Ancestral man is predicted to have eaten a diet high in fibre, K, complex carbohydrates and protein and low in Na, refined sugars and energy density. Typically, a palaeolithic diet provided a plant-to-animal energy ratio of 1:1, with the net acid-load being alkaline( Reference Eaton, Konner and Cordain 1 , Reference Cordain, Eaton and Sebastian 2 ). Analyses of the diets of modern hunter–gatherer populations support these predictions( Reference Cordain, Eaton and Sebastian 2 , Reference Strohle, Hahn and Sebastian 3 ). Since this time, when physiological and metabolic systems were evolving, there has been a gradual transition away from this palaeolithic diet. With the emergence of agriculture (about 7–5000 years ago) through to the industrial revolution (about last 100 years), the ‘modern diet’ has rapidly become low in fibre and high in Na, simple sugars and energy density( Reference Popkin 4 ). When superimposed on the palaeolithic genotype and physiology, the modern diet has resulted in an increased incidence of non-communicable diseases (NCD), which is estimated to account for 60 % of all deaths worldwide( Reference Daar, Singer and Persad 5 ). The economic impact of NCD is vast: $558, $237 and $33 billion in China, India and the UK, respectively( 6 ), whereas $750 billion is spent annually in the USA for diabetes and hypertension alone( Reference Narayan, Ali and Koplan 7 ).
Modification of diet offers an achievable and economically beneficial prevention strategy for NCD. Short-term consumption of a ‘palaeolithic’ diet produces significant reductions in blood pressure, cholesterol, TAG and insulin resistance( Reference Lindeberg, Jonsson and Granfeldt 8 ). In addition, reduced salt intake (e.g. 3 g/d) is predicted to reduce all-cause mortality in the USA by 44–92 000 individuals, saving an estimated $10–24 billion annually( Reference Bibbins-Domingo, Chertow and Coxson 9 ). Reducing sugar-sweetened beverage consumption by 1 serving/d reduced systolic blood pressure by 1·8 mmHg( Reference Chen, Caballero and Mitchell 10 ). Earlier dietary intervention, for example to pregnant mothers or those considering pregnancy, may have added benefit, as an adverse periconceptional and/or prenatal nutritional exposure has been shown to increase the risk of NCD (e.g. CVD or metabolic disease) in the adult offspring( Reference Crispi, Bijnens and Figueras 11 – Reference Whincup, Kaye and Owen 13 ) – a paradigm referred to as the developmental programming of health and disease.
The majority of developmental programming studies to recapitulate either a ‘Westernised’ or under/over-nourished diet in experimental models have used a low-protein or a high-fat and/or a high-sugar paradigm( Reference Langley-Evans, Phillips and Jackson 14 – Reference Sedova, Seda and Kazdova 16 ). In the UK, although higher than optimal (reference nutrient intake) intake of SFA is observed, high total fat intake is not. Indeed, data from the National Diet and Nutrition Survey suggest that total fat consumption is close to recommended but that fructose and salt intake remain high( 17 ). In the USA, a similar dietary pattern of high fructose and high salt intake has been observed, raising concerns about increased CVD risk( Reference Johnson, Appel and Brands 18 , Reference Bernstein and Willett 19 ).
The delayed programming effect of a maternal diet high in simple sugars (e.g. fructose( Reference Alzamendi, Castrogiovanni and Gaillard 20 )) or salt has been considered( Reference Contreras, Wong and Henderson 21 , Reference Porter, King and Honeycutt 22 ). Feeding sucrose to pregnant rats can influence hepatic metabolism and reduce offspring birth weight( Reference Soria, Chicco and Mocchiutti 23 ), and fructose-feeding during lactation renders the resultant adult offspring vulnerable to cardiometabolic risk( Reference Alzamendi, Castrogiovanni and Gaillard 20 ). A maternal diet high in salt is one of the few dietary challenges to repeatedly produce hypertensive offspring( Reference Contreras, Wong and Henderson 21 , Reference Gray, Al-Dujaili and Sparrow 24 ). More importantly, increased intake of salt in (or added to) food potentiates intake of simple sugars (e.g. from drinking sugar-sweetened beverages)( Reference He, Marrero and MacGregor 25 ). As each is known to influence cardiovascular health, it is important to consider their potential interaction experimentally. Sex-specific effects are widely observed in developmental programming studies( Reference Ojeda, Grigore and Alexander 26 ); sex is an important consideration with regard to disease susceptibility,( Reference Duma, Collins and Chou 27 ) and there has been recent criticism of sex bias (in favour of male offspring) in translational medicine studies( Reference Zucker and Beery 28 , Reference Kim, Tingen and Woodruff 29 ). It is therefore important to also consider potential sex-specific responses after maternal dietary intervention with respect to offspring cardiovascular function.
