The metabolic syndrome (MS) is a highly prevalent pro-thrombotic and pro-atherogenic condition characterised by abdominal obesity, hypertension, dyslipidaemia and insulin resistance(Reference Grundy, Cleeman and Daniels1). Although raised plasma cholesterol concentration is not one of its typical features, the MS has also been associated with reduced intestinal cholesterol absorption and enhanced endogenous cholesterol synthesis(Reference Grundy, Cleeman and Daniels1, Reference Gylling, Hallikainen and Kolehmainen2). While weight loss is recommended as the first step for the treatment of the MS, diet is being increasingly recognised as a key aetiological factor as well(Reference Grundy, Cleeman and Daniels1). The Mediterranean-type diet (MedDiet) has been proposed as the optimal diet for the prevention of the MS(Reference Meydani3), having favourable effects on several risk factors including plasma LDL-cholesterol (LDL-C) concentrations(Reference Richard, Couture and Desroches4). Little is known about the mechanisms that are implicated in the LDL-C-lowering effects of the MedDiet and how weight loss modulates these effects in the context of the MS. It has been suggested that the high phytosterol content of the MedDiet may on its own contribute to reduce plasma LDL-C concentrations by inhibiting intestinal absorption of cholesterol(Reference Rudkowska and Jones5). On the other hand, weight loss has been associated with reduced cholesterol synthesis, while having little or no effect on intestinal cholesterol absorption(Reference Santosa, Varady and AbuMweis6).
Lathosterol is a cholesterol precursor and its plasma concentrations have been shown to reflect endogenous cholesterol synthesis(Reference Simonen, Gylling and Miettinen7). Plasma phytosterol concentrations, mostly campesterol and β-sitosterol, have been shown to be a valid surrogate marker of intestinal cholesterol absorption(Reference Simonen, Gylling and Miettinen7). Moreover, studies have shown that markers of cholesterol synthesis and absorption may be stronger predictors of the risk of CVD than traditional plasma lipid concentrations(Reference Matthan, Giovanni and Schaefer8). The proprotein convertase subtilisin/kexin-9 (PCSK9) also plays a key role in whole-body cholesterol homeostasis by regulating the intra-cellular degradation of the LDL receptor in the liver, small intestine and kidney(Reference Seidah9). Elevated plasma PCSK9 concentrations have been associated with a reduced clearance rate of LDL and hence with elevated plasma LDL-C concentrations(Reference Chan, Lambert and Barrett10).
The primary aim of the present study was to assess the impact of the MedDiet in the absence of weight change on surrogate markers of cholesterol absorption, synthesis and clearance using plasma phytosterols, lathosterol and PCSK9 concentrations, respectively, in male patients with the MS. The intervention was also designed to examine the extent to which body weight reduction modifies the impact of the MedDiet on these markers of cholesterol homeostasis.
Subjects and methods
The study design has already been described in detail previously(Reference Richard, Couture and Desroches4). Briefly, men (18–65 years) from the Quebec City metropolitan area, who met the National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP) III criteria for the MS(Reference Grundy, Cleeman and Daniels1), were recruited for the study. All study procedures were approved by the Research Ethics Committee of Laval University (Quebec, QC, Canada). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and written informed consent was obtained from all participants enrolled in the study. Men with a previous history of CVD or type 2 diabetes, on lipid-lowering or hypertension medication and smokers were excluded. Men had also to have a stable body weight for at least 6 months.
Diets and study design
Fig. 1 shows the study design(Reference Richard, Couture and Desroches4). The subjects' diet was first standardised to a baseline North American control diet that they consumed for 5 weeks under isoenergetic, weight-maintaining conditions. The participants then consumed the MedDiet for 5 weeks under isoenergetic, weight-maintaining conditions. This first part of the study in controlled feeding conditions (all foods were provided to the participants) allowed us to investigate the impact of the MedDiet per se in the absence of weight loss. The participants subsequently underwent a 20-week weight-loss period in free-living conditions during which they were given advice on how to create a 2092 kJ (500 kcal) deficit in their daily energy intake. This was achieved by recommending a reduction in portion size as well as by promoting low-energy density food choices in free-living conditions. Finally, the subjects consumed the MedDiet for the second time for 5 weeks under controlled feeding, weight-stabilising conditions. This allowed us to investigate the combined impact of the MedDiet and weight loss on markers of cholesterol homeostasis.

