegian code of practice for the care and use of animals for scientific purposes. There are no aspects of this trial that would cause aggravated or unnecessary harm or stress to the fish involved.
Experimental diets and feeding
Following a factorial (two-way 3 × 2) experimental design, six diets were formulated at three different dietary protein/fat levels and two different levels of FO substitution. Regarding protein/lipid levels, the diets contained either: (1) 350 g/kg protein and 350 g/kg lipid (high protein; HP); (2) 330 g/kg protein and 360 g/kg lipid (medium protein; MP); (3) 290 g/kg protein and 380 g/kg lipid (low protein; LP). For the oil source, FO or RO was used within each dietary protein:fat level, where crude RO comprised 60 % of the total added oil in the RO diets, the remainder being FO. All diets were isoenergetic (gross energy 25 kJ/g). The diets were formulated to meet all known nutritional requirements of salmonid fish(3) and were produced as practical-type extruded pellets (9 mm) at the BioMar TechCentre (Brande, Denmark). The ingredients, proximate and FA compositions of the experimental diets are shown in Tables 1 and 2, respectively. Feeding was carried out by hand to satiation on a daily basis, including two daily meals with a minimum of 4 h between them. A lift-up system was used to collect uneaten feed, which was recorded for accurate calculations of feed intake and feed conversion ratio (FCR).
Table 1 Diet formulations, proximate compositions (g/kg) and energy content (kJ/g) of the six experimental diets fed to Atlantic salmon (Salmo salar L.) for 10 weeks
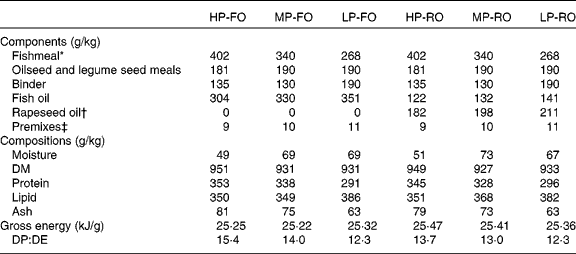
HP-FO, high-protein fish oil; MP-FO, medium-protein fish oil; LP-FO, low-protein fish oil; HP-RO, high-protein rapeseed oil; MP-RO, medium-protein rapeseed oil; LP-RO, low-protein rapeseed oil; DP, digestible protein; DE, digestible energy.
* South American, Anchoveta oil.
† European, non-GM, double-low-quality rapeseed oil.
‡ Vitamin and mineral premixes prepared according to the BioMar AS commercial standards, which include crystalline amino acids and Carophyll pink to provide 40 mg/kg astaxanthin (DSM Roche, Basel, Switzerland).
Table 2 Fatty acid compositions (g/100 g total fatty acids) of the six experimental diets fed to Atlantic salmon (Salmo salar L.) for 10 weeks

HP-FO, high-protein fish oil; MP-FO, medium-protein fish oil; LP-FO, low-protein fish oil; HP-RO, high-protein rapeseed oil; MP-RO, medium-protein rapeseed oil; LP-RO, low-protein rapeseed oil.
* Includes 15 : 0.
† Includes 16 : 1n-9 and 20 : 1n-7.
‡ Includes 18 : 3n-6, 20 : 3n-6 and 22 : 4n-6.
§ Includes 20 : 3n-3 and 22 : 4n-3.
Sampling procedure
At the start of the trial, the fish were bulk weighed. At the end (10th week), the fish were individually weighed after being anaesthetised in MS-222 (metacain, 8 mg/l). At the end of the trial, another three fish/cage were sampled at random for lipid and FA composition of muscle, liver and pyloric caeca PL and for β-oxidation determination. Fish were killed with a sharp blow to the head, and samples of the liver and pyloric caeca were dissected and immediately frozen in liquid N2. Muscle samples, representative of the edible portion, were obtained by cutting a steak between the dorsal and ventral fins (Norwegian Quality Cut), which were then skinned, deboned and homogenised. For β-oxidation determination, samples of liver, red and white muscle were taken separately and immediately frozen in liquid N2. Samples from the six experimental diets were also taken to determine proximate and FA compositions. All samples were kept at − 20°C until further analysis.
Lipid extraction and fatty acid analyses
Total lipids of tissues and diet samples were extracted by homogenisation in 20 vol. of chloroform–methanol (2:1, v/v) containing butylated hydroxytoluene (0·01 %, w/w) as an antioxidant(Reference Folch, Lees and Sloane Stanley38). FA methyl esters were prepared from total lipid by acid-catalysed transesterification using 2 ml of 1 % H2SO4 in methanol plus 1 ml toluene, as described by Christie(Reference Christie39), and FA methyl ester extraction and purification, as described by Tocher & Harvie(Reference Tocher and Harvie40). FA methyl esters were separated and quantified by GLC (Carlo Erba Vega 8160; Carlo Erba Instrumentazone, Milan, Italy) using a 30 m × × 0·32 mm capillary column (CP wax 52CB; Chrompak Limited, London, UK). The carrier gas was H2, and the temperature programming used was from 50 to 150°C at 40°C/min and then to 225°C at 2°C/min. Individual methyl esters were identified by comparison with known standards and by reference to published data(Reference Ackman and Connell41).
