1. Introduction
Familial isolated hyperparathyroidism (FIHP) is a rare disorder encountered in a minority (∼1%) of all patients with primary hyperparathyroidism (Simonds et al., Reference Simonds, James-Newton, Agarwal, Yang, Skarulis, Hendy and Marx2002). At times, FIHP is associated with a syndromic clustering of other endocrine tumours, jaw tumours, or is associated with hypocalciuria. In these cases, germline mutations in the multiple endocrine neoplasia type 1 (MEN1) gene (MIM#613733) (Chandrasekharappa et al., Reference Chandrasekharappa, Guru, Manickam, Olufemi, Collins, Emmert-Buck, Debelenko, Zhuang, Lubensky, Liotta, Crabtree, Wang, Roe, Weisemann, Boguski, Agarwal, Kester, Kim, Heppner, Dong, Spiegel, Burns and Marx1997; Bassett et al., Reference Bassett, Forbes, Pannett, Lloyd, Christie, Wooding, Harding, Besser, Edwards, Monson, Sampson, Wass, Wheeler and Thakker1998), the HPRT2 gene (MIM# 145001) that causes hyperparathyroidism and jaw tumour (HPT–JT) syndrome (Carpten et al., Reference Carpten, Robbins, Villablanca, Forsberg, Presciuttini, Bailey-Wilson, Simonds, Gillanders, Kennedy, Chen, Agarwal, Sood, Jones, Moses, Haven, Petillo, Leotlela, Harding, Cameron, Pannett, Höög, Heath, James-Newton, Robinson, Zarbo, Cavaco, Wassif, Perrier, Rosen, Kristoffersson, Turnpenny, Farnebo, Besser, Jackson, Morreau, Trent, Thakker, Marx, Teh, Larsson and Hobbs2002; Howell et al., Reference Howell, Haven, Kahnoski, Khoo, Petillo, Chen, Fleuren, Robinson, Delbridge, Philips, Nelson, Krause, Hammje, Dralle, Hoang-Vu, Gimm, Marsh, Morreau and Teh2003; Cavaco et al., Reference Cavaco, Guerra, Bradley, Carvalho, Harding, Oliveira, Santos, Sobrinho, Thakker and Leite2004; Cetani et al., Reference Cetani, Pardi, Borsari, Viacava, Dipollina, Cianferotti, Ambrogini, Gazzerro, Colussi, Berti, Miccoli, Pinchera and Marcocci2004), or the calcium sensing receptor CaSR gene (MIM# 601199) that leads to familial hypercalcaemic hypocalciuria (FHH; MIM #145980) (Pollak et al., Reference Pollak, Brown, Chou, Hebert, Marx, Steinmann, Levi, Seidman and Seidman1993) can be encountered. Rarely, mutations in additional genes (e.g. CDKN1B – MIM# 600778) have been noted in families with a MEN1 phenotype (Pellegata et al., Reference Pellegata, Quintanilla-Martinez, Siggelkow, Samson, Bink, Höfler, Fend, Graw and Atkinson2006). However, in some FIHP families the causative gene remains elusive (Marx, Reference Marx2000). FIHP is a diagnosis of exclusion made in kindreds with familial clustering of hyperparathyroidism in the absence of any clinical, biochemical or radiological evidence of MEN1, HPT–JT or FHH. Over the past 15 years we have identified (Olchovsky et al., Reference Olchovsky, Hobbs, Pras, Shimon, Silver, Irmin and Friedman2001) and followed a multigenerational Jewish family of Georgian origin with a seemingly autosomal dominantly inherited syndrome hallmarked by multiglandular FIHP, hypercalciuria and nephrolithiasis with no clinical, biochemical or imaging modality evidence of additional endocrinopathies. The present study aimed to define the genetic basis of FIHP in that family, using a whole exome capture and massive parallel sequencing approach with Sanger sequencing confirmation and co-segregation analyses.
2. Materials and methods
General – The study was approved by the institutional review board, and each participant gave an informed consent.
Case report – A multigenerational FIHP kindred was studied (Fig. 1), the specific pedigree and relevant medical history were previously reported by us (Olchovsky et al., Reference Olchovsky, Hobbs, Pras, Shimon, Silver, Irmin and Friedman2001), and the main clinical features of this family are reiterated in the results section.

Fig. 1. Pedigree phenotype and genotype in the FIHP family. Filled symbols, clinical and biochemical diagnosis of HPT; M, mutant allele; WT, wild-type allele.