Fig. 1 Study design as shown in Richard et al. (Reference Richard, Couture and Desroches4). * Daily weighing and adjustment of energy intake (controlled feeding phases of the study). † Food journals and physical activity records (3 d). ↑ Blood samples taken for assessment of study outcomes. MedDiet, Mediterranean diet.
Diets in the absence of weight loss
The control diet was devised to reflect the current macronutrient intake averages in North American men(Reference Gray-Donald, Jacobs-Starkey and Johnson-Down11). The MedDiet was formulated to be concordant with the characteristics of the traditional Mediterranean eating pattern(Reference Matalas, Zampelas and Stavrinos12). For the study, 7 d cyclic menus were developed and have already been presented(Reference Richard, Couture and Desroches4). The servings of various food categories consumed weekly during the control North American diet and the MedDiet for a 10 460 kJ (2500 kcal) intake is presented in Table 1. The control diet provided 34 % of the energy as fat with 13 % as SFA, 2 % as trans-fatty acids, 13 % as MUFA and 5 % as PUFA; 48 % of the energy as carbohydrates with a significant proportion of these coming from foods enriched in refined sugars; 17 % of the energy as proteins and 2 % of the energy as alcohol. The respective percentages of energy derived from lipids, carbohydrates, protein and alcohol in the MedDiet were 32, 50, 17 and 5 %, respectively. The MedDiet provided a MUFA:SFA ratio of 2·7 compared with a ratio of 1·0 in the control diet. The MedDiet also provided twice as much dietary fibre as the control diet, and virgin olive oil (commercially available) was the main source of fat in the diet. The experimental diets were formulated by using the Nutrition Data System software (version 4.03_31; Nutrition Coordinating Center, Minneapolis, MN, USA). Since the Nutrition Data System software does not provide amounts of phytosterols contained in foods, the phytosterol content of each diet was calculated using data from the United States Department of Agriculture National Nutrient Database for Standard Reference and from published tables(Reference Kritchevsky13–Reference Normen and Johnsson15).
Table 1 Servings of various food categories consumed weekly during the control North American diet and the Mediterranean diet (MedDiet) for a 10 460 kJ (2500 kcal) menu
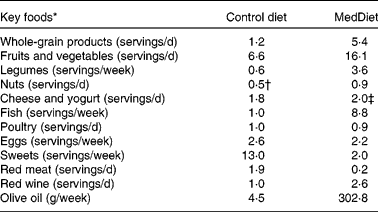
* Olive oil, g/week; portion size for whole-grain products, 125 ml (brown rice, brown pasta, bulgur and couscous), one bread piece or 30 g cereal; for fruits and vegetables, 125 ml; for legumes, 175 ml; for nuts, 30 g; for fish, poultry and red meat, 75 g; for eggs, 100 g; for dairy products, 50 g cheese, 175 g yogurt and 250 ml milk; for one glass of red wine, 75 ml.
† Mostly peanut butter on the control diet.
‡ Low-fat cheese and yogurt on the MedDiet.
The control diet was first assigned using pre-determined levels of energy intake estimated using 3 d food records and revised according to body weight fluctuations measured daily during the isoenergetic phases of the study. All meals, foods and beverages including red wine were provided to participants at the clinical investigation unit during the isoenergetic phases of the study, thereby optimising control for energy and macronutrient intake. The men were instructed to only consume the meals provided and to maintain their usual levels of physical activity during all isoenergetic phases of the study (15 weeks). A checklist was provided to all participants to identify the prescribed foods that had not been consumed when eating outside of the clinical investigation unit (mostly dinners and breakfasts) or had been consumed in addition to the formulated diets. Information on physical activity was collected using a daily checklist.