In the case of PL FA composition of the pyloric caeca, a PL fraction was prepared from 0·5 mg of total lipid applied to a 20 × 20 cm silica gel 60 TLC plate (VWR, Lutterworth, UK) and developed in isohexane–diethyl ether–acetic acid (80:20:1, by vol.) and dried for a few minutes at room temperature. The plate was sprayed lightly with 2,7-dichlorofluorescein (0·1 %, w/v) in 97 % methanol (v/v), and the PL bands on the origin were scraped from the plate and placed in a 15 ml test-tube. FA methyl esters were prepared by acid-catalysed transesterification in 2 ml of 1 % H2SO4 in methanol at 50°C overnight(Reference Christie39). The samples were neutralised with 2·5 ml of 2 % KHCO3 and extracted with 5 ml isohexane–diethyl ether (1:1, v/v) plus butylated hydroxytoluene. The samples were then re-extracted with 5 ml isohexane–diethyl ether (1:1), and the combined extracts were dried and dissolved in 0·3 ml of isohexane before FA analysis.
Peroxisomal β-oxidation capacity
Liver and red and white muscle were weighed and homogenised in 20 % (w/v) ice-cold buffered sucrose solution containing 0·25 m-sucrose, 0·04 m-potassium phosphate buffer (pH 7·4), 0·15 m-KCl, 40 mm-KF and 1 mm-N-acetyl cysteine. The resulting total homogenates were then centrifuged at 1880 g for 10 min at 2°C. The resulting post-nuclear fractions were collected, and portions were used immediately to determine β-oxidation capacity. The latter was determined as acid-soluble products using radiolabelled [1-14C]palmitoyl-CoA as a substrate as described by Frøyland et al. (Reference Frøyland, Asiedu and Vaagenes42).
Briefly, 250 μl of the assay medium were added to 2 ml Eppendorf tubes. Then, 10 μl of the [1-14C]palmitoyl-CoA substrate (0·1 μCi/100 μm) were added to each tube. The samples were pre-incubated at room temperature for 2 min. The reaction was started by the addition of the homogenate (30–50 μl for liver and red muscle and 300–500 μl for white muscle, homogenised in 20 % (w/v) ice-cold buffered sucrose solution as described above), and the reaction continued for 10 min. The reaction was stopped by the addition of 150 μl of 1·5 m-KOH. Then, 25 μl of FA-free bovine serum albumin (100 mg/ml) were added, the tubes were vortexed and 500 μl of ice-cold 4 m-perchloric acid were added. The tubes were centrifuged at 1880 g for 10 min. Aliquots of 500 μl were placed in scintillation vials, 2·5 ml of the scintillant were added and radioactivity was determined in a scintillation counter. The protein content of the samples was determined according to the Lowry method(Reference Lowry, Rosebrough and Farr43).
Calculations and statistical analysis
The following formulae were applied to the data:
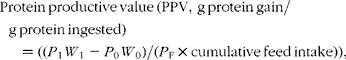
where W 0 and W 1 are the initial and final fish mean weights in g; P 0 and P 1 are the initial and final protein concentrations of the fish; P F is the protein concentration of the feed on a DM basis, and cumulative feed intake was determined in grams on a DM basis.
Factorial (two-way) ANOVA was used to analyse the effects of the protein:fat ratio (protein level), dietary RO inclusion (oil source) and their interactions on FA composition of tissues and β-oxidation. When the interaction of the two factors was significant, multiple comparison testing was performed for both factors to investigate the simple main effects, i.e. the main effect of one factor at a given level of the other, while the main effects were not taken into account(Reference Lowry, Rosebrough and Farr43). Data, which were identified as non-homogeneous (Levene's test), were subjected to square root, log or arcsine transformation before analysis. Differences were regarded as significant when P < 0·05(Reference Zar44). All data are presented as means and standard deviations (n 3), and all statistical analyses were performed using SPSS 14.0 (2005; SPSS, Inc., Chicago, IL, USA). The graphs were created using Prism 4 (Graphpad Software, Inc., San Diego, CA, USA).