To date, no study has considered the delayed cardiovascular consequences on adult offspring (male and female) of the combined intake of fructose and salt by the dam. Excess salt in the diet increases fluid intake; disappointingly, this tends to be of sugar-sweetened beverages( Reference He, Marrero and MacGregor 25 ). We anticipate that high maternal intake of fructose and salt renders adult offspring prone to hypertension and hypersensitive to further consumption of salt or fructose. The aim of the present study was to characterise the cardiovascular health of adult male and female rat offspring after maternal consumption of a high salt diet (SD) and/or fructose diet (FD) before and during her pregnancy and for the duration of her lactation. Baseline cardiovascular health of all offspring was assessed 24/7 by radiotelemetry, as previously described by us after maternal salt intake( Reference Gray, Al-Dujaili and Sparrow 24 ). Cardiovascular hypersensitivity in vivo was assessed during four further experimental studies: (1) during sympathetic activation induced by anxiety-related isolation, (2) during nitric oxide blockade with N G-nitro-l-arginine methyl ester (l-NAME), (3) during dietary salt loading or (4) dietary fructose loading to determine whether postnatal response is conditioned by prenatal exposure. During each challenge, all data recorded were submitted for further non-linear regression analyses to determine potential effects on cardiovascular function through the circadian cycle. Finally, offspring hearts were studied ex vivo using the perfused Langendorff system to assess isolated cardiac function. For all outcome measures, we have assessed cardiovascular responses in different-sex siblings.
Methods
Ethics
Animal procedures were carried out under license and in accordance with the Home Office animals (Scientific Procedures) Act 1986 and approved by the local animal welfare and ethical review board of the University of Nottingham.
Diet design
In brief, Sprague–Dawley dams (190–200 g; 8–10 weeks of age) were kept in a temperature (20–22°C)- and humidity (55–65 %)-controlled environment and subjected to a 12-h light/dark cycle (07.00–19.00 h). Rats were randomly assigned to one of four treatment (diet) groups: (1) control diet (CD; n 6), fed purified standard chow (TD.08164; Teklad Harlan) and tap water; (2) SD (n 6), fed purified standard chow with 4 % NaCl added (TD.08162; Teklad Harlan) and tap water; (3) FD (n 6), fed purified standard chow (TD.08164) and tap water with 10 % fructose (Sigma-Aldrich) added; (4) fructose/salt diet (FSD; n 6), fed purified SD (TD.08162) and tap water with 10 % fructose added. Diet composition has been published previously( Reference Gray, Long and Green 30 ). All rats were fed the experimental diets ad libitum for at least 28 d before conception and throughout gestation and lactation.
Radiotelemetry and baseline cardiovascular recording
At 9 weeks of age, one male and one female offspring from each litter were surgically instrumented for radiotelemetric recording of blood pressure (TA11PA-C40; DSI) from the descending abdominal aorta, as described previously( Reference Kramer and Kinter 31 ). In brief, the rats were fully anaesthetised (fentanyl citrate; Sublimaze, Janssen-Cilag and medetomidine hydrochloride; Domitor, Pfizer; 300 µg/kg of each i.p.) for probe implantation (TA11PA-C40; DSI). Anaesthesia was reversed (Antisedan, Pfizer; 1 mg/kg) and analgesia was administered (buprenorphine; Buprecare, Animalcare; 0·02 mg/kg s.c.) together with a long-acting antibiotic (Amoxycare LA; 0·05 ml i.m.). All twenty-four rats that underwent surgery completed the study, and all were subsequently housed with a same-sex sibling to minimise stress. Cardiovascular variables were recorded (Dataquest GOLD version 4.02; DSI) at intervals (15×2 s periods per 15 min) during a 5–7-d recovery and baseline period and during cardiovascular challenges, which each lasted for further 5–7-d periods. Male and female siblings were recorded simultaneously, each with a same-sex cage mate present at all times, but challenges were conducted in a random order. At the end of all experiments, rats were euthanised in a sealed chamber using a rising concentration of CO2, followed by cervical dislocation after confirmation of cardiac arrest.