Weight loss period and stabilisation on the Mediterranean diet
After the first isoenergetic phase, the participants received instructions on how to reduce their usual energy intake by 2092 kJ (500 kcal)/d, in order to generate a minimum of 5–10 % reduction in body weight over a period of 20 weeks. To be consistent with our second objective, only participants who had lost at least 5 % of their body weight were eligible for the last isoenergetic phase of the study during which they consumed the MedDiet for 5 weeks again under isoenergetic, weight-stabilising conditions, with all foods provided to them as in the first isoenergetic phase.
Laboratory measurements
The lipid profile and lipoprotein–lipid concentrations as well as insulin and glucose levels were assessed after a 12 h fast at the end of each experimental isoenergetic diet according to previously described methods(Reference Richard, Couture and Desroches4). Anthropometric measures were assessed according to standardised procedures(Reference Lohman, Roche and Martorell16). Plasma concentrations of lathosterol, β-sitosterol and campesterol were quantified using a GC method similar to the one described previously(Reference Matthan, Giovanni and Schaefer8). Total plasma phytosterol concentrations were calculated as the sum of plasma campesterol and β-sitosterol concentrations. All values have been expressed as ratios relative to plasma total cholesterol concentrations ( × 102 μmol/mmol of cholesterol)(Reference Simonen, Gylling and Miettinen7). Plasma PCSK9 concentrations were measured in fasting plasma at the end of each experimental isoenergetic diet by an in-house ELISA as described in Dubuc et al. (Reference Dubuc, Tremblay and Pare17).
Statistical analysis
Data are reported as means and standard deviations and percentage of changes from the control diet unless stated otherwise. The main analysis compared the values of each outcome measured at the end of the control diet, MedDiet without weight loss and MedDiet with weight loss. Data were analysed using the PROC MIXED procedure for repeated measures in SAS (version 9.2; SAS Institute, Inc., Cary, NC, USA). The Tukey adjustment in the MIXED model was used to account for multiple comparisons of the three diets a posteriori. The extent to which initial values (on the control diet) modified the change in risk factors in response to the MedDiet with and without weight loss was also investigated using appropriate interaction terms in the MIXED model. When the interaction terms were found to be significant, the subgroups were created using the median value for the selected variable measured after the control diet to better illustrate this interaction. Variables with a skewed distribution were log10 transformed before statistical analysis. Differences at P ≤ 0·05 (two-sided) were considered significant.
Results
Of the twenty-nine men recruited in the study, two subjects dropped out and eight subjects were excluded because of non-compliance with the study protocol and requirements(Reference Richard, Couture and Desroches4). Of these subjects, seven did not achieve the 5 % body weight loss prescription defined a priori. The mean weight loss in these seven subjects was 1·2 (sd 1·7) % and was therefore not sufficient for including these subjects in the last phase of the study. The characteristics at screening of the nineteen participants included in the analyses are shown in Table 2. The overall compliance with the pre-determined diets during feeding in the isoenergetic phases of the study calculated from the food checklist in these subjects was 97·9 (sd 1·8) %.
Table 2 Physical characteristics and plasma lipid profile at screening of the nineteen male subjects included in analyses
(Mean values and standard deviations)
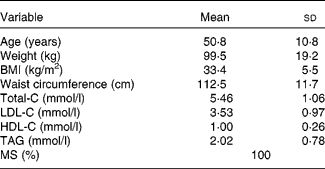
C, cholesterol; MS, metabolic syndrome.
As shown previously, the MedDiet in the absence of weight loss significantly reduced plasma total cholesterol ( − 7·8 %), LDL-C ( − 9·9 %) and LDL-apo B ( − 10·4 %) compared with the control diet (all P < 0·01, Table 3)(Reference Richard, Couture and Desroches4). Although the estimated intake in dietary phytosterol increased by 288·0 (sd 48·5) mg/d compared with the control diet, consumption of the MedDiet in the absence of weight loss led to a significant 9·7 % reduction in the plasma phytosterol:cholesterol ratio (P = 0·007). There was no significant correlation between changes in phytosterol intake and changes in the plasma phytosterol:cholesterol ratio (not shown). Finally, plasma PCSK9 concentrations were reduced by 11·7 % after the MedDiet in the absence of weight loss compared with the control diet (P = 0·004). Changes in plasma PCSK9 concentrations after the MedDiet in the absence of weight loss correlated significantly with concurrent reduction in plasma LDL-C (r 0·40, P = 0·088) and in LDL–apoB concentrations (r 0·51, P = 0·026).
