Introduction
Patient-to-patient transmission and widespread use (and misuse) of antimicrobials has led to an increased incidence of antimicrobial-resistant bacteria, including multidrug-resistant organisms (MDROs). At present, MDROs worsen morbidity and mortality for patients,
Reference Barrasa-Villar, Aibar-Remón, Prieto-Andrés, Mareca-Doñate and Moliner-Lahoz1
though there is concern that MDROs could represent a threat to the very core of our healthcare system, as pathogens resistant to all antibiotics continue to spread across the globe. In 2019 the CDC updated their antibiotic resistance threats report, which includes sobering data about the breadth of the problem in the United States, with 2.8 million yearly infections from antibiotic-resistant infections, and 35,000 yearly deaths from antibiotic-resistant infections.
2
National initiatives to slow the spread of MDROs have increased in their scope in the past decade. The White House released a National Action Plan for Combatting Antibiotic-resistant Bacteria (CARB) for 2020–2025, representing a broad collaboration across multiple government agencies. Broadly speaking, the goals of CARB are to slow the emergence and prevent the spread of MDROs through improved diagnostics, antimicrobial research/development, antimicrobial stewardship, and fostering international collaboration.
3
The Veterans Health Administration (VHA) is the largest integrated health system in the US, with over 1,300 care facilities serving over 9 million patients. Notably, the VHA has a long history of combatting MDROs through efforts to reduce patient-to-patient transmission. In 2007, the VHA implemented a methicillin-resistant Staphylococcus aureus (MRSA) prevention bundle which was associated with sustained declines in infection rates for not just MRSA, but other MDROs such as Clostridioides difficile and carbapenem-resistant Enterobacteriaceae (CRE).
Reference Jain, Kralovic and Evans4,Reference Goto, O’Shea and Livorsi5
Given its national footprint, its prior history of combatting MDROs, and its involvement as a CARB collaborator, the VHA is a national leader in MDRO research efforts. In 2017, a collaboration among VHA researchers outlined an agenda for MDRO research within the VHA. That research agenda was set by 37 national experts and outlined the five-year research needs for combating MDROs, including transmission dynamics, antimicrobial stewardship, the microbiome, and special populations.
Reference Perencevich, Harris and Pfeiffer6–Reference Kates, Tischendorf and Schweizer10
This document is a follow-up of the 2017 research agenda collaborative and is designed as a companion piece for an accompanying 2024 antimicrobial stewardship research agenda.
Reference Livorsi, Drekonja and Eschevarria11
The primary goal of this collaboration is to assess research progress in the domain of MDRO transmission prevention since 2017 and utilize this information to identify current MDRO research needs for the VHA system over the next 5 years. When possible, there is a focus on research conducted within the VHA. Our hope is that this document will serve as a roadmap for VHA transmission prevention research during the next five years.
This transmission prevention research agenda is a collaboration between 20 VHA research leaders across the country. Committee members were divided into subgroups tailored to their areas of expertise, with subgroups formed around the core topics of active surveillance/isolation, hand hygiene, environmental cleaning/disinfection, special populations, and biosurveillance. Subgroups all met multiple times and performed independent literature reviews of their topic areas during a six month period, then identified high-need research areas in their respective domains. Once finalized, all topics were reviewed by all member-authors.
Active surveillance
Programs which screen patients to determine whether they are colonized with a specific organism, known as active surveillance (AS), are designed to monitor and control the spread of MDROs. When an organism of interest is detected via AS, it should prompt a response with patient isolation, decolonization, or another intervention with the goal of decreasing the risk of infection in the colonized patient and/or transmission of the organism to other patients. Active surveillance is a common vertical strategy to reduce transmission;
Reference Jain, Kralovic and Evans4
for example, the recent joint practice recommendations from the Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and Association for Professionals in Infection Control and Epidemiology (APIC) lists AS as an “additional recommendation” for prevention of MRSA infections, meaning that AS should be considered in select locations and populations.
Reference Popovich, Aureden and Ham12
However, evidence about the efficacy of AS and its associated interventions is conflicting, with some clinical trials showing no difference in acquisition of vancomycin-resistant enterococci (VRE) or MRSA when AS plus expanded use of barrier precautions was compared to no intervention.
Reference Huskins, Huckabee and O’Grady13
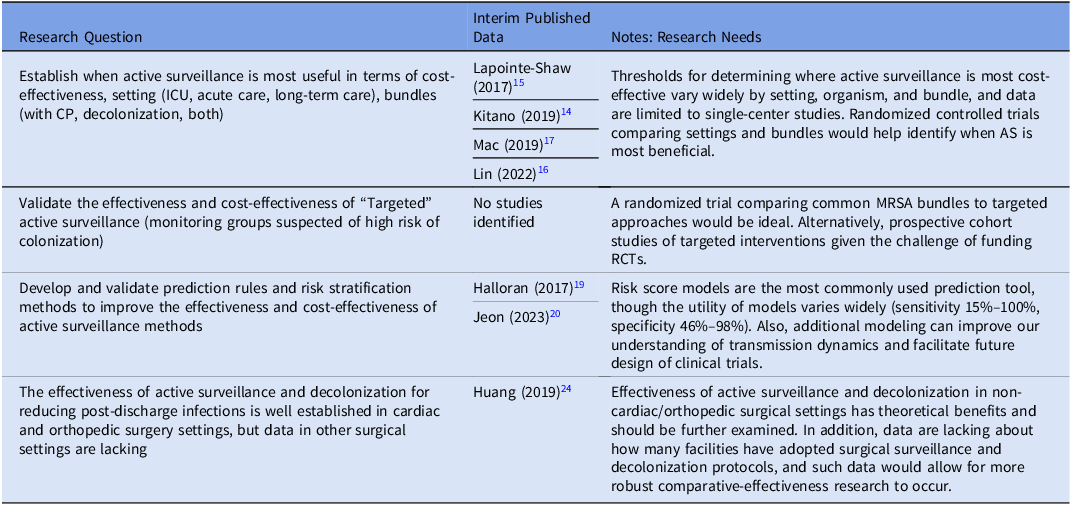
In the prior research agenda, several research gaps were identified that still have not been sufficiently addressed (Table 1). For example, it is still unclear when AS is most cost-effective. Studies have occurred in various settings (such as intensive care units, acute care units), with various MDROs of interest (MRSA, VRE, etc), and with various bundles (AS + contact precautions [CP], AS + decolonization, AS + CP + decolonization), so there is great complexity in understanding when AS is most effective.