Radiotelemetry and stimulated cardiovascular recording
CV challenge 1: Isolation-induced anxiety – after a recovery period, the untelemetered sibling was removed from the cage for a 24-h period, and blood pressure and heart rate were recorded continuously (i.e. ×2 15 s periods/min; 2880 data points in total). Thereafter, siblings were reunited and recording continued at intervals. With 5–7-d recovery and wash-out periods between each challenge, telemetered rats were subjected to three further experimental studies in a randomised manner, each lasting 5 d with a further 2 d of recording during recovery; CV challenge 2: Nitric oxide blockade – the drinking water was substituted for fresh water with l-NAME dissolved at a concentration of 150 µg/ml (equivalent to 4·1 mg l-NAME/d); CV challenge 3: Salt loading – standard chow was substituted for purified chow with 4 % NaCl (TD.08162); and CV challenge 4: Fructose loading – the drinking water was substituted for fresh water with 10 % fructose solution.
The isolated heart (Langendorff) preparation
One male and one female offspring from each control or salt-exposed dams (offspring of fructose-fed dams were not included) were randomly selected, anaesthetised (3 % isofluorane in 2 litres/min O2) and killed by cervical dislocation. Within 90 s, the heart was excised and cannulated via the aorta to Langendorff perfusion apparatus (AD Instruments) and reverse-perfused with Krebs Henseleit buffer (118 mm-NaCl, 4·7 mm-KCl, 1·2 mm-KH2PO4, 1·2 mm-MgSO4, 25 mm-NaHCO3, 11 mm-glucose and 1·25 mm-CaCl2, pH 7·4, bubbled with 95 %/5 % O2/CO2). Perfusion was maintained at a constant pressure of 60 mmHg, with the perfusate warmed to 37·4°C and the heart immersed in a water-jacketed temperature-controlled glass chamber set at 37·4°C, therefore ensuring normothermia throughout the perfusion protocol. Contractile function (left ventricular developed pressure) was determined by an intravascular balloon, adjusted to an end diastolic pressure of 5–10 mmHg. Data were recorded for a 30-min baseline period after 15–30 min stabilisation via transducers (Senso-Nor 844; AD Instruments) using the Powerlab Acquisition System (AD Instruments).
Statistics
The study was designed with a 2 (±fructose)×2 (±salt) factorial structure and was analysed by a general linear model (GLM) approach for normally distributed data or after log transformation for a skewed error distribution (GenStat version 16; VSNi). All data are presented as mean values with their standard errors or standard error of the difference between means (for a more conservative estimate of the contrast variance). Although P≤0·050 was accepted as indicating statistical significance, values of P from 0·06 to 0·09 are also presented to indicate effects falling close to the arbitrary significance boundaries. Using one male or female offspring per litter per determination avoids complicating the statistical model with shared intra-litter variance. For offspring cardiovascular analyses, data were either tested as summary measures (e.g. hourly means of blood pressure) or, for circadian analyses, by incorporating all recorded cardiovascular data (e.g. 2880 datapoints per animal; 14 400–17 280 data points per group (n 5–6 animals of each sex) into a non-linear regression model fitting a Fourier curve (Y=α+βsin(2π(X+ε)/w)) to derive four parameters – α, set-point; β, amplitude; w, wavelength; and ε, offset – which were analysed by GLM.
Results
Maternal food intake
At conception, food intake was similar in rats fed SD but marginally reduced in those with fructose-sweetened water available (CD, 10·3 (sem 1·0) g/d; SD, 10·9 (sem 0·9) g/d; FD, 9·36 (sem 0·8) g/d; FSD, 7·02 (sem 0·9) g/d; P fructose=0·01). Food intake increased with advancing gestational age: by day 20 of gestation (term approximately day 21), rats were eating approximately double the quantity at conception, and those rats with fructose-sweetened water available were still consuming marginally less food (CD, 22·6 (sem 2·2) g/d; SD, 21·8 (sem 2·0) g/d; FD, 18·2 (sem 1·9) g/d; FSD, 16·6 (sem 1·9) g/d; P fructose=0·02). Nevertheless, using the AIN-93G formulation and despite a marginal reduction in food intake in those rats with fructose available, the diets (TD.08164 and TD.08162) still met macronutrient and micronutrient requirements for pregnant rats( 32 ).