(i) Genetic analyses
Whole exome sequencing (WES) – DNA extracted from two sibs and one offspring from a single family, all affected with multiglandular hyperparathyroidism was subjected to whole exome capturing and sequencing using the Roche NimbleGen V2 chip (Madison, WI) and the Illumina HiSeq2000 sequencing platform (Hayward, CA).
Variant calling and annotation – For each sequenced sample, raw sequence files were prepared using the Genome Analysis Tool Kit (GATK) suggested practice (McKenna et al., Reference McKenna, Hanna, Banks, Sivachenko, Cibulskis, Kernytsky, Garimella, Altshuler, Gabriel, Daly and DePristo2010). Briefly, each fastq file was aligned against the human hg19/GRCh37 reference genome. PCR duplicates were removed using Picard (http://picard.sourceforge.net/), reads around known and detected indels were realigned, and base quality was recalibrated using GATK. In order to call variants from the processed BAM files, we employed the GATK-recommended variant calling pipeline. Regional information including whether a variant is up/down stream of a known gene, whether it is located in a gene's intron, exon, splice junction or untranslated regions (UTRs) was added using ANNOVAR (Wang et al., Reference Wang, Li and Hakonarson2010) and the Ensembl gene annotation (Flicek et al., Reference Flicek, Amode, Barrell, Beal, Brent, Carvalho-Silva, Clapham, Coates, Fairley, Fitzgerald, Gil, Gordon, Hendrix, Hourlier, Johnson, Kahari, Keefe, Keenan, Kinsella, Komorowska, Koscielny, Kulesha, Larsson, Longden, McLaren, Muffato, Overduin, Pignatelli, Pritchard, Riat, Ritchie, Ruffier, Schuster, Sobral, Tang, Taylor, Trevanion, Vandrovcova, White, Wilson, Wilder, Aken, Birney, Cunningham, Dunham, Durbin, Fernandez-Suarez, Harrow, Herrero, Hubbard, Parker, Proctor, Spudich, Vogel, Yates, Zadissa and Searle2011). Information regarding other functional regions such as miRNA and other non-coding RNA sites was gathered from the wgRNA table downloaded from the UCSC Genome Browser site (Kent et al., Reference Kent, Sugnet, Furey, Roskin, Pringle, Zahler and Haussler2002). Information about the known and predicted miRNA binding sites was downloaded from TargetScan (Lewis et al., Reference Lewis, Burge and Bartel2005). Allele frequencies were retrieved from designated public databases such as dbSNP (Day, Reference Day2010), the 1000 genomes project (Nothnagel et al., Reference Nothnagel, Herrmann, Wolf, Schreiber, Platzer, Siebert, Krawczak and Hampe2011), the NHLBI Exome Sequencing Project (http://evs.gs.washington.edu/EVS/) and the Complete Genomics database (Drmanac et al., Reference Drmanac, Sparks, Callow, Halpern, Burns, Kermani, Carnevali, Nazarenko, Nilsen, Yeung, Dahl, Fernandez, Staker, Pant, Baccash, Borcherding, Brownley, Cedeno, Chen, Chernikoff, Cheung, Chirita, Curson, Ebert, Hacker, Hartlage, Hauser, Huang, Jiang, Karpinchyk, Koenig, Kong, Landers, Le, Liu, McBride, Morenzoni, Morey, Mutch, Perazich, Perry, Peters, Peterson, Pethiyagoda, Pothuraju, Richter, Rosenbaum, Roy, Shafto, Sharanhovich, Shannon, Sheppy, Sun, Thakuria, Tran, Vu, Zaranek, Wu, Drmanac, Oliphant, Banyai, Martin, Ballinger, Church and Reid2010). Additional allele frequency information was added using our own database, which includes past exome sequencing data of 50 unrelated individuals. Variants were annotated with their conservation levels and their inferred constraint levels using GERP++ (Davydov et al., Reference Davydov, Goode, Sirota, Cooper, Sidow and Batzoglou2010), PhyloP (Siepel et al., Reference Siepel, Bejerano, Pedersen, Hinrichs, Hou, Rosenbloom, Clawson, Spieth, Hillier, Richards, Weinstock, Wilson, Gibbs, Kent, Miller and Haussler2005) and PhastCons (Cooper et al., Reference Cooper, Stone, Asimenos, Green, Batzoglou and Sidow2005). Information regarding the predicted severity of each variant (namely, how deleterious a variant is predicted to be) was gathered using SIFT (Ng & Henikoff, Reference Ng and Henikoff2003), PolyPhen2 (Adzhubei et al., Reference Adzhubei, Schmidt, Peshkin, Ramensky, Gerasimova, Bork, Kondrashov and Sunyaev2010) and Mutation Taster (Schwarz et al., Reference Schwarz, Rödelsperger, Schuelke and Seelow2010). Clinical associations were gathered from the National Human Genome Research Institute catalogue of published Genome-Wide Association Studies (NHGRI-GWAS catalogue) (Hindorff et al., Reference Hindorff, Sethupathy, Junkins, Ramos, Mehta, Collins and Manolio2009), the Online Mendelian Inheritance in Man database (OMIM; http://omim.org/) and Uniprot's Human polymorphisms and disease mutations index (www.uniprot.org/docs/humsavar).