Reference Kitano, Takagi and Arai14–Reference Mac, Fitzpatrick, Johnstone and Sander17
Future research should strive to improve the quality of this data, ideally with cluster-randomized trials, though realistically with less expensive cohort and quasi-experimental studies.
Reference Perencevich and Lautenbach18
Modeling could serve as a useful adjunct,
Reference Halloran, Auranen and Baird19
and recent literature has tended to focus on risk score models as a prediction tool for determining cost-effectiveness of AS, though the utility of these modeling methods has varied greatly.
Reference Jeon, Chavda, Rennert-May and Leal20
An important example of how AS varies by setting can be seen in the peri-operative domain. The use of AS coupled with decolonization in the cardiac and orthopedic surgery settings is well established as a tool to reduce MRSA surgical site infections,
Reference Schweizer, Perencevich and McDanel21,Reference Saraswat, Magruder and Crawford22
however apart from intra-abdominal surgeries,
Reference Huttner, Robicsek and Gervaz23
the efficacy of AS plus decolonization protocols is lacking in other procedures. Additionally, given the potential benefit of AS plus decolonization protocols in non-operative settings,
Reference Huang, Singh and McKinnell24,Reference Miller, McKinnell and Singh25
this is an area in need of further study.
Table 1. Veterans Healthcare Administration research agenda for transmission prevention research: active surveillance
Isolation measures
The principal tools of isolation are the use of contact precautions (CP) and patient cohort isolation. Contact precautions involve the use of personal protective equipment (PPE) such as gowns and gloves when healthcare workers enter a patient room, while cohort isolation involves moving patients colonized or infected with an MDRO to be separated from non-colonized and non-infected patients. These interventions can be implemented universally
Reference Harris, Pineles and Belton26
or in a targeted approach (eg, guided by AS, or in a syndrome-based manner such as for patients with uncontained wounds).
Reference Huang, Septimus and Kleinman27
From a research standpoint, a principle challenge is linking policy (eg, CP) to outcomes (eg, reduction in infection rates) given their distant temporal relationship.
MRSA is the best-studied organism in the domain of CP, and national guidelines favor the implementation of CP for MRSA. The aforementioned joint guidelines from SHEA/IDSA/APIC updated in 2022 recommend universal contact precautions for MRSA as an “essential practice” that should be adopted by all hospitals.
Reference Popovich, Aureden and Ham12
It is worth mentioning that there remains active discussion about the necessity of universal CP for MRSA.
Reference Morgan, Wenzel and Bearman28,Reference Diekema, Nori, Stevens, Smith, Coffey and Morgan29
This controversy primarily arises from inadequate data as well as the difficulty in separating the effect of CP alone from other interventions often bundled with CP (eg, hand hygiene).
Reference Fitzpatrick and Perencevich30
Due to the relatively rare detected transmission of MDRO organisms, large sample sizes are needed over long periods to optimally measure effectiveness,
Reference Khader, Thomas and Stevens31,Reference Blanco, Harris and Magder32
and modeling is often used as an adjunct to study transmission.
Reference Khader, Thomas and Stevens31
It is unlikely that large-scale clinical trials will ever obtain the requisite funding to fully study this issue, so most of the literature in this domain is limited to non-randomized, quasi-experimental studies. Recent data from the VHA, one of the larger data sources available to answer this question, continues to indicate that MRSA isolation practices are associated with lower rates of MRSA infection.
Reference Evans, Simbartl and McCauley33
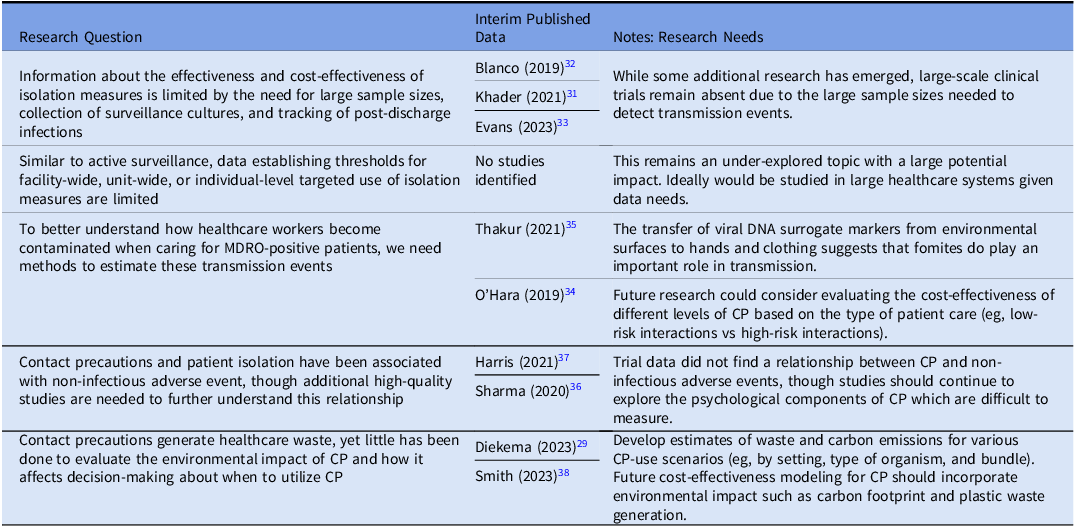
Due to the obstacles associated with studying CP, important questions remain unanswered (Table 2). A topic of great importance is establishing when to utilize targeted CP vs universal CP, as the ability to perform targeted interventions could result in significant cost-savings for healthcare organizations. Some recent work has been done to explore transmission of MDROs,
Reference O’Hara, Calfee and Miller34,Reference Thakur, Alhmidi and Cadnum35
and future studies could examine what level of CP is needed for certain types of patient interaction (eg, low-risk vs high-risk). Another aspect of the CP discussion relates to non-infectious adverse events. Data continues to emerge about the psychological aspects of patient isolation,
Reference Sharma, Pillai and Lu36
though trial data suggests that CP has minimal impact on non-infectious adverse events.