Resting cardiovascular status of adult offspring
Prenatal exposure to SD significantly increased blood pressure in male offspring, with systolic, mean and diastolic pressures being 15 mmHg higher than age-matched CD (Table 1; Fig. 1(a)). In contrast, female siblings tended to be hypotensive, with systolic, mean and diastolic pressures being 10 mmHg lower than dietary controls (Table 1; Fig. 1(b)). Circadian analyses of pressure and heart rate, incorporating all measured data points for each animal within each diet group, suggested less dipping of nocturnal heart rate in male offspring exposed in utero to high maternal salt (Fig. 1(c)) and in female offspring exposed in utero to high maternal fructose (Fig. 1(d)). The latter, additionally, exhibited less dipping of nocturnal blood pressure (Fig. 1(e)). Such effects, despite the absence of excessive dietary intake postnatally, suggest long-term programming of cardiovascular sensitivity and reactivity in the offspring. We then tested this hypothesis in a number of experiments:
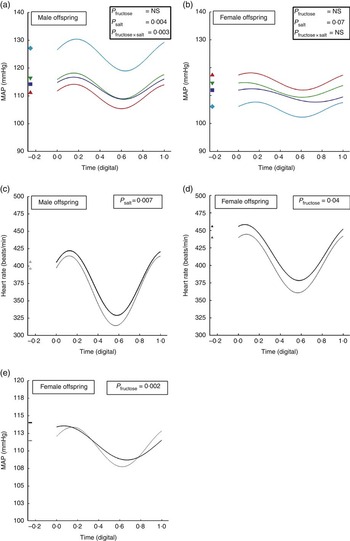
Fig. 1 Circadian analyses of pressure and heart rate in adult male and female offspring from dams fed fructose or salt. Circadian variation in mean arterial pressure (MAP; a, b and e) and heart rate (c, d) derived from Fourier curves in adult male and female offspring of dams fed (1) control diet and water ad libitum (CD, n 6 male/female offspring), (2) CD and 10 % fructose in water ad libitum (fructose diet (FD), n 5 male/female offspring), (3) 4 % salt diet (SD) and water ad libitum (SD, n 5 male/female offspring) and (4) 4 % SD and 10 % fructose in water ad libitum (fructose/salt diet (FSD), n 5 male/female offspring). Fourier plots represent predicted mean regression curve for each group (GenStat version 16; VSNi Ltd). Digital time is 00.00=0·0000 and 23 h 59 min 59 s=0·9999. FSD, fructose/salt diet. ![]() , SD;
, SD; ![]() , FD;
, FD; ![]() , FSD;
, FSD; ![]() , CD;
, CD; ![]() , +ve salt;
, +ve salt; ![]() −ve salt;
−ve salt; ![]() , +ve fructose;
, +ve fructose; ![]() , −ve fructose;
, −ve fructose; ![]() , +ve fructose;
, +ve fructose; ![]() , −ve fructose.
, −ve fructose.
Table 1 Summary measures analysis of resting cardiovascular status of adult male and female offspring from dams consuming salt and/or fructose (Mean values with their standard errors of the difference between means)

Fr×S, interaction of fructose×salt.
Blood pressures and heart rate were derived from radiotelemetric signals and reflect average values during the ‘resting’ period (i.e. day time; 07.00 to 20.00 hours) over a 7-d period. Mean values for the comparison from n 5–6 male or female offspring per dietary group (n 5–6 dams per dietary group). Data were analysed by 2 (salt, yes/no)×2 (fructose, yes/no) factorial ANOVA within each sex (GenStat version 13). Statistical significance was accepted at P<0·05.
Stimulated cardiovascular responses: isolation-induced stress
Immediately upon removal of their sibling from the cage, the single-housed telemetered offspring exhibited a robust cardiovascular response (Fig. 2(a)–(d)). Despite differing baselines, the magnitude of the change in pressure and heart rate was similar between dietary groups, but when the slopes of the relationship between paired values were analysed, the male, but not female, offspring of dams fed salt diet exhibited a significantly steeper response; the calculated slopes for male offspring were CD, 3·26 (95 % CI 3·02, 3·49); FD, 2·81 (95 % CI 2·63, 2·99); SD, 5·36 (95 % CI 5·17, 5·55); FSD, 5·38 (95 % CI 5·15, 5·60) beats/min per mmHg; P<0·001, and for female offspring the slopes were CD, 4·77 (95 % CI 4·59, 5·08); FD, 4·26 (95 % CI 3·91, 4·60); SD, 4·47 (95 % CI 4·25, 4·69); FSD, 3·30 (95 % CI 3·12, 3·48) beats/min per mmHg (Fig. 2(e) and (f)). In short, the male offspring of dams fed a high-SD are hypertensive, with greater short-term cardiovascular reactivity to anxiety-related stimuli that leads on, in the long term, to less dipping of heart rate at night. We then assessed whether such a phenotype was underpinned by programmed cardiovascular changes in (a) the periphery, by examining cardiovascular function on a background of tonic endothelial nitric oxide blockade, and in (b) the heart, by using the Langendorff technique in isolated hearts.