Gene annotation – After all variants were gathered we reviewed and annotated them using the following sources: (i) Pathway annotation, which includes all the pathways in which a given gene product has reportedly been involved in. Pathway information was gathered from the Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa et al., Reference Kanehisa, Goto, Sato, Furumichi and Tanabe2012), Biocarta (http://www.biocarta.com) and REACTOM (Matthews et al., Reference Matthews, Gopinath, Gillespie, Caudy, Croft, de Bono, Garapati, Hemish, Hermjakob, Jassal, Kanapin, Lewis, Mahajan, May, Schmidt, Vastrik, Wu, Birney, Stein and D'Eustachio2009). Gene biological process properties were gathered from the Gene Ontology (GO) database (Ashburner et al., Reference Ashburner, Ball, Blake, Botstein, Butler, Cherry, Davis, Dolinski, Dwight, Eppig, Harris, Hill, Issel-Tarver, Kasarskis, Lewis, Matese, Richardson, Ringwald, Rubin and Sherlock2000). (ii) Gene–gene and protein–protein interactions: Interactions annotations were gathered from the BioGRID interaction database (Stark et al., Reference Stark, Breitkreutz, Chatr-Aryamontri, Boucher, Oughtred, Livstone, Nixon, Van Auken, Wang, Shi, Reguly, Rust, Winter, Dolinski and Tyers2011), STRING protein functional interactions database (Szklarczyk et al., Reference Szklarczyk, Franceschini, Kuhn, Simonovic, Roth, Minguez, Doerks, Stark, Muller, Bork, Jensen and von Mering2011) and the human protein–protein interaction prediction database (PIPs) (McDowall et al., Reference McDowall, Scott and Barton2009). Following the aforementioned steps of variant and gene annotation and classification, we reviewed the data manually in order to produce the final candidate gene table and summarize any supporting evidence for their putative involvement in familial hyperparathyroidism.
Validation of the sequencing data – Validation of the sequencing data was carried out using Sanger sequencing of PCR products, using primers that flank the mutation site. This approach was employed in order to ascertain co-segregation of any seemingly pathogenic mutation with the hyperparathyroidism phenotype in this family.
3. Results
Case report – Briefly, affected individuals from two generations from a non-consanguineous, Georgian Jewish family were the focus of this study. In three cases, uni-glandular parathyroidectomy undertaken at ages 18–45 years was performed (indicated by recurring nephrolithiasis and hypercalciuria). This intervention resulted in normocalcaemia that lasted 3–5 years. In all three cases, hypercalcaemia recurred and additional parathyroid adenomas were located prior to second surgery. Subsequent surgery, indicated by high levels of hypercalcaemia, was subtotal parathyroidectomy. In one case, recurrence of hypercalcaemia within 9 months led to finding a fifth intramediastinal parathyroid gland that was subsequently surgically removed. Analysis of all parathyroid glands removed from these three patients (n=13) showed parathyroid adenomas with no cystic features or evidence of malignancy. Affected individuals from the same family who were evaluated (n=6) all had hypercalcaemia (ranging from 13·1 to 15·3 mg%), hypercalciuria (ranging from 576 to 898 mg/24 h), 4/6 patients had recorded evidence of nephrolithiasis at age range from 18 to 33 years. Initial evaluation in 1998 and subsequently in 2010 in all six affected individuals, revealed no biochemical evidence of abnormal hormone secretion or other biochemical abnormalities (PRL, GH, IGF-1, TSH, ACTH, cortisol, FSH, LH, insulin, gastrin, vasoactive intestinal peptide (VIP), glucagon, serotonin, adrenalin, noradrenalin and glucose concentrations), no pituitary abnormalities by MRI, no pancreatic or adrenal masses by CT ultrasound and MRI, normal chest X-rays and jaw orthopentography. Of note one affected woman (III-10 in Fig. 1) had a transient hyperprolactinaemia that was spontaneously resolved within 12 months. Thus, the diagnosis of FIHP was made in this family based on the evidence of primary hyperparathyroidism in three generations with a seemingly autosomal dominant mode of transmission, histological evidence for multiglandular disease and the lack of any clinical, biochemical or radiological evidence of additional endocrinopathies.