Reference Harris, Morgan, Pineles, Magder, O’Hara and Johnson37
Another non-infectious adverse event of increasing relevance is the environmental impact of contact precautions
Reference Diekema, Nori, Stevens, Smith, Coffey and Morgan29,Reference Smith, Singh and Sherman38
– this is an under-explored topic and research is needed to quantify the environmental impact of different CP scenarios (eg, universal vs targeted CP), and future modeling work should ideally incorporate environmental sustainability metrics (eg, carbon footprint, plastic waste burden) into cost-effectiveness models.
Table 2. Veterans Healthcare Administration research agenda for transmission prevention research: isolation measures
Hand hygiene
Hand hygiene remains the cornerstone of transmission prevention in healthcare settings and is the foundational horizontal intervention included in almost all prevention bundles.
Reference Bhatt and Collier39
The COVID-19 pandemic raised the profile of infection prevention and control, including hand hygiene, and was associated with higher healthcare worker hand hygiene compliance.
Reference Wang, Yang and Qiao40
However, in the majority of published studies hand hygiene rates remain low. For example, in the recent multinational trial involving intensive care unit central venous catheter bloodstream infections that included a bundled hand hygiene intervention, compliance only increased to 59%.
Reference van der Kooi, Sax and Grundmann41
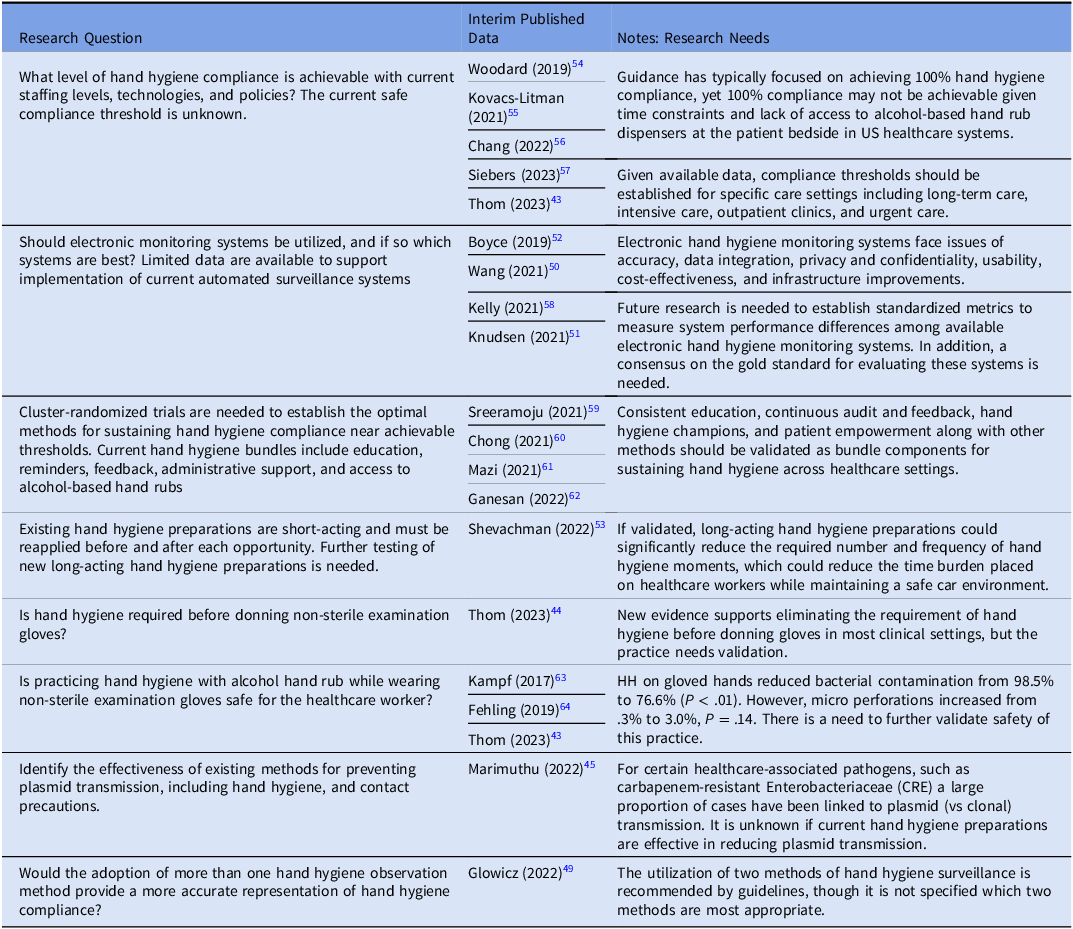
Thus, significant research focus on hand hygiene includes efforts to determine if 100% hand hygiene compliance is achievable with current policies and technologies. Table 3 lists several research questions for consideration.
Table 3. Veterans healthcare administration research agenda for transmission prevention research: hand hygiene
Although transmission-based precautions include other interventions such as contact precautions, discussed above, hand hygiene interacts with glove use in several ways. For example, current guidance requires practicing hand hygiene prior to donning non-sterile gloves and recommends against practicing hand hygiene with alcohol hand rub while wearing gloves.
42
However, recent studies suggest that hand hygiene prior to donning non-sterile examination gloves might be an unnecessary barrier in most settings and that allowing hand hygiene while wearing gloves greatly improves compliance compared to standard practice.
Reference Thom, Rock and Robinson43,Reference Thom, Rock and Robinson44
However, there are concerns with both practices that warrant further investigation in specific settings (ie, emergency rooms) and validating the safety for healthcare workers. Some MDROs such as CRE spread via plasmid transmission, with studies suggesting nearly half of CRE spreading via this mechanism.
Reference Marimuthu, Venkatachalam and Koh45
It is unclear if current hand hygiene methods or preparations are effective in reducing plasmid transmission in healthcare settings.