Results
Diet proximate and fatty acid composition
The analysed protein:lipid contents of the diets were 349 g/kg protein and 350 g/kg lipid for HP, 333 g/kg protein and 358 g/kg lipid for MP, and 293 g/kg protein and 384 g/kg lipid for LP (Table 1). The digestible protein:digestible energy ratio was 14·5, 13·5 and 12·3 for the HP, MP and LP diets, respectively; digestible protein and digestible energy were calculated using the apparent digestibity coefficient values for protein and energy found in this trial(Reference Karalazos, Bendiksen and Bell37). The diets contained either 100 % FO or a blend of 40 % FO and 60 % RO, with a consequential effect on the total lipid FA profiles (Table 2). Briefly, the FO diets contained approximately 36 % total SFA, largely 16 : 0 (approximately 20 %), except for the HP-FO diet which had a slightly higher total SFA content (40·4 %). The total monoenes were 26 %, predominantly 18 : 1n-9 and 16 : 1n-7. The total n-6 PUFA were low (4·5 %), half of which was 18 : 2n-6. Lastly, the total n-3 PUFA were as high as 32 %, mainly as EPA and DHA (18·5 and 8 %, respectively). The 60 % inclusion of RO resulted in the reduction of 16 : 0, and consequently of the total SFA, by half, compared with the FO diets. The total monoenes almost doubled, largely due to the high amount of 18 : 1n-9 (38·5 %). The total n-6 PUFA in the RO diets increased threefold, up to 14 %, mainly as 18 : 2n-6 (13 % of total FA). EPA decreased by 70 % and DHA by more than half (values were 6 and 3 %, respectively), and hence the total n-3 PUFA were reduced by half (17 %). The n-3:n-6 PUFA ratio was 7 and 1·2 for the FO and RO diets, respectively.
Growth
At the beginning of the trial, fish had a mean weight of 2053 g. At the end of the trial, all groups showed good performance, mean weight ranging from 3340·2 to 3664·2 g, for HP-RO and MP-RO, respectively (Table 3). The oil source had a significant effect (two-way ANOVA; P < 0·05) on growth; specifically, fish fed the RO diets had higher final weight, specific growth rate and thermal growth coefficient compared with fish fed the FO diets. There was no significant effect due to the protein level, and no significant interactions were shown. FCR was not affected by any of the factors and varied from 0·99 to 1·10. Lastly, PPV was significantly affected by both factors. The LP diets had significantly a higher PPV than the other two groups (0·41, 0·43 and 0·47 for HP, MP and LP, respectively), while the RO groups had a higher PPV compared with the FO groups (0·42 v. 0·46 for FO and RO, respectively). These results are described in detail in Karalazos et al. (Reference Karalazos, Bendiksen and Bell37).
Table 3 Growth and performance of Atlantic salmon (Salmo salar L.) fed the six experimental diets for 10 weeks
(Mean values and standard deviations, n 3)

HP-FO, high-protein fish oil; MP-FO, medium-protein fish oil; LP-FO, low-protein fish oil; HP-RO, high-protein rapeseed oil; MP-RO, medium-protein rapeseed oil; LP-RO, low-protein rapeseed oil; FCR, feed conversion ratio; SGR, specific growth rate; TGC, thermal growth coefficient; PPV, protein productive value.
Tissue fatty acid compositions
The total lipid content and the FA composition of muscle and liver from fish fed the six experimental diets for 10 weeks are shown in Tables 4 and 5, respectively. The muscle total lipid varied from 138·0 to 156·5 mg lipid/g tissue. The liver lipid content was much lower, compared with that of muscle, ranging from 50·1 to 64·9 mg lipid/g tissue. Neither the dietary protein level nor the RO inclusion affected the muscle and liver lipid contents, and no significant interactions were shown by two-way ANOVA.
Table 4 Total lipid (mg lipid/g tissue) and fatty acid compositions (g/100 g total fatty acids) of muscle from Atlantic salmon (Salmo salar L.) fed the experimental diets for 10 weeks
(Mean values and standard deviations, n 3)

HP-FO, high-protein fish oil; MP-FO, medium-protein fish oil; LP-FO, low-protein fish oil; HP-RO, high-protein rapeseed oil; MP-RO, medium-protein rapeseed oil; LP-RO, low-protein rapeseed oil.
* Includes 15 : 0, 20 : 0 and 22 : 0.
† Includes 16 : 1n-9 and 20 : 1n-7.
‡ Includes 18 : 3n-6, 22 : 4n-6 and 22 : 5n-6.
§ Includes 20 : 3n-3 and 22 : 4n-3.
Table 5 Total lipid (mg lipid/g tissue) and fatty acid compositions (g/100 g total fatty acids) of liver from Atlantic salmon (Salmo salar L.) fed the experimental diets for 10 weeks
(Mean values and standard deviations, n 3)

HP-FO, high-protein fish oil; MP-FO, medium-protein fish oil; LP-FO, low-protein fish oil; HP-RO, high-protein rapeseed oil; MP-RO, medium-protein rapeseed oil; LP-RO, low-protein rapeseed oil.
* Includes 15 : 0, 20 : 0 and 22 : 0.
† Includes 16 : 1n-9 and 20 : 1n-7.
‡ Includes 18 : 3n-6, 22 : 4n-6 and 22 : 5n-6.
§ Includes 20 : 3n-3 and 22 : 4n-3.