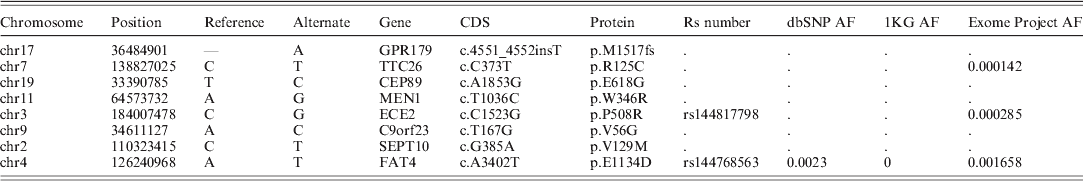
Genetic analysis results – Variant calling from WES phase of the three genotyped patients resulted in a total of 762 791 detected variants, of which 732 235 (96%) were found in all three patients: 9539 of these shared sequence variants were either amino acid (AA) (8659) or splice junction (880) altering mutations. Of these, 324 were considered rare (allele frequency <1% in all of the tested databases). Of these rare variants, eight were considered deleterious since they either led to a truncated protein (as a result of a premature stop codon) or were predicted to be deleterious by all prediction tools employed. Finally, we compiled a list of genes associated with hyperparathyroidism using a combination of the OMIM (http://www.ncbi.nlm.nih.gov/omim) and HuGENet (http://www.cdc.gov/genomics/hugenet/) databases (Supplementary Table S1). The average coverage in these genes was 21·6×, 25·8× and 22·6× for individuals II-1, II-2 and III-3, respectively (Fig. 1). Of the eight deleterious variants, only one mutation altered one of the HPT-associated genes (MEN1). The mutation (chr11: 64573732: c.T1021C: p.W341R) was found in a heterozygous state in all three patients and resulted in a change from a large size and aromatic AA (tryptophan) to a large size and basic AA (arginine). Additionally, the mutation was found in a region highly conserved among mammals and predicted to deleteriously alter protein function by all prediction tools employed. Therefore, this variant presented the most likely causative variant. Since this was the only HPT-associated gene mutation, we elected not to further pursue co-segregation analysis for the other seven seemingly pathogenic variants (Table 1). Supplementary Table S1 details non-synonymous mutations in other putative HPT-associated genes.
Table 1. Deleterious mutations following all filtration stages

Validation of the whole exome sequencing data – Sequencing the W341R MEN1 missense mutation showed that all tested individuals with the primary HPT phenotype exhibited the mutation, and three unaffected family members showed the wild-type allele only. Notably, 6/7 affected family members who agreed to participate in the study were all mutation carriers. The seventh affected member (Patient III-2 in Fig. 1) and three unaffected family members (III-1, III-5 and III-9 in Fig. 1) all refused participation.