Direct observations remain the gold standard method for observing hand hygiene compliance in the healthcare setting. Historical and recent studies demonstrate that there is a clear Hawthorne effect, with an increase in hand hygiene compliance observed utilizing direct observations, which skews compliance rates.
Reference Purssell, Drey, Chudleigh, Creedon and Gould46–Reference Jeanes, Coen, Gould and Drey48
Due to this, recent guidance has suggested that utilizing two methods of observation may be appropriate and more effective. However, specific methods were not recommended,
Reference Glowicz, Landon and Sickbert-Bennett49
thus creating another research question and opportunity for research.
The utilization of automated hand hygiene surveillance systems continues to increase nationwide, but the effectiveness of these methods remains in question after multiple studies. Although increased compliance has been found when using automated methods, one of the glaring issues with automated systems is the lack of standardization of technology and the inability to accurately assess and compare technologies.
Reference Wang, Jiang and Yang50,Reference Knudsen, Kolle, Hansen and Møller51
This is in part due to lack of a gold standard to measure the quality and effectiveness, which makes it difficult to determine if the large cost and identified risks of using automated systems would be cost-effective for individual facilities or healthcare systems, such as the VHA. A large gap also needs to be bridged between accuracy of the data and intelligence of the system, with issues identified regarding an automated system’s lack of intelligence during clinical emergencies when high hand hygiene compliance may not be achievable.
Reference Boyce, Laughman, Ader, Wagner, Parker and Arbogast52
Hand hygiene bundles remain effective tools for increasing hand hygiene compliance in the hospital setting. These bundles are multifaceted interventions that include increased access to hand hygiene products, education, audit and feedback, and administrative support.
Reference Glowicz, Landon and Sickbert-Bennett49
Although these bundled interventions have been proven to be very effective in the short term, there is mixed evidence on what is needed to make them more sustainable. Some studies have found that champions and consistent re-enforcement have proven highly effective in sustaining hand hygiene compliance for several years. However, other studies have found that auditing and consistent meetings with healthcare workers did not sustain high hand hygiene compliance numbers. More work is needed to determine what factors influence long term sustainment of hand hygiene compliance.
One of the drawbacks to current hand hygiene solutions is that they are short-acting. There have been some recent trials on a new longer-acting hand prep solution which has been shown in clinical trials to inactivate COVID-19 and bacteria up to 4 hours after application with no reports of skin irritation.
Reference Shevachman, Mandal, Gelston, Mitragotri and Joshi53
This product and others should be evaluated using mixed-method hybrid study designs in various clinical settings to establish effectiveness and optimal implementation strategies.
Environmental cleaning/disinfection and management
The healthcare environment plays a key role in the transmission and persistence of healthcare-associated pathogens.
Reference Peters, Schmid and Parneix65
For instance, several recent publications have highlighted that patients are at higher risk of C. difficile infection (CDI) if the prior occupant of the room they are in in had CDI.
Reference Cohen, Cohen, Løyland and Larson66
Environmental management is a critical aspect of effective infection prevention, and it is essential for infection prevention and control teams to collaborate and partner closely with environmental management services (EMS) staff. The recent SHEA compendiums on MRSA and CDI prevention and the 2022 update on CDI prevention
Reference Popovich, Aureden and Ham12,Reference Kociolek, Gerding and Carrico67
provide a set of recommendations for cleaning of patient rooms and acknowledge that the quality of evidence for many of the recommendations is low. Cleaning and disinfection of patient rooms is a complex activity that is an interplay of several possible discrete tasks (daily vs at discharge, high-touch surfaces vs all surfaces), tools (such as microfiber cloths and a variety of available products), technologies (such as ultraviolet light), healthcare personnel (nursing vs EMS), and physical layout (single vs multiple-occupancy rooms, isolation vs non-isolation patients). Moreover, environmental cleaning and disinfection of a patient’s room must often be completed under intense time pressure to have the room ready for the next patient. EMS staff, the personnel at the center of this complex set of behaviors, are often underappreciated and undertrained.
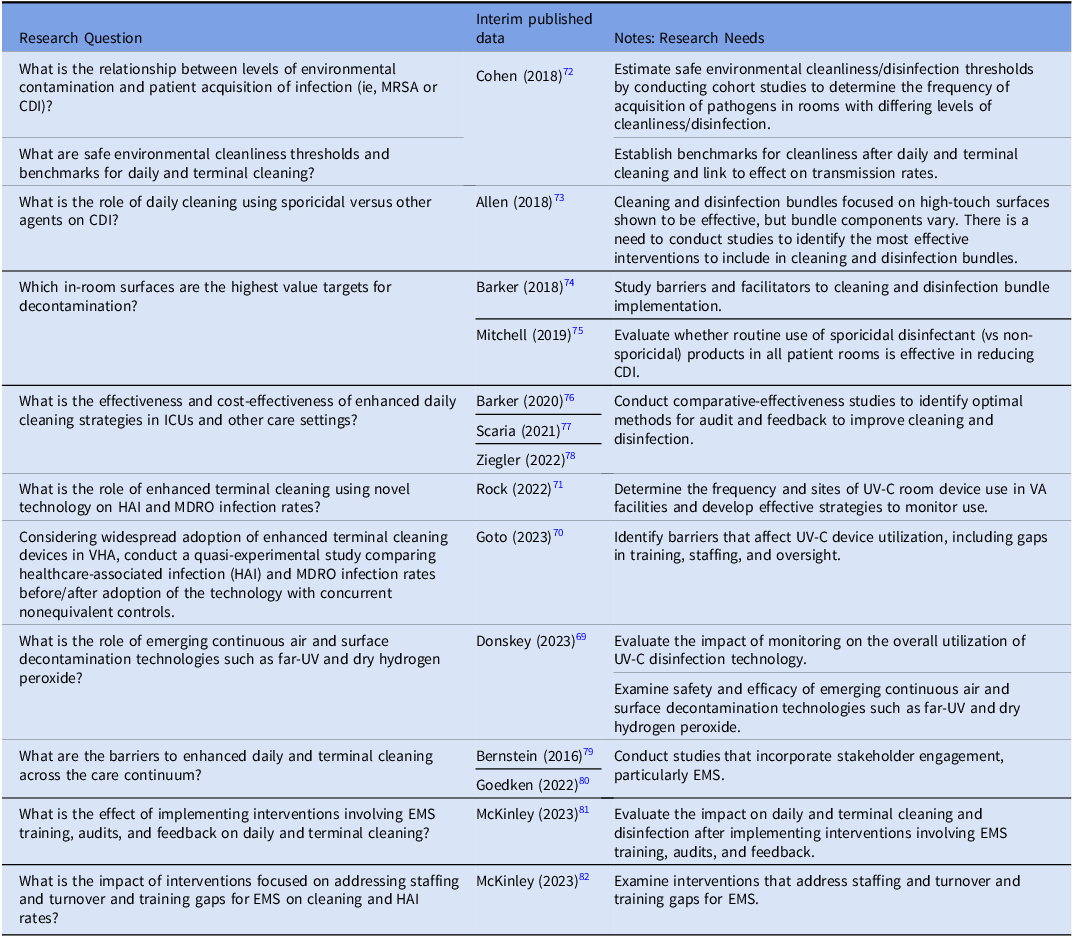
In the prior research agenda, several research gaps were identified, some of which have been addressed, but new, important questions have arisen (Table 4). For example, although it is increasingly evident that cleaning and disinfection are important for reducing the risk of infection to hospitalized patients, the intensity, frequency, technique, choice of product, and the role of novel existing and emerging technologies are all unanswered questions.