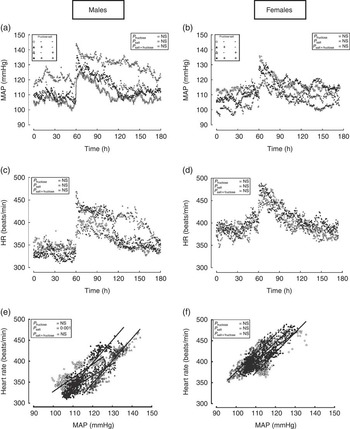
Fig. 2 Mean arterial pressure (MAP) (a, b), heart rate (c, d) and slopes of the relationship (e, f) between mean arterial pressure and heart rate in male and female offspring at approximately 10 weeks of age from dams fed fructose and/or salt. Data are (○) control diet (CD) and water ad libitum (n 6), (▴) CD and 10 % fructose in water ad libitum (n 5), (▵) 4 % salt diet (SD) and water ad libitum (n 5), (●) 4 % SD and 10 % fructose in water ad libitum (n 5) for male and female offspring. Data were measured continuously (i.e. sampled at 2 outputs per min) by telemetry for a 1-h baseline period and subsequently for 2 h after removal of their sibling from the cage. Regression lines were generated in Graphpad Prism 5·0.
Stimulated cardiovascular responses: nitric oxide blockade
Upon consumption of l-NAME, the mean arterial pressure increased significantly in both sexes of all groups (Fig. 3(a) and (b)), with the magnitude of change (i.e. increase from baseline) being similar between groups and sexes pooled estimate, 43·3 (sem 2·6 mmHg). The oscillation in heart rate increased with the duration of l-NAME treatment in both male and female offspring – i.e. the β-coefficient increased from 37·1 (sem 3·1) beats/min (days 1–2) to 50·1 (sem 3·0) beats/min (days 4–5) for male and female offspring alike (Fig. 3(c) and (d)). Despite l-NAME treatment, circadian analyses indicated heart rate to remain elevated in male, but not female, offspring of salt-fed dams (352 v. 337 (sem 2·1) beats/min; P<0·001; Fig. 3(e)). In addition, the reduced dipping of heart rate at night in the male offspring from salt-loaded dams was retained (Fig. 3(e)). Similarly, adult female, but not male, offspring of fructose-fed dams retained higher average heart rates: 384 v. 362 (sem 2·1) beats/min (Fig. 3(f)). Programmed sex-specific pathways in the adult offspring, which independently influence adult cardiovascular control after maternal salt or fructose loading, were therefore beginning to emerge: for male offspring, maternal high-SD renders them reactive to further cardiovascular stressors as adults; for female offspring, maternal high fructose has a similar effect. Each was apparently independent of endothelial NOx status.
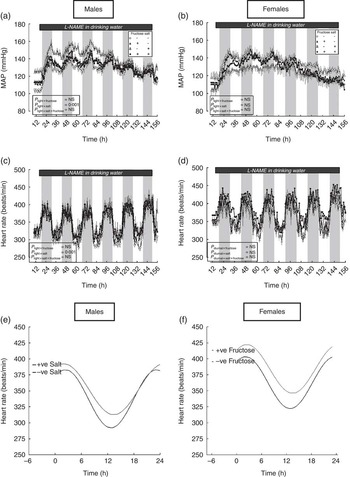
Fig. 3 Mean arterial pressure (MAP; a, b), heart rate (c, d) and Fourier curves (e, f) for circadian variation in heart rate in response to N G-nitro-l-arginine methyl ester (l-NAME) in the male and female offspring of dams fed fructose and/or salt. Data are (○) control diet (CD) and water ad libitum (n 6), (▴) CD and 10 % fructose in water ad libitum (n 5), (▵) 4 % salt diet (SD) and water ad libitum (n 5), (●) 4 % SD and 10 % fructose in water ad libitum (n 5) for male and female offspring. Data were measured intermittently (for 30 s every 15 min for 7 d) by telemetry, and hourly means were calculated as a summary measure of the cardiovascular response. Data were analysed within sex by general linear mixed model (GenStat version 13). l-NAME was provided in the drinking water (150µg/ml).
Adult offspring isolated heart function at 8 weeks of age
With hearts mounted on the Langendorff apparatus, heart rate was higher (P=<0·001) in the female offspring of dams fed SD (male, 312 v. 308; female, 310 v. 330 beats/min for CD v. SD, respectively) but left ventricular developed pressure (male, median 39 (IQR 17–45) v. 39 (IQR 35–53); female, 51 (IQR 46–56) v. 48 (IQR 39–50) mmHg for CD v. SD, respectively) and the maximal positive derivative of the rate of change in developed pressure (+dp/dt) were not different between groups (male offspring, median 1076 (IQR 609–1449) v. 1617 (IQR 1481–1670); female offspring, 1448 (IQR 1271–1700) v. 1568 (IQR 1475–1744) mmHg of CD v. SD, respectively).