Regarding the FA composition of muscle and liver, they were significantly affected by the RO but not by the protein level, and no significant interactions between the two factors were shown. Specifically, the inclusion of RO resulted in a significant increase of 18 : 1n-9, total monoenes, 18 : 2n-6, 20 : 2n-6, total n-6 PUFA and 18 : 3n-3. On the other hand, all saturates, including total SFA, 16 : 1n-7, 22 : 1, arachidonic acid, EPA, DHA, total n-3 FA and the n-3:n-6 ratio, were significantly reduced when the fish were fed the diets containing RO.
Notably in muscle, EPA was reduced by half (10·4 v. 5·2 % for the FO v. RO, respectively), while the reduction in DHA was more moderate (7·8 v. 5·0 % for FO v. RO, respectively). 18 : 1n-9 increased from 20·9 to 35·1 %, 18 : 2n-6 from 6·6 to 11·6 % and 18 : 3n-3 from 2·0 to 4·6 % for the FO and RO groups, respectively. Similarly in the liver, EPA was reduced from 16·0 to 9·9 % and DHA from 16·2 to 13·2 % for the FO and RO groups, respectively. The increase between the FO and RO groups for 18 : 1n-9, 18 : 2n-6 and 18 : 3n-3 was 13·8 v. 27·9 %, 1·9 v. 7·6 % and 0·5 v. 2·9 %, respectively.
The differences (Δ) between diet and muscle FA concentrations for the six experimental diets are shown in Table 6, where negative Δ values indicate lower values in muscle compared with the diet, whereas positive values indicate accumulation in tissues relative to the diet. Thus, the SFA, arachidonic acid, EPA and the total n-3 PUFA were utilised to a higher extent by the fish fed the FO diets compared with the RO groups. DHA appeared to be slightly utilised in the FO groups, but was accumulated in the muscle in the RO groups. On the contrary, 18 : 1n-9, 18 : 2n-6 and 18 : 3n-3 were found in higher concentrations in the muscle in the FO groups but were utilised in the RO groups. Likewise, the differences (Δ) between diet and liver FA concentrations for the six experimental diets are shown in Table 7. In the liver, the 14 : 0 and 16 : 0 were utilised in all groups, although lower Δ values were found in the FO groups. Similar to the muscle, 18 : 1n-9, 18 : 2n-6 and 18 : 3n-3 in the liver were much more utilised in the RO groups compared with the FO ones. Lastly, 18 : 0, arachidonic acid and DHA were accumulated in the liver in all groups.
Table 6 Differences (Δ)* between diet and muscle fatty acid concentrations (g/100 g total fatty acids) for the six experimental treatments
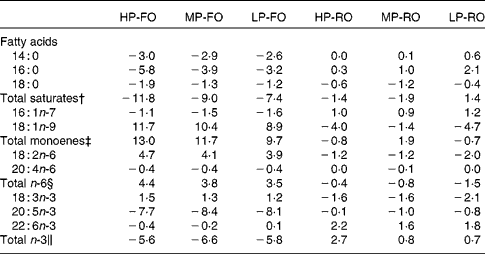
HP-FO, high-protein fish oil; MP-FO, medium-protein fish oil; LP-FO, low-protein fish oil; HP-RO, high-protein rapeseed oil; MP-RO, medium-protein rapeseed oil; LP-RO, low-protein rapeseed oil.
* Negative Δ values indicate lower values in muscle compared with the diet, whereas positive values indicate accumulation in muscle relative to the diet.
† Includes 15 : 0, 20 : 0 and 22 : 0.
‡ Includes 16 : 1n-9, 18 : 1n-7, 20 : 1n-9, 20 : 1n-7, 22 : 1 and 24 : 1n-9.
§ Includes 18 : 3n-6, 20 : 2n-6, 20 : 3n-6, 22 : 4n-6 and 22 : 5n-6.
∥ Includes 18 : 4n-3, 20 : 3n-3, 20 : 4n-3, 22 : 4n-3 and 22 : 5n-3.
Table 7 Differences (Δ)* between diet and liver fatty acid concentrations (g/100 g total fatty acids) for the six experimental treatments
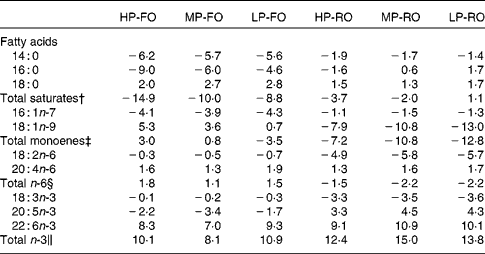
HP-FO, high-protein fish oil; MP-FO, medium-protein fish oil; LP-FO, low-protein fish oil; HP-RO, high-protein rapeseed oil; MP-RO, medium-protein rapeseed oil; LP-RO, low-protein rapeseed oil.
* Negative Δ values indicate lower values in liver compared with diet, whereas positive values indicate accumulation in liver relative to diet.