Reference Rutala, Donskey and Weber68,Reference Donskey69
For example, Ultraviolet technology has been studied with positive results for reducing some, but not all, pathogens. In the VHA System, use of Ultraviolet C (UV-C) was associated with a 19% lower incidence of hospital-onset gram-negative bloodstream infection,
Reference Goto, Hasegawa, Balkenende, Clore, Safdar and Perencevich70
but no decrease in hospital-onset CDI. Similarly, daily and at post-discharge UV-C added to standard cleaning and disinfection did not reduce VRE or CDI rates in non-VA cancer and solid organ transplant units.
Reference Rock, Hsu and Curless71
Table 4. Veterans Healthcare Administration (VHA) research agenda for transmission prevention research: environmental cleaning/disinfection and management
Future studies should systematically examine the set of complex interventions that constitute environmental management with input by stakeholders to address barriers to effective cleaning/disinfection and incorporate innovations in this area. These questions may be well suited to mixed-methods approaches. Moreover, a fundamental gap exists in our understanding of what constitutes effective environmental cleaning and disinfection as it relates to the risk of pathogen transmission and what are the optimal monitoring methods to adopt. It is also important to identify whether or not sporicidal agents are needed for routine daily cleaning and disinfection or whether they should be targeted for high risk areas or for certain pathogens such as C. difficile. Given the permutations possible in the various environmental cleaning bundles, simulation modeling could be very useful to identify and narrow down promising approaches for further testing in trials (ideally cluster-randomized trials).
Special populations and settings
Transmission prevention strategies vary by healthcare settings. Most research has focused on acute care settings, but patients receive care across multiple settings such as in nursing homes,
Reference Sturm, Flood, Montoya, Mody and Cassone83,Reference Wong, Huang, Wei, Wong and Kwok84
ambulatory care,
Reference Harris, Chandramohan, Awali, Grewal, Tillotson and Chopra85,Reference Reynolds, Sexton, Pivo, Humphrey, Leslie and Gerba86
home care,
Reference Keller, Hannum and Weems87–92
and specialty units such as dialysis
Reference D’Agata, Lindberg and Lindberg93–Reference Johansen, Gilbertson, Wetmore, Peng, Liu and Weinhandl98
and rehabilitation or spinal cord injury.
Reference Hughes, Evans and Ray99–Reference Ramanathan, Fitzpatrick and Suda101
Policies and general acute care guidelines focusing on prevention of healthcare-associated infections (HAIs) and MDROs may not be appropriate for these patient populations and settings. For example, patients with limited mobility such as those with spinal cord injury may depend on healthcare workers to enter rooms more frequently and have many more opportunities for patient contact than patients with more mobility. As a result, standard protocols may need to be modified for these types of interactions when MDROs are involved.
Reference Lones, Ramanathan and Fitzpatrick102
The evidence base on infection prevention in special populations or alternative care settings has evolved over the past five years, but significant research gaps remain.