Table 3 Effect of the Mediterranean diet (MedDiet) with and without weight loss on plasma lipid concentrations and surrogate markers of cholesterol (C) homeostasis in men with the metabolic syndrome
(Mean values, standard deviations and percentages)

PCSK9, proprotein convertase subtilisin/kexin 9.
* P value from the main effect of diet in the MIXED model. P values were obtained using the PROC MIXED procedure in SAS (SAS Institute, Inc., Cary, NC, USA).
† Significantly different from the control diet (P < 0·05).
‡ Significantly different from the Mediterranean diet in the absence of weight loss (P < 0·05).
§ Analysis was performed on log-transformed values.
∥ Campesterol+β-sitosterol.
The 20-week energy restriction phase and further stabilisation on the MedDiet for 5 weeks reduced the body weight by 10·2 (sd 2·9) % and waist circumference by 8·6 (sd 3·3) cm compared with the values measured after the control diet (P < 0·001)(Reference Richard, Couture and Desroches4). Combining weight loss with the MedDiet had no further impact on plasma total-cholesterol, LDL-C and PCSK9 concentrations. However, combining weight loss with the MedDiet significantly reduced the plasma lathosterol:cholesterol ratio compared with the MedDiet in the absence of weight loss ( − 18·2 %, P = 0·004) and the control diet ( − 17·5 %, P = 0·007). The magnitude of the reduction in the plasma lathosterol:cholesterol ratio on the MedDiet with weight loss from values on the control diet was positively correlated with parallel changes in body weight, TAG and insulin (0·46 < r <0·52, all P < 0·05). Finally, combining weight loss to the MedDiet tended to increase the plasma phytosterol:cholesterol ratio compared with the values on the MedDiet in the absence of weight loss (8·3 %, P = 0·075).
Fig. 2 illustrates how baseline plasma PCSK9 concentrations on the control diet modulated both the PCSK9 and the LDL–apoB response to the MedDiet with and without weight loss. The MedDiet, irrespective of weight loss, was associated with a significant reduction in plasma PCSK9 and LDL–apoB concentrations only in men with arbitrarily higher plasma PCSK9 concentrations at baseline (P < 0·04 for both interactions).

Fig. 2 (a) Proprotein convertase subtilisin/kexin-9 (PCSK9) and (b) LDL–apoB response to the Mediterranean diet (MedDiet) with and without weight loss according to baseline values of PCSK9. The P value for the interaction was obtained from the corresponding interactions terms in the MIXED model, using each variable as a continuous variable. The median value of PCSK9 (57·8 ng/ml) measured after the control diet was used to categorise individuals with high or low levels at baseline and to present this interaction graphically. * Values were significantly different from those of control diet (P < 0·05). Baseline PCSK9: –♦–, high; –□–, low. (a) P interaction < 0·001 and (b) P interaction = 0·037.
Discussion
Elevated plasma LDL-C concentrations are typically not considered a central feature of the MS. However, studies show that insulin resistance and the MS are associated with a perturbed cholesterol homeostasis encompassing reduced intestinal cholesterol absorption and increased whole-body cholesterol synthesis(Reference Gylling, Hallikainen and Kolehmainen2). We believe that this is the first nutritional intervention documenting the impact of the MedDiet in the absence and presence of weight loss on surrogate markers of cholesterol homeostasis in men with the MS. To the best of our knowledge, this is the first study of the MedDiet undertaken under tightly controlled feeding conditions as all previous studies are either epidemiological in nature or of interventions with dietary recommendations.