† Includes 15 : 0, 20 : 0 and 22 : 0.
‡ Includes 16 : 1n-9, 18 : 1n-7, 20 : 1n-9, 20 : 1n-7, 22 : 1 and 24 : 1n-9.
§ Includes 18 : 3n-6, 20 : 2n-6, 20 : 3n-6, 22 : 4n-6 and 22 : 5n-6.
∥ Includes 18 : 4n-3, 20 : 3n-3, 20 : 4n-3, 22 : 4n-3 and 22 : 5n-3.
Pyloric caeca phospholipid fatty acid composition
Pyloric caeca PL FA composition (Table 8) was significantly affected mainly by the dietary oil source, although in some cases, a significant main effect due to the protein level and/or significant interactions between the two factors were also shown (two-way ANOVA; P < 0·05). Pyloric caeca PL comprised almost half as n-3 PUFA (41·6–49·9 %), mainly DHA and EPA, followed by SFA (24·3–30·5 %), largely 16 : 0, and monoenes (15·0–24·0 %), while n-6 PUFA were less than 9 % (5·3–9·0 %). DHA was the most abundant FA varying from 18·9 to 25·8 % and being affected significantly both by the oil source (FO>RO) and by the protein content (reduced with lower protein content). EPA was also found at high levels (16·1–22·1 %) and was affected significantly by both factors, while significant interactions were also found. As shown in Fig. 1, the RO groups had a significantly lower EPA content at all protein levels, whereas EPA content was significantly higher for the LP diet compared with the HP diet but only when FO was the oil source. Regarding total SFA, there was a significant effect due to the oil source (FO>RO), and the same pattern was shown for 16 : 0. Total monoenes, and mainly 18 : 1n-9, were significantly increased due to the dietary inclusion of RO. However, significant interactions were found for 18 : 1n-9, showing an effect of protein content (higher 18 : 1n-9 level for the LP diet v. HP and MP) only for the RO diets. Total n-6 FA, mainly 18 : 2n-6, were increased in fish fed the RO diet, while arachidonic acid was decreased. Noticeably, all n-6 FA were at relatively low levels. Significant interactions were shown for 18 : 2n-6, resulting in a significantly higher content in fish fed LP diets compared with HP and MP for the RO diets.
Table 8 Fatty acid compositions (g/100 g total fatty acids) of total phospholipids of the pyloric caeca from Atlantic salmon (Salmo salar L.) fed the experimental diets for 10 weeks
(Mean values and standard deviations, n 3)

HP-FO, high-protein fish oil; MP-FO, medium-protein fish oil; LP-FO, low-protein fish oil; HP-RO, high-protein rapeseed oil; MP-RO, medium-protein rapeseed oil; LP-RO, low-protein rapeseed oil.
* Includes 15 : 0, 20 : 0 and 22 : 0.
† Includes 16 : 1n-9 and 20 : 1n-7.
‡ Includes 18 : 3n-6 and 22 : 4n-6.
§ Includes 20 : 3n-3 and 22 : 4n-3.

Fig. 1 Means of 18 : 1n-9 (A) and 20 : 5n-3 (B) (g/100 g total fatty acids (FA)) of total phospholipids of pyloric caeca from Atlantic salmon (Salmo salar L.) fed the six experimental diets, in a two-way ANOVA, showing the effects of the two factors and their interaction. a,b Mean values with unlike letters were significantly different (P < 0·05) for each oil source: A,B fish oil (FO; ○); a,b rapeseed oil (RO; ●), respectively. * Mean values were significantly different (P < 0·05) between the FO and RO values within each protein level. HP, high protein; MP, medium protein; LP, low protein.
Peroxisomal β-oxidation capacity
The peroxisomal palmitoyl-CoA oxidation capacity in the liver, red and white muscle is shown in Table 9. The peroxisomal β-oxidation capacity in the liver ranged from 6·6 to 12·5 pmol/min per mg protein, in red muscle from 26·7 to 36·3 pmol/min per mg protein and in white muscle from 1·1 to 1·6 pmol/min per mg protein. In the liver and red muscle, β-oxidation capacity was significantly affected by the oil source (P = 0·035 and 0·034 for the liver and red muscle, respectively). Specifically, RO inclusion resulted in significantly higher β-oxidation in both the liver (7·2 v. 9·7 for FO and RO, respectively) and red muscle (28·2 v. 33·7 for FO and RO, respectively). However, in white muscle, there was a significant interaction of the two factors (protein level and oil source), and hence the simple main effects of the two factors were tested, as demonstrated in Fig. 2. Specifically, the HP group had a significantly higher β-oxidation capacity than MP and LP when FO was the oil source, whereas in contrast, the β-oxidation capacity in HP was lower than the other two groups when RO was included in the diet. Regarding the effects of the oil source on β-oxidation capacity at the three protein levels, the ranking was FO>RO for HP, RO>FO for MP, while FO and RO did not significantly differ at LP.