4. Discussion
In this study, we combined WES with prior information regarding HPT-associated genes in order to effectively pinpoint the causative mutation in a proband with FIHP. Although WES focuses on the functional regions of the genome, each sequencing run was associated with tens of thousands of variations per sequenced individual. In order to narrow down the list of candidate variants, we initially annotated them using various data sources that enabled the stratification of variants by frequency, coding sequence effects and predicted functional alterations. Focusing our search only on rare (allele frequency <1% in all of the databases) AA altering variations that are either non-sense mutations or predicted to alter protein function, resulted in eight final variants. Comparing the list of genes affected by these variants with our compiled list of HPT-associated genes, only one variant matched: a rare change from tryptophan to arginine in position 341 of the MEN1 protein. The same mutation was previously reported in two non-familial cases displaying an MEN1 phenotype: one individual of French origin presented with primary HPT, pancreatic endocrine tumour and a non-secreting adrenal tumour (Giraud et al., Reference Giraud, Zhang, Serova-Sinilnikova, Wautot, Salandre, Buisson, Waterlot, Bauters, Porchet, Aubert, Emy, Cadiot, Delemer, Chabre, Niccoli, Leprat, Duron, Emperauger, Cougard, Goudet, Sarfati, Riou, Guichard, Rodier, Meyrier, Caron, Vantyghem, Assayag, Peix, Pugeat, Rohmer, Vallotton, Lenoir, Gaudray, Proye, Conte-Devolx, Chanson, Shugart, Goldgar, Murat and Calender1998) and another patient of Chinese ancestry who harboured the same mutation, was diagnosed with primary HPT, endocrine pancreatic, thyroid and pituitary tumours (Tso et al., Reference Tso, Rong, Lo, Tan, Tiu, Wat, Xu, Villablanca, Larsson, Teh and Lam2003). An additional study, reviewing 170 probands with at least one MEN1 syndrome-associated feature, reported the same mutation in one proband with FIHP (Wautot et al., Reference Wautot, Vercherat, Lespinasse, Chambe, Lenoir, Zhang, Porchet, Cordier, Béroud and Calender2002). The reasons for the different phenotypes of the family reported herein from two other individuals who carry the same mutation remain elusive. Although some studies (e.g. Kassem et al., Reference Kassem, Kruse, Wong, Larsson and Teh2000) suggested that mutations in exons 3–7 of the MEN1 gene result in isolated HPT without additional endocrinopathies, this claim of an established genotype–phenotype correlation in MEN1 is not universally accepted (Lemos & Thakker, Reference Lemos and Thakker2008).
Given the diverse ethnic origin of the previously reported individuals who harbour the mutation (French, Chinese and Jewish-Georgian) it seems unlikely that this mutation represents a founder mutation, and in all likelihood reflects a mutational hot spot. Although the W341R*MEN1 gene mutation is in all likelihood the causative mutation in the family presented herein, the final proof of causation is still lacking. Only functional analyses of the effect that this mutation has on the MEN1 protein function may enable firm establishment of the pathogenic role of this mutation in the FIHP phenotype. Other genes that may be associated with the same phenotype do not show co-segregation and are thus unlikely to harbour any deleterious mutation relevant to the phenotype.
Reviewing the additional seven candidate mutations, none of the genes that harbour these mutations had a clear association with HPT, and their interacting proteins or the biological processes and pathways they are involved with are not associated with the pathogenesis of HPT (Table 1).
We also reviewed the mutation spectra in other HPT-associated genes. One interesting variant, detected in a heterozygous state in all three patients, was found in the calcium-sensing, G-protein-coupled cell-surface receptor (CASR; c.G2956T: p.A986S). The variant is a known polymorphism (rs1801725) with a known association with increased extracellular calcium levels (Cole et al., Reference Cole, Peltekova, Rubin, Hawker, Vieth, Liew, Hwang, Evrovski and Hendy1999). Another HPT-associated polymorphism shared by all three patients was a synonymous heterozygous variant in the parathyroid hormone gene (PTH; c.C247A:p.R83R; rs6256) associated with lower levels of serum PTH (Kanzawa et al., Reference Kanzawa, Sugimoto, Kobayashi, Kobayashi and Chihara1999). Yet both variants are polymorphic and thus unlikely to contribute to the observed FIHP phenotype.
Given the limited number of genes that have been implicated in familial hyperparathyroidism phenotype, the question of the screening approach used for detecting the causative mutation, namely whole exome sequencing, needs to be addressed. From the cost-effectiveness analysis aspect, the costs of genotyping each of the three genes known in three affected individuals would be ∼$15 000 on a commercial basis. Thus, it made more sense to try and encompass the known genes and yet unknown genes in a single, less expensive experiment. Moreover, as exemplified by the recent report by Nesbit et al. (Reference Nesbit, Hannan, Howles, Babinsky, Head, Cranston, Rust, Hobbs, Heath and Thakker2013), novel genes may still be detected as underlying a closely related phenotype.
In conclusion, a combined approach of WES and targeted candidate gene-filtering approach facilitated uncovering the most likely causative mutation in the MEN1 gene in a family with FIHP in addition to a comprehensive characterization of the mutation spectra in the entire coding region which may modulate the MEN1 mutation phenotype.
The research reported herein was not funded by any funding agency. We thank the family members for their participation in this research project.
5. Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0016672313000141.
6. Conflict of interest statement
All authors declare that they have no conflict of interest regarding the data published.