Mediterranean diet in the absence of weight loss
Our data suggest that part of the cholesterol-lowering properties of the MedDiet may be attributed to the reduced intestinal cholesterol absorption. The 8–10 % reduction in plasma total cholesterol and LDL-C concentrations observed with the MedDiet in the present study(Reference Richard, Couture and Desroches4) is comparable in magnitude with data from previous studies(Reference Jula, Marniemi and Huupponen18). Escurriol et al. (Reference Escurriol, Cofan and Serra19) have suggested that even small increases in the intake of phytosterols from traditional Mediterranean foods may play a role in the cholesterol-lowering effect of the MedDiet. β-Sitosterol, the most abundant phytosterol in the MedDiet, has been shown to reduce the expression of Niemann-Pick C1-like 1(Reference Jesch, Seo and Carr20), the major intestinal cholesterol transporter, and this mechanism may indeed be evoked to explain the reduction in cholesterol absorption with the MedDiet in the absence of weight loss. The MedDiet in the present study only provided an extra 288 mg/d of dietary phytosterols compared with the control diet, and it is thought that several hundred fold increase in the dietary load of phytosterols only doubles the plasma β-sitosterol concentrations in humans(Reference Miettinen, Tilvis and Kesaniemi21). We also stress that total plasma phytosterol concentrations were reduced with the MedDiet in the absence of weight loss despite this small increase in dietary phytosterol intake, and there was no correlation between variations in plasma phytosterol concentrations and dietary intake. Although some concerns have been raised regarding how variations in dietary phytosterol may affect the interpretation of results based on plasma phytosterol concentrations as surrogate markers of cholesterol absorption, we argue that the MedDiet-induced variation in plasma phytosterol concentrations are still likely to reflect whole diet effects rather than changes in dietary phytosterol intake per se. On the other hand, increased dietary intake of MUFA, PUFA, fish oils and fibres have been associated with reduced cholesterol absorption(Reference Wang22). Therefore, the higher intake of these nutrients may also partly explain the reduction in cholesterol absorption with the MedDiet.
PCSK9 plays a determining role in modulating the intracellular degradation of the LDL receptor(Reference Seidah9). PCSK9 knockout mice have reduced plasma total cholesterol concentrations compared with wild-type mice due to an increased LDL receptor activity that accelerates clearance of LDL particles(Reference Rashid, Curtis and Garuti23). The MedDiet in the absence of weight loss led to a significant reduction in plasma PCSK9 concentrations compared with the control diet, and the magnitude of the PCSK9 reduction was significantly correlated with the concurrent reduction in plasma LDL–apoB concentrations. This correlation remained significant even after adjustment for concurrent variations in plasma phytosterol concentrations with the MedDiet (not shown). This suggests that a significant proportion of the reduction in plasma LDL-C and LDL–apoB concentrations observed with the MedDiet in the absence of weight loss may be due to an enhanced uptake of LDL from the bloodstream by various tissues including the liver, independent of the apparent reduction in cholesterol absorption.
The MedDiet in the absence of weight loss had no effect on cholesterol synthesis as estimated by plasma lathosterol concentrations. Reduction in cholesterol absorption is generally counterbalanced by increased cholesterol synthesis(Reference Santosa, Varady and AbuMweis6). The 3-hydroxy-3methylglutaryl-CoA reductase, the rate-limiting enzyme in the cholesterol synthesis pathway, seems to be mostly regulated by intracellular cholesterol concentrations through negative feedback mechanisms(Reference Liscurn, Dennis and Jean24). We hypothesise that an increased uptake of LDL particles by the liver, thought to occur because of a reduced PCSK9 expression, may have contributed to maintaining the intracellular cholesterol pool that may have otherwise been reduced due to lower cholesterol absorption following the consumption of the MedDiet in the absence of weight loss(Reference Santosa, Varady and AbuMweis6, Reference Liscurn, Dennis and Jean24). Replacing dietary SFA with MUFA has been shown to increase LDL clearance, which could be partly and indirectly responsible for the neutral effect of the MedDiet on cholesterol synthesis(Reference Gill, Brown and Caslake25). Among other dietary factors known to affect endogenous cholesterol synthesis, plant sterols and PUFA have been associated with increased cholesterol synthesis(Reference Jones26).