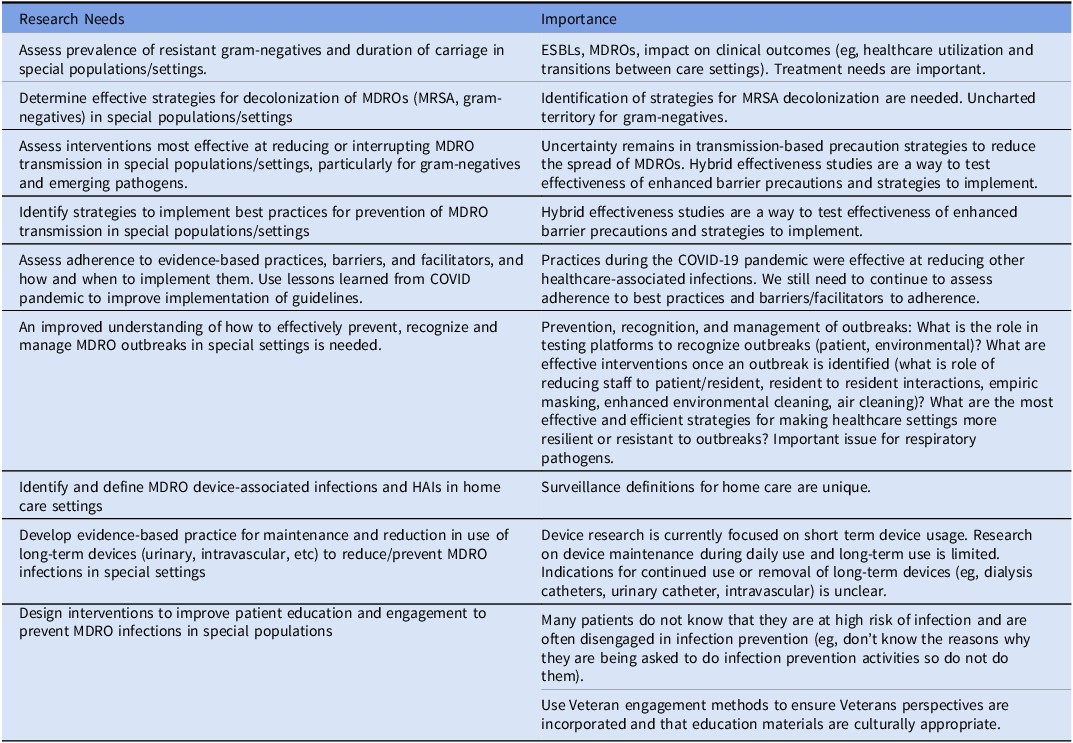
Special populations have cross-cutting themes across healthcare settings and populations in relation to MDROs. For example, there is a need for additional surveillance activities and definitions of infections in home care. The growing burden and morbidity due to multidrug-resistant gram-negative infections are of particular significance in patients with spinal cord injury and those in long-term care where there is a need to assess prevalence and risk factors and identifying interventions to reduce or interrupt transmission for infections caused by these organisms. Other areas of continued research needs include understanding outbreaks and using lessons learned from the COVID-19 pandemic (long-term care/rehabilitation, dialysis), developing evidence-based practice for maintenance, stopping use of long-term devices (home care) including dialysis catheters, and designing interventions that incorporate patient engagement (dialysis). Additionally, emerging evidence suggests inequities exist in who is affected by HAIs and MDROs (eg, by race, ethnicity, and rurality), but further study is needed to understand the drivers of these inequities.
Reference Chen, Khazanchi, Bearman and Marcelin103
Table 5 highlights the aforementioned research themes needed for special population settings.
Table 5. Veterans healthcare administration research agenda for transmission prevention research: special populations and settings
Biosurveillance
Biosurveillance, a systematic framework for the comprehensive monitoring, collection, analysis, interpretation, and dissemination of health-related data, plays a foundational role in MDRO epidemiology research.
Reference Wagner, Moore and Aryel104
Integrating large healthcare systems with comprehensive electronic health records (EHRs) opened the door for near-real-time data collection from diverse care settings, with VHA leading the nation by establishing the Corporate Data Warehouse (CDW), which includes health data from across the country.
105,Reference Kolodner106
CDW also includes microbiology data and has become a fundamental resource in MDRO epidemiology research and operations, including early detection of resistant strains, identifying hotspots and risk factors, tracking antimicrobial use, and evaluating the effectiveness of programs and policies.
Reference Goto, O’Shea and Livorsi5,Reference Khader, Thomas and Huskins107–Reference Nelson, Evans and Simbartl109
VHA’s informatics infrastructure can serve as a model for many large, multi-facility healthcare systems.
There are also several areas where VHA may be able to improve upon its groundbreaking efforts. First, most current biosurveillance activities within the VHA continue to rely on structured data elements from the EHR. However, there is a vast quantity of unstructured information also present in the EHR. Expanding utilization of unstructured data elements, such as free-text documentation by healthcare providers, is a potential avenue to conducting more comprehensive surveillance. Second, the expansion of the VHA community care program in recent years allowed Veterans to seek care outside of VHA and improved access to care,
Reference Schlosser, Kollisch, Johnson, Perkins and Olson110
but the lack of integration across information systems potentially creates fragmentation of care and gaps in health information, making longitudinal surveillance more challenging.
Reference Tsai, Orav and Jha111
Lastly, the data sets currently accessible to researchers are largely limited to patient-level health data and structural information (eg, facility characteristics). Although VHA collects operational data for healthcare environments (eg, facility water quality monitoring) or daily operations activities (eg, availability and consumption of PPE),
Reference Gamage, Jinadatha and Coppin112,113
research access to those data sources and integration with patient care data are relatively limited at this point.
To support advancement in healthcare epidemiology, there are several biosurveillance areas where improvements may be beneficial. First, the aforementioned unstructured data can be harnessed using natural language processing and large language models. VHA established the National Artificial Intelligence Institute (NAII) to facilitate the adaptation of advanced analytic technologies, which should be expanded to include healthcare epidemiology research.
Reference Atkins, Makridis, Alterovitz, Ramoni and Clancy114
Second, the timely integration of healthcare data from VHA and non-VHA community partners through improved interoperability and information exchange can help researchers understand the global picture of the VHA patient population. Third, expanded research access to existing environmental and operational data, as well as new modalities of disease surveillance if adopted by VA (eg, facility wastewater monitoring),
Reference Gamage, Jinadatha and Rizzo115
may help facilitate a better understanding of transmission dynamics. Fourth, expanded access to computational resources within the VHA firewall to support the efforts mentioned above is needed to advance science while protecting privacy and data security.
116
VHA recently launched the VA Enterprise Cloud, partnering with Amazon Web Services and Microsoft Azure platforms,
117
and expanding access to these cloud-based elastic computational resources can accommodate the needs of cutting-edge research. Lastly, VHA is in the midst of a monumental and unprecedented transition of its EHR system with a sequenced roll-out nationwide. These processes could potentially cause disruptions in data access and raise the need for new models of data collection, especially during the roll-out period, projected to last several years. This may hinder the ability of VHA researchers to conduct comprehensive and longitudinal analyses for some time into the future.
Conclusion
This document represents collaboration between national research and operations experts to identify key research goals in MDRO transmission prevention for 2024–2028. Subtopics include AS, contact precautions, hand hygiene, environmental cleaning/disinfection, special populations, and biosurveillance. There is great need for additional research in these areas, and the VHA is well suited to be a national leader in these MDRO transmission prevention domains.