Without any obvious programmed alteration to tonic endothelial (nitric oxide) activity or cardiac function, we next tested whether male and female offspring were rendered differentially reactive to the same inducing dietary stimulus in their mothers.
Stimulated cardiovascular responses: salt sensitivity
There was little measurable effect of high salt intake on cardiovascular status in the male and female offspring of all dietary groups. Circadian analyses did indicate, however, that with salt loading the offspring of fructose-exposed dams exhibited significantly blunted nocturnal dipping of pressure (β-coefficient male offspring; 3·9 v. 5·3 (sem 0·4) mmHg; F=3·8; P light×salt=0·001) and heart rate (β-coefficient male offspring, 39·5 v. 45·3(sem 2·1) mmHg; F=6·3; P light×fructose=0·001; female offspring, 37·1 v. 41·9 (sem 1·7) mmHg; F=2·5; P light×fructose=0·01).
Stimulated cardiovascular responses: fructose sensitivity
Consumption of fructose per se had little cardiovascular effect in control offspring (CD effect size, 1·0 (sem 2·3) mmHg). In male offspring from salt-loaded dams, high fructose intake elicited a significant pressor response (SD effect size, 6 (sem 2·6) mmHg; P=0·002), which was greater in male offspring from fructose-loaded dams (FD effect size, 8·1 (sem 2·6) mmHg; Fig. 4(a)). For female offspring, high fructose intake increased pulse pressure (effect size, +5·2 (sem 3·1) mmHg; P=0·005), but this effect was 2-fold greater if their dams had also been fructose-loaded (FD effect size, 10·3 (sem 3·1 mmHg; Fig. 4(b)). Heart rate varied with the light/dark cycle, as in the unchallenged state, but it was not overly influenced by 5-d fructose consumption (Fig. 4(c) and (d)). In male rats, which were previously exposed to maternal salt loading, the increase in heart rate from day to night as the rats became active was diminished (a change of 54 v. 60 beats/min ±5; P salt<0·005).

Fig. 4 Mean arterial pressure (MAP) (a), pulse pressure (b) and summary measures of heart rate (c, d) during fructose ingestion in the male and female offspring of dams fed fructose and/or salt. Data are (○) control diet (CD) and water ad libitum (n 6), (▴) CD and 10 % fructose in water ad libitum (n 5), (▵) 4 % salt diet (SD) and water ad libitum (n 5), (●) 4 % SD and 10 % fructose in water ad libitum (n 5) for male and female offspring. Data were measured intermittently (for 30 s every 15 min for 7 d) by telemetry and hourly means were calculated as a summary measure of the cardiovascular response. Data were analysed within sex by general linear mixed model (GenStat version 13). Fructose was provided in the drinking water (10 % solution). ![]() , Water;
, Water; ![]() , 10 % fructose.
, 10 % fructose.
Heart rate variability
During all challenges, heart rate variability (HRV) was calculated. HRV exhibited marked circadian and ultradian patterns under control conditions, which was unaffected by l-NAME treatment, salt loading or high intake of fructose (Fig. 5(a)–(f)). However, notably, regardless of the challenge, HRV distinctly peaked at 20.00 hours in all groups (Fig. 5(a)–(f)).

Fig. 5 Heart rate variability (HRV) in male and female offspring from dams fed (○) control diet (CD) and water ad libitum (n 6), (▴) CD and 10 % fructose in water ad libitum (n 5), (▵) 4 % salt diet (SD) and water ad libitum (n 5), (●) 4 % SD and 10 % fructose in water ad libitum (n 5) for male and female offspring during 5 d of (a, b) N G-nitro-L-arginine methyl ester (L-NAME) treatment, (c, d) 4 % salt loading and (e, f) 10 % fructose in drinking water. Heart rate was derived from the radiotelemetric pressure pulse and recorded intermittently (for 30 s every 15 min) for the duration (7 d) of each nutritional challenge. HRV was calculated as the variance (SD 2) in heart rate for each hour of recording. Data were highly positively skewed and were therefore analysed by general linear mixed model with a gamma error distribution and logarithm-link function; back-transformed predicted means are presented (GenStat version 16).