Mediterranean diet in the presence of weight loss
The study also allowed us to specifically investigate the combined effect of weight loss and the MedDiet on surrogate markers of cholesterol homeostasis. Total plasma phytosterol concentrations on the MedDiet after weight loss tended to be higher than values before weight loss and were back to values measured after the control diet. The MedDiet combined with weight loss led to a significant reduction in the estimated cholesterol synthesis compared with the control diet and the MedDiet in the absence of weight loss, and this is consistent with studies having shown that weight loss frequently leads to reduction in cholesterol synthesis(Reference Santosa, Varady and AbuMweis6). Insulin resistance, on the other hand, has been associated with an increased endogenous cholesterol synthesis(Reference Pihlajamaki, Gylling and Miettinen27). This is consistent with our data showing that the reduction in cholesterol synthesis on the MedDiet with weight loss correlated with the reductions in body weight and in plasma TAG and insulin concentrations, which altogether predicts reduced secretion of apoB-containing lipoproteins by the liver. Such conclusions must be made with caution, however, without any confirmatory kinetic data from stable isotope studies. Cholesterol synthesis was also inversely correlated with cholesterol absorption after the MedDiet with weight loss (not shown). Based on these observations, we suggest that estimated cholesterol absorption may have been up-regulated after the MedDiet with weight loss v. before weight loss to compensate for the weight loss-induced reduction in endogenous cholesterol synthesis, thus attenuating any further change in plasma cholesterol concentrations.
Finally, previous studies have shown that weight loss on a low-fat diet is associated with an increased LDL clearance in patients with the MS(Reference Ng, Watts and Barrett28). As indicated earlier, the MedDiet in the absence of weight loss reduced plasma PCSK9 concentrations and the addition of weight loss had no further impact on this surrogate marker of LDL clearance. This suggests that weight loss per se may have no influence on estimated LDL clearance when changes have already occurred through significant dietary modifications with the MedDiet. Indeed, comparing the values before and after weight loss measured in both instances after controlled feeding of the MedDiet provides information on the effect of weight loss per se on cholesterol homeostasis markers since the dietary pattern and hence nutrient intake are unchanged. Taken together, the present results suggest that change in estimated LDL clearance with diet may be a more important determinant of the plasma LDL-C and apoB response than change in cholesterol synthesis.
Inter-individual variation in the cholesterol response to the MedDiet was quite important, and our data revealed that baseline PCSK9 concentrations were a significant determinant of this variation. Specifically, patients with a low estimated LDL clearance (reflected by high plasma PCSK9 levels) showed a greater reduction in plasma LDL–apoB concentrations on the MedDiet with and without weight loss than patients with an elevated clearance to start with. Although the significance of varying plasma PCSK9 in plasma is not known, the present results suggest that plasma PCSK9 may represent an interesting and novel biomarker predicting the LDL response to the MedDiet in men with MS.
In summary, the reduction in plasma LDL-C concentrations with the MedDiet even in the absence of weight loss appears to be primarily due to an increased LDL clearance combined with reduced intestinal cholesterol absorption, rather than to any change in endogenous cholesterol synthesis. Adding weight loss to the MedDiet has no further effect on plasma cholesterol concentrations, most probably because there is no further change in LDL clearance and the reduction in cholesterol synthesis with weight loss may have been counterbalanced by a reciprocal elevation in cholesterol absorption. However, these conclusions have to be interpreted with caution without the kinetic data on apoB-containing lipoproteins and cholesterol with stable isotopes.
Acknowledgements
B. L., P. C., A. H. L. and S. D. have designed and obtained funding for the present study. P. C. is a recipient of a scholarship from the FRSQ. N. G. S. is a recipient of Canada Research Chair in Precursor Proteolysis (no. RCHS0164). C. R. contributed to the coordination of the clinical study, analysed the data and wrote the manuscript, which was reviewed critically by all authors. B. L. is a Canada Research Chair in Nutrition and Cardiovascular Health. S. D. is a FRSQ Junior 1 scholar. We are grateful to the subjects for their excellent collaboration and to the dedicated staff of the Institute on Nutraceutical and Functional Food. The present study was supported by an operating grant from the Canadian Institutes for Health Research (MOP-68866, MOP-102741). Provigo/Loblaws donated the foods used in the present study. The authors report no conflict of interest in relation to the present study.