Introduction
Patient-to-patient transmission and widespread use (and misuse) of antimicrobials has led to an increased incidence of antimicrobial-resistant bacteria, including multidrug-resistant organisms (MDROs). At present, MDROs worsen morbidity and mortality for patients, Reference Barrasa-Villar, Aibar-Remón, Prieto-Andrés, Mareca-Doñate and Moliner-Lahoz1 though there is concern that MDROs could represent a threat to the very core of our healthcare system, as pathogens resistant to all antibiotics continue to spread across the globe. In 2019 the CDC updated their antibiotic resistance threats report, which includes sobering data about the breadth of the problem in the United States, with 2.8 million yearly infections from antibiotic-resistant infections, and 35,000 yearly deaths from antibiotic-resistant infections. 2
National initiatives to slow the spread of MDROs have increased in their scope in the past decade. The White House released a National Action Plan for Combatting Antibiotic-resistant Bacteria (CARB) for 2020–2025, representing a broad collaboration across multiple government agencies. Broadly speaking, the goals of CARB are to slow the emergence and prevent the spread of MDROs through improved diagnostics, antimicrobial research/development, antimicrobial stewardship, and fostering international collaboration. 3
The Veterans Health Administration (VHA) is the largest integrated health system in the US, with over 1,300 care facilities serving over 9 million patients. Notably, the VHA has a long history of combatting MDROs through efforts to reduce patient-to-patient transmission. In 2007, the VHA implemented a methicillin-resistant Staphylococcus aureus (MRSA) prevention bundle which was associated with sustained declines in infection rates for not just MRSA, but other MDROs such as Clostridioides difficile and carbapenem-resistant Enterobacteriaceae (CRE). Reference Jain, Kralovic and Evans4,Reference Goto, O’Shea and Livorsi5
Given its national footprint, its prior history of combatting MDROs, and its involvement as a CARB collaborator, the VHA is a national leader in MDRO research efforts. In 2017, a collaboration among VHA researchers outlined an agenda for MDRO research within the VHA. That research agenda was set by 37 national experts and outlined the five-year research needs for combating MDROs, including transmission dynamics, antimicrobial stewardship, the microbiome, and special populations. Reference Perencevich, Harris and Pfeiffer6–Reference Kates, Tischendorf and Schweizer10
This document is a follow-up of the 2017 research agenda collaborative and is designed as a companion piece for an accompanying 2024 antimicrobial stewardship research agenda. Reference Livorsi, Drekonja and Eschevarria11 The primary goal of this collaboration is to assess research progress in the domain of MDRO transmission prevention since 2017 and utilize this information to identify current MDRO research needs for the VHA system over the next 5 years. When possible, there is a focus on research conducted within the VHA. Our hope is that this document will serve as a roadmap for VHA transmission prevention research during the next five years.
This transmission prevention research agenda is a collaboration between 20 VHA research leaders across the country. Committee members were divided into subgroups tailored to their areas of expertise, with subgroups formed around the core topics of active surveillance/isolation, hand hygiene, environmental cleaning/disinfection, special populations, and biosurveillance. Subgroups all met multiple times and performed independent literature reviews of their topic areas during a six month period, then identified high-need research areas in their respective domains. Once finalized, all topics were reviewed by all member-authors.
Active surveillance
Programs which screen patients to determine whether they are colonized with a specific organism, known as active surveillance (AS), are designed to monitor and control the spread of MDROs. When an organism of interest is detected via AS, it should prompt a response with patient isolation, decolonization, or another intervention with the goal of decreasing the risk of infection in the colonized patient and/or transmission of the organism to other patients. Active surveillance is a common vertical strategy to reduce transmission; Reference Jain, Kralovic and Evans4 for example, the recent joint practice recommendations from the Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and Association for Professionals in Infection Control and Epidemiology (APIC) lists AS as an “additional recommendation” for prevention of MRSA infections, meaning that AS should be considered in select locations and populations. Reference Popovich, Aureden and Ham12 However, evidence about the efficacy of AS and its associated interventions is conflicting, with some clinical trials showing no difference in acquisition of vancomycin-resistant enterococci (VRE) or MRSA when AS plus expanded use of barrier precautions was compared to no intervention. Reference Huskins, Huckabee and O’Grady13
In the prior research agenda, several research gaps were identified that still have not been sufficiently addressed (Table 1). For example, it is still unclear when AS is most cost-effective. Studies have occurred in various settings (such as intensive care units, acute care units), with various MDROs of interest (MRSA, VRE, etc), and with various bundles (AS + contact precautions [CP], AS + decolonization, AS + CP + decolonization), so there is great complexity in understanding when AS is most effective. Reference Kitano, Takagi and Arai14–Reference Mac, Fitzpatrick, Johnstone and Sander17 Future research should strive to improve the quality of this data, ideally with cluster-randomized trials, though realistically with less expensive cohort and quasi-experimental studies. Reference Perencevich and Lautenbach18 Modeling could serve as a useful adjunct, Reference Halloran, Auranen and Baird19 and recent literature has tended to focus on risk score models as a prediction tool for determining cost-effectiveness of AS, though the utility of these modeling methods has varied greatly. Reference Jeon, Chavda, Rennert-May and Leal20 An important example of how AS varies by setting can be seen in the peri-operative domain. The use of AS coupled with decolonization in the cardiac and orthopedic surgery settings is well established as a tool to reduce MRSA surgical site infections, Reference Schweizer, Perencevich and McDanel21,Reference Saraswat, Magruder and Crawford22 however apart from intra-abdominal surgeries, Reference Huttner, Robicsek and Gervaz23 the efficacy of AS plus decolonization protocols is lacking in other procedures. Additionally, given the potential benefit of AS plus decolonization protocols in non-operative settings, Reference Huang, Singh and McKinnell24,Reference Miller, McKinnell and Singh25 this is an area in need of further study.