Discussion
The adverse metabolic consequences of increased consumption of extrinsic sugars, in particular fructose, has been widely reported( Reference Tappy and Le 33 – Reference Stanhope, Schwarz and Keim 36 ). Only one study in mice( Reference Huang, Boini and Friedrich 37 ) and one in rats( Reference Song, Hu and Shi 38 ) have described the cardiovascular effects of additional dietary salt on cardiovascular function; none has considered their interaction when fed to pregnant dams and, subsequently, to their offspring. In the current study, we reveal some clear circadian and sex-specific effects of high maternal intake of salt or fructose on cardiovascular physiology in the adult offspring. Two independent sex-specific phenotypes emerge that are retained despite no significant consumption of salt or fructose postnatally; maternal salt loading has distinct and marked hypertensive effects on male offspring, and maternal fructose loading appears to have greater cardiovascular effects on female offspring. Importantly, for fructose in particular, these effects in the female offspring are exacerbated by further fructose intake, as would naturally occur in human populations.
The adverse cardiovascular effects of increased consumption of salt have long been recognised( Reference Bang, Bechgaard and Nielsen 39 ); for fructose, the deleterious consequences are the subject of much recent debate( Reference Johnson, Perez-Pozo and Sautin 40 ). Taking an evidence-based approach, however, would favour the hypothesis that increased consumption of fructose after the introduction of high fructose maize syrup and sugar-sweetened beverages has had a negative impact on cardiovascular health( Reference Jalal, Smits and Johnson 35 , Reference Fung, Malik and Rexrode 41 ). When considering the impact of diet on health (including offspring health), the relativity is all important; early hominids evolved eating approximately 0·25 g/d salt and no more than 2 % energy/d from simple sugars. The current estimated average consumption is 8–12 g/d salt and 18–25 % energy/d from simple sugars. Relative to our ancestral diet, during which our physiology was moulded over many thousands of years, the current average diet represents a considerable physiological burden. In the context of developmental programming, in which maternal malnutrition may influence fetal development to result in adaptations that become deleterious in a westernised nutritional environment, it is unsurprising that such a physiological burden is not without effect. Using an animal model to recapitulate a westernised dietary pattern, the current study illustrates how this burden may translate to the offspring, and how these responses are sex- and nutrient-specific. For salt-loaded dams, effect size in sibling offspring is approximately 25 mmHg (male offspring are hypertensive (approximately 15 mmHg above controls), and female offspring hypotensive (approximately 10 mmHg below controls)). Such large sex-specific effect sizes are rarely observed( Reference Sinclair, Allegrucci and Singh 42 , Reference Grigore, Ojeda and Alexander 43 ).
Sex-specific effects are often observed within the developmental programming paradigm( Reference Aiken and Ozanne 44 ) but, to our knowledge, none as marked as in the current study. This study was designed to illustrate potential sex-specific, delayed developmental effects but not to interrogate potential mechanisms should they arise. For example, whilst a number of models have inferred sex-specific effects of programming by adopting the relatively crude approach of gonad removal, a more appropriate intervention would be to use highly specific and reversible sex-hormone antagonists longitudinally. Some excellent recent studies that have shown programming of a sex-specific cardiovascular phenotype (such as increased blood pressure in male but not female offspring) have identified an absence of oestrogen in male offspring as a causal factor( Reference Sinclair, Allegrucci and Singh 42 , Reference Grigore, Ojeda and Alexander 43 ); in effect, oestrogen acts as a ‘pro-survival factor’ mitigating (perhaps epigenetically) the adverse consequences of a nutritionally poor developmental environment until concentrations decline in middle-age and morbidity and mortality rates (e.g. for cardiovascular outcomes) in female offspring begin to rise – the basis for oestrogen replacement therapy( Reference Aiken and Ozanne 44 ). However, being genetically male or female and interacting differently with the immediate (e.g. intra-uterine) environment could be important; for example, periconceptional exposure to a maternal methyl-deficient diet for only 6 d (day 0–6 gestation) revealed significant sex-specific differential DNA methylation of CpG islands in the fetal livers at day 90 of gestation – i.e. of the altered loci as a result of the dietary treatment, 53 % were specific to male and only 12 % were specific to female( Reference Khan, Dekou and Douglas 15 ).