Table 9 Peroxisomal β-oxidation capacity (pmol/min per mg protein) of liver, red and white muscle from Atlantic salmon (Salmo salar L.) fed the experimental diets for 10 weeks
(Mean values and standard deviations, n 3)

HP-FO, high-protein fish oil; MP-FO, medium-protein fish oil; LP-FO, low-protein fish oil; HP-RO, high-protein rapeseed oil; MP-RO, medium-protein rapeseed oil; LP-RO, low-protein rapeseed oil.

Fig. 2 Means of the peroxisomal β-oxidation capacity (pmol/min per mg protein) of white muscle from Atlantic salmon fed the six experimental diets, in a two-way ANOVA, showing the effects of the two factors and their interaction. a,b Mean values with unlike letters were significantly different for each oil source; A,B, fish oil (FO; ○); a,b, rapeseed oil (RO; ●), respectively. * Mean values were significantly different (P < 0·05) between the FO and RO values within each protein level. HP, high protein; MP, medium protein; LP, low protein.
Discussion
Growth
The effects and interactions of the two factors on growth are thoroughly discussed in Karalazos et al. (Reference Karalazos, Bendiksen and Bell37). Briefly, it was shown that, in agreement with previous studies(Reference Bendiksen, Berg and Jobling23, Reference Azevedo, Leeson and Cho30–Reference Solberg34), no negative effects on growth and FCR were observed when the fish were fed with low-protein/high-lipid diets, even at protein levels below 300 g/kg. This is much lower than what had been previously tested and of significant importance regarding the tolerance in low-protein diets and the utilisation of lipid for energy. Moreover, the dietary inclusion of RO at the expense of FO had a positive effect on final weight, specific growth rate and thermal growth coefficient. Such an effect had been reported by a couple of studies(Reference Bendiksen, Berg and Jobling23, Reference Karalazos, Bendiksen and Dick24) and possibly relates to the positive effect of low-n-3 FA diets towards higher growth for large salmon(Reference Torstensen, Bell and Rosenlund21, Reference Menoyo, Lopez-Bote and Bautista45). This effect is probably explained by the higher digestibility of RO, and other VO, FA, and hence better utilisation of the dietary oil for energy by the fish. This theory is confirmed by the digestibility results of the present study(Reference Karalazos, Bendiksen and Bell37). Moreover, the increased β-oxidation and the changes in the pyloric caeca PL discussed below could also have played a significant role.
Lastly, a positive effect of increased dietary lipid content on protein retention and, hence, on protein sparing has been previously reported(Reference Bendiksen, Berg and Jobling23, Reference Karalazos, Bendiksen and Dick24, Reference Einen and Roem32, Reference Hillestad, Johnsen and Austreng33) and was also confirmed in the present study as the LP diets showed a higher PPV. The inclusion of RO at the expense of FO resulted in a positive effect on PPV also. The potential effects of dietary VO in protein sparing in fish are largely unknown; however, the results of the present study are supported by the increased β-oxidation capacity that was shown in tissues of Atlantic salmon fed with the RO diets compared with the FO diets. The results showing increased catabolism of FA for energy production may suggest a protein-sparing effect. The β-oxidation results are discussed further below.
Tissue fatty acid composition
Tissue total lipid FA composition reflects the FA composition of the diet, usually following linear correlations between the concentrations of individual FA in the diet and the tissues(Reference Bell, McEvoy and Tocher16, Reference Bell, McGhee and Campbell17, Reference Torstensen, Frøyland and Lie19, Reference Torstensen, Bell and Rosenlund21, Reference Karalazos, Bendiksen and Dick24, Reference Bell, Tocher and Henderson46, Reference Rosenlund, Obach and Sandberg47). The results of the present study are in agreement with previous studies, showing a reduction in SFA, 16 : 1n-7, 20 : 4n-6, EPA, DHA and n-3:n-6 ratio, respective to the reductions in the dietary FA, with inclusion of RO. Similarly, the increase of 18 : 1n-9, 18 : 2n-6 and 18 : 3n-3 in the diets containing RO, compared with the FO diets, was reflected in muscle and liver FA. It is clear that, since the FA compositions of the diets were affected by the oil source only, the changes in the muscle and liver FA compositions were also due to the dietary RO inclusion and, as expected, the dietary protein level had no significant effect on the tissue FA composition.