Table 1. Veterans Healthcare Administration research agenda for transmission prevention research: active surveillance
Isolation measures
The principal tools of isolation are the use of contact precautions (CP) and patient cohort isolation. Contact precautions involve the use of personal protective equipment (PPE) such as gowns and gloves when healthcare workers enter a patient room, while cohort isolation involves moving patients colonized or infected with an MDRO to be separated from non-colonized and non-infected patients. These interventions can be implemented universally Reference Harris, Pineles and Belton26 or in a targeted approach (eg, guided by AS, or in a syndrome-based manner such as for patients with uncontained wounds). Reference Huang, Septimus and Kleinman27 From a research standpoint, a principle challenge is linking policy (eg, CP) to outcomes (eg, reduction in infection rates) given their distant temporal relationship.
MRSA is the best-studied organism in the domain of CP, and national guidelines favor the implementation of CP for MRSA. The aforementioned joint guidelines from SHEA/IDSA/APIC updated in 2022 recommend universal contact precautions for MRSA as an “essential practice” that should be adopted by all hospitals. Reference Popovich, Aureden and Ham12 It is worth mentioning that there remains active discussion about the necessity of universal CP for MRSA. Reference Morgan, Wenzel and Bearman28,Reference Diekema, Nori, Stevens, Smith, Coffey and Morgan29 This controversy primarily arises from inadequate data as well as the difficulty in separating the effect of CP alone from other interventions often bundled with CP (eg, hand hygiene). Reference Fitzpatrick and Perencevich30 Due to the relatively rare detected transmission of MDRO organisms, large sample sizes are needed over long periods to optimally measure effectiveness, Reference Khader, Thomas and Stevens31,Reference Blanco, Harris and Magder32 and modeling is often used as an adjunct to study transmission. Reference Khader, Thomas and Stevens31 It is unlikely that large-scale clinical trials will ever obtain the requisite funding to fully study this issue, so most of the literature in this domain is limited to non-randomized, quasi-experimental studies. Recent data from the VHA, one of the larger data sources available to answer this question, continues to indicate that MRSA isolation practices are associated with lower rates of MRSA infection. Reference Evans, Simbartl and McCauley33
Due to the obstacles associated with studying CP, important questions remain unanswered (Table 2). A topic of great importance is establishing when to utilize targeted CP vs universal CP, as the ability to perform targeted interventions could result in significant cost-savings for healthcare organizations. Some recent work has been done to explore transmission of MDROs, Reference O’Hara, Calfee and Miller34,Reference Thakur, Alhmidi and Cadnum35 and future studies could examine what level of CP is needed for certain types of patient interaction (eg, low-risk vs high-risk). Another aspect of the CP discussion relates to non-infectious adverse events. Data continues to emerge about the psychological aspects of patient isolation, Reference Sharma, Pillai and Lu36 though trial data suggests that CP has minimal impact on non-infectious adverse events. Reference Harris, Morgan, Pineles, Magder, O’Hara and Johnson37 Another non-infectious adverse event of increasing relevance is the environmental impact of contact precautions Reference Diekema, Nori, Stevens, Smith, Coffey and Morgan29,Reference Smith, Singh and Sherman38 – this is an under-explored topic and research is needed to quantify the environmental impact of different CP scenarios (eg, universal vs targeted CP), and future modeling work should ideally incorporate environmental sustainability metrics (eg, carbon footprint, plastic waste burden) into cost-effectiveness models.
Table 2. Veterans Healthcare Administration research agenda for transmission prevention research: isolation measures
Hand hygiene
Hand hygiene remains the cornerstone of transmission prevention in healthcare settings and is the foundational horizontal intervention included in almost all prevention bundles. Reference Bhatt and Collier39 The COVID-19 pandemic raised the profile of infection prevention and control, including hand hygiene, and was associated with higher healthcare worker hand hygiene compliance. Reference Wang, Yang and Qiao40 However, in the majority of published studies hand hygiene rates remain low. For example, in the recent multinational trial involving intensive care unit central venous catheter bloodstream infections that included a bundled hand hygiene intervention, compliance only increased to 59%. Reference van der Kooi, Sax and Grundmann41 Thus, significant research focus on hand hygiene includes efforts to determine if 100% hand hygiene compliance is achievable with current policies and technologies. Table 3 lists several research questions for consideration.
Table 3. Veterans healthcare administration research agenda for transmission prevention research: hand hygiene
Although transmission-based precautions include other interventions such as contact precautions, discussed above, hand hygiene interacts with glove use in several ways. For example, current guidance requires practicing hand hygiene prior to donning non-sterile gloves and recommends against practicing hand hygiene with alcohol hand rub while wearing gloves. 42 However, recent studies suggest that hand hygiene prior to donning non-sterile examination gloves might be an unnecessary barrier in most settings and that allowing hand hygiene while wearing gloves greatly improves compliance compared to standard practice. Reference Thom, Rock and Robinson43,Reference Thom, Rock and Robinson44 However, there are concerns with both practices that warrant further investigation in specific settings (ie, emergency rooms) and validating the safety for healthcare workers. Some MDROs such as CRE spread via plasmid transmission, with studies suggesting nearly half of CRE spreading via this mechanism. Reference Marimuthu, Venkatachalam and Koh45 It is unclear if current hand hygiene methods or preparations are effective in reducing plasmid transmission in healthcare settings.
Direct observations remain the gold standard method for observing hand hygiene compliance in the healthcare setting. Historical and recent studies demonstrate that there is a clear Hawthorne effect, with an increase in hand hygiene compliance observed utilizing direct observations, which skews compliance rates. Reference Purssell, Drey, Chudleigh, Creedon and Gould46–Reference Jeanes, Coen, Gould and Drey48 Due to this, recent guidance has suggested that utilizing two methods of observation may be appropriate and more effective. However, specific methods were not recommended, Reference Glowicz, Landon and Sickbert-Bennett49 thus creating another research question and opportunity for research.
The utilization of automated hand hygiene surveillance systems continues to increase nationwide, but the effectiveness of these methods remains in question after multiple studies. Although increased compliance has been found when using automated methods, one of the glaring issues with automated systems is the lack of standardization of technology and the inability to accurately assess and compare technologies. Reference Wang, Jiang and Yang50,Reference Knudsen, Kolle, Hansen and Møller51 This is in part due to lack of a gold standard to measure the quality and effectiveness, which makes it difficult to determine if the large cost and identified risks of using automated