Programmed alterations of cardiovascular control in salt-exposed offspring appears independent of tonic endothelial nitric oxide; if this were the case, then l-NAME treatment should have revealed differences in short-term responses (i.e. the magnitude of increase in first 8–12 h) or long-term regulation. However, a simple procedure to induce temporal anxiety – removing the cage mate for a 24-h period – does reveal marked differences in male, salt-exposed offspring. This has two important consequences: first, the generation of curves of the coupling between pressure and heart rate at this time indicates that salt-exposed hypertensive male offspring, but not non-hypertensive female siblings, have a greater rate of rise of heart rate per unit pressure relative to female salt-exposed offspring. This suggests a centrally mediated alteration at the level of the brain or peripheral autonomic nervous system and/or an effect on cardiac function. The latter can be ruled out, as ex vivo cardiac function, as shown by the Langendorff preparation, was not significantly different. Furthermore, we have previously shown that the offspring of salt-loaded dams have altered set-points for osmolar regulation – a phenotype indicative of alterations at the level of the brain( Reference Gray, Al-Dujaili and Sparrow 24 ). In addition, the data clearly indicate that measurements of resting blood pressure in telemetered rats should always be conducted with same-sex sibling cage mates in order to achieve a true ‘resting or ambulatory reading’; single-housed rats are easily stressed, which has a marked negative impact on resting cardiovascular variables.
For the first time, we provide evidence that increased maternal fructose consumption has important effects on adult offspring cardiovascular control. Resting blood pressure was unaltered by increased maternal fructose intake, but the circadian oscillation in pressure and heart rate was significantly blunted, reflective of a ‘non-dipping’ nocturnal pattern – previously identified as a significant risk factor for later CVD( Reference de la Sierra, Redon and Banegas 45 ). This finding is intriguing considering the limited exposure to fructose; none had consumed any fructose, as they were weaned at 3 weeks of age. A number of studies have previously reported a pressor effect of fructose either given acutely, using high doses (66 % of total energy intake( Reference Hwang, Ho and Hoffman 46 )), or chronically (using lower doses( Reference Jalal, Smits and Johnson 35 )) and others reporting no effects( Reference D’Angelo, Elmarakby and Pollock 47 ). Furthermore, our data suggest that maternal diet renders offspring (in particular female offspring) with a residual, increased sensitivity to further fructose intake. Mean arterial or pulse pressure in male and female offspring increased significantly more in prenatally fructose-exposed groups relative to control animals. The fact that chronic l-NAME treatment did not reveal any difference in fructose-exposed groups suggests no residual involvement of tonic nitric oxide activity. A recent study demonstrated that an altered pattern of vascular smooth muscle prostanoid release may be a contributing factor to fructose-induced vascular sensitivity( Reference Puyo, Zabalza and Mayer 48 ), but equally up-regulation of other vasoconstrictor, anti-natriuretic or diminished vasodilatory pathways may be causal. We have measured a number of fructose-induced advanced glycation end products such as fructosamine (an indicator of fructose-induced protein glycosylation), uric acid and glucose and found no difference in the basal state to account for alterations in fructose sensitivity. Acute fructose ingestion has been shown to increase blood pressure, likely through an effect on cardiac sympathetic sensitivity( Reference Brown, Dulloo and Yepuri 49 ). The current study illustrates that the effects of fructose ingestion after being exposed in utero to a maternal diet high in fructose have a distinct sex-specific bias, with female offspring being more fructose-sensitive.
Finally, the current study clearly illustrates that moderate over-consumption of salt and/or fructose by dams during pregnancy and lactation is able, in the offspring, to recapitulate many of the known pathophysiological effects of these micronutrients despite little exposure of the offspring to these diets. This has marked implication for NCD in Western populations. Continued intake of refined, low nutritional-quality diets in the next generation, following maternal over-consumption, has the potential to vertically transmit adverse health outcomes through generations. Reversal of this trend is going to require preventative action before birth, and as a result it will also take generations to effect a response. Given the implications for human populations, we would also strongly endorse recent commentaries and initiatives to reduce both the quantity of salt( Reference He, Jenner and MacGregor 50 ) and fructose( Reference Johnson, Appel and Brands 18 ) consumed as part of the modern Western diet.
Acknowledgements
The authors express their gratitude for the help and support provided by the Bioscience Research Unit on the Sutton Bonington Campus and Julie March for performing the radiotelemetry transmitter implantations. All work in this manuscript was performed on the Sutton Bonington Campus, University of Nottingham.
This work was supported by a BBSRC doctoral training grant to C. G. (50:50) with The University of Nottingham. Grant support from The Nutricia Research Foundation and The School of Veterinary Medicine and Science is gratefully acknowledged.
D. S. G., C. G. and S. M. G. designed research; C. G. and D. S. G. conducted the research; S. M. G. provided essential materials; and D. S. G., C. G. and S. M. G. wrote and critically evaluated the paper. D. S. G. has primary responsibility for its final content.
The authors declare that they have no conflicts of interest.