The changes in muscle and liver FA indicate selective utilisation or retention of individual FA. It has been shown previously that when specific FA are in abundance in the diet, they are selectively utilised for energy production, via β-oxidation, and perhaps to a lesser extent for desaturation and elongation. In contrast, when FA, and especially n-3 highly unsaturated FA, are limited in the diet, they are retained or deposited in the tissues(Reference Bell, McEvoy and Tocher16, Reference Bell, McGhee and Campbell17, Reference Torstensen, Frøyland and Lie19). For example, in the present study, 18 : 1n-9 was increased almost fourfold, 18 : 2n-6 more than fivefold and 18 : 3n-3 ninefold in the diets when FO was replaced with RO; however, the respective increases in muscle were less than or about twofold for all of the above FA, while in the liver, it was approximately twofold for 18 : 1n-9, less than fourfold for 18 : 2n-6 and less than sixfold for 18 : 3n-3, indicating selective utilisation of these FA in the RO groups. On the other hand, although the reduction in EPA and DHA was more than 60 % in the RO diets, the decrease in muscle was approximately 50 % for EPA and 35 % for DHA, while in the liver, the reduction was approximately 35 % for EPA and 20 % for DHA, indicating a selective retention of these FA in the tissues when the dietary supply was reduced. These results are also supported by the Δ values for muscle and liver, i.e. the differences between diet and tissue FA concentrations. It was shown that, when provided at high concentrations, 18 : 1n-9, 18 : 2n-6 and 18 : n-3 were highly utilised in both muscle and liver, and EPA and DHA were accumulated in the tissues when dietary supply was reduced. In other animals, the selective retention of essential FA in specific tissues is believed to occur by a mechanism of reacylation of sn-2-monoacylglycerols by hepatic microsomal activity of monoacylglycerol acyltransferase, during lipolysis(Reference Xia, Mostafa and Bhat48). However, the moderate reductions in EPA and DHA shown in the present study may also be partially affected by the enhanced endogenous desaturation and elongation of dietary 18 : 3n-3(Reference Tocher, Bell and Dick49–Reference Tocher, Bell and McGhee51).
Furthermore, it is noteworthy that the results of the present trial suggest that the inclusion of RO up to 60 %, at the expense of FO, in diets of Atlantic salmon at various dietary protein:lipid levels caused only moderate reductions in tissue EPA and DHA. This was also shown by Karalazos et al. (Reference Karalazos, Bendiksen and Dick24), where fish were reared at low water temperatures. Reductions in n-3 highly unsaturated FA affect the quality of the final product, compromise its high nutritional value for the human consumer and should be avoided(Reference Bell, Waagbø, Holmer, Black, Duarte, Marbà and Karakassis13). Hence, such results, leading to moderate reductions in EPA and DHA, are promising for the use of VO in commercial diets, although the present trial was conducted over a short time period, which could have masked the full extent of the FA changes that could occur over the whole production cycle of salmon.
Pyloric caeca phospholipid fatty acid composition
PL are of importance in lipid digestion in fish, playing a significant role in the structure of cell membranes of lipoproteins for the transport of lipids in the blood and lymph and also in forming intra-luminal mixed micelles along with bile salts and dietary lipids(Reference Tocher, Bendiksen and Campbell52). PL in fish contain mainly 16 : 0 and 18 : 1n-9 at the sn-1 position and 20 : 5n-3 and 22 : 6n-3 at the sn-2 position(Reference Sargent, Tocher, Bell, Halver and Hardy26). Hence, the intestinal PL FA composition and the consequent alterations occurring due to dietary FA changes may affect the uptake and digestibility of lipids and ultimately the utilisation of diets, especially when high-lipid diets are used. Pyloric caeca are major sites in the intestine duct for nutrient uptake and the most significant section for lipid uptake after their digestion(Reference Denstadli, Vegusdal and Krogdahl53). In the present study, focus was given to the interactive effects of the protein:lipid level and oil source in the FA composition of the pyloric caeca.
In the present study, it was shown that, regardless of the dietary treatment, n-3 PUFA were the major FA group in pyloric caeca PL, consisting mainly of DHA and EPA, followed by SFA, largely 16 : 0, and monoenes (15·0–24·0 %), while n-6 PUFA were less than 9 (5·3–9·0)%, which is in accordance with previous reports(Reference Sargent, Tocher, Bell, Halver and Hardy26). The high abundance of these FA could have also been enhanced by their high recovery rate into enterocyte PL(Reference Oxley, Tocher and Torstensen54, Reference Pérez, Rodríguez and Henderson55).
However, the dietary changes had significant effects on the PL FA, mainly due to the oil source but also due to the protein:lipid level, although to a small extent, while significant interactions were also observed. Specifically, the dietary inclusion of RO at the expense of FO resulted in significant reductions in DHA, EPA, 16 : 0 and 20 : 4n-6, and significant increases of 18 : 1n-9 and 18 : 2n-6. On the other hand, the effect of the protein content was also significant in some cases with contradicting results, including 18 : 0 (HP < LP) and DHA (HP>LP). Lastly, significant interactions of the two factors were also revealed by two-way ANOVA for some FA, including 18 : 1n-9 and 18 : 2n-6, with a significant increase due to the protein content (HP>LP) only for the RO groups, while EPA had a significant decrease due to the protein content (HP < LP) only for the FO groups (for these FA, the effect of the oil source was significant at all protein:lipid levels). These changes reflected the changes in the dietary FA compositions, similar to the muscle and liver total lipid FA compositions, although to a relatively smaller extent. However, altering the relative proportions of FA in intestinal PL, as an effect of the use of VO in the diets and more interestingly of the protein:lipid level or the interactive effects of the two factors, is most likely to affect their structure and consequently their role in the digestion and uptake of lipids and nutrients. The present study showed that lipid digestibility was improved due to RO inclusion but also (for FO only) due to low-protein/high-lipid diets (data presented and discussed in Karalazos et al. (Reference Karalazos, Bendiksen and Bell37)). However, the clarification of the exact mechanisms involved requires further investigation.
Peroxisomal β-oxidation capacity
The peroxisomal β-oxidation activity was measured in liver, red and white muscle. Conducting the assay onsite was not possible, and hence the samples of tissues had to be frozen on dry ice and transferred to the Institute of Aquaculture in Scotland where analysis took place. Therefore, the measurement of the total β-oxidation activity was not possible, and the results obtained represent the peroxisomal β-oxidation capacity. Previous studies in Atlantic salmon have shown that different tissues/organs have very different β-oxidation capacities as a result of their unique and different energy requirements, depending on their functions(Reference Frøyland, Lie and Berge56–Reference Torstensen, Lie and Frøyland61). In agreement with that, between the three tissues assessed in the present study, red muscle had the highest β-oxidation activity and white muscle the lowest. However, it should be noted that the β-oxidation capacities of these tissues, and the consequent ranking, were expressed on a tissue protein content basis. Considering that white muscle accounts for more than 60 % of the total body mass of Atlantic salmon, it becomes clear that its role in energy production for the fish is the most significant(Reference Frøyland, Lie and Berge56, Reference Stubhaug, Frøyland and Torstensen57).
It is well documented that tissue β-oxidation capacities are affected by various factors, including the diet and especially the dietary FA composition(Reference Tocher, Bell and McGhee51, Reference Stubhaug, Frøyland and Torstensen57, Reference Stubhaug, Lie and Torstensen58, Reference Torstensen, Lie and Frøyland61, Reference Torstensen and Stubhaug62). Specific FA, such as 16 : 0, 18 : 1n-9, 22 : 1n-11 and 20 : 1n-9, are readily catabolised, although 18 : 3n-3, 18 : 2n-6 and even EPA and DHA are also good substrates for β-oxidation, especially when provided at high levels(Reference Stubhaug, Frøyland and Torstensen57, Reference Henderson and Tocher60, Reference Stubhaug, Tocher and Bell63). Hence, dietary changes, incorporating VO, could affect the β-oxidation capacities of tissues. However, the results of previous studies are contradictory. Tocher et al. (Reference Tocher, Bell and McGhee51) showed that β-oxidation capacity was not affected either by the oil content or by the oil type in diets of Atlantic salmon. On the contrary, Stubhaug et al. (Reference Stubhaug, Frøyland and Torstensen57) reported that dietary RO inclusion had a positive effect on β-oxidation. The results of the present study showed a significant increase in liver and red muscle β-oxidation capacities due to RO inclusion. This could explain, at least partially, the better performance that was shown for the RO groups and the enhanced protein-sparing effect. However, it remains unclear whether this was primarily due to changes in dietary FA composition rather than due to other dietary factors. For instance, an interactive effect of the protein level and the oil source was shown in white muscle, suggesting a higher β-oxidation capacity for the FO groups than the RO ones at the HP level, whereas the RO groups had higher values for the other two protein levels, although the difference was significant only for the MP diet. The higher β-oxidation capacity of the HP-FO group could be due to the higher content of SFA in that diet; although if the hypothesis is correct, it remains unclear why such an effect was not reflected in the other two tissues.
Conclusions
In conclusion, the investigation of the interactive effects of the dietary protein:lipid level and FO replacement showed that low-protein/high-lipid diets can be used safely in large Atlantic salmon nutrition with regard to the growth and FCR, while the inclusion of RO at the expense of FO can enhance the growth of the fish by increased protein sparing and β-oxidation. In terms of the tissue FA compositions, they were significantly affected by the RO inclusion, reflecting the FA composition of the diets. However, the reduction in EPA and DHA, resulting from the dietary FA changes, was only moderate, and hence the impact on the final product quality, in terms of the nutritional value for the human consumer, was limited. Further studies on the longer-term use of diets are therefore warranted.
Acknowledgements
The present study was carried out with support from BioMar AS. V. K. was supported by the State Scholarships Foundation (IKY), Greece. V. K. wrote the manuscript with assistance from all other authors, especially J. G. B. and E. Å. B.; E. Å. B. was responsible for all aspects of the feeding trial, with assistance from V. K. in sample collection. V. K. was responsible for all aspects of analysis, with assistance and technical advice for β-oxidation analysis from J. R. D. and D. R. T. All authors read and approved the findings of the study. None of the authors had a conflict of interest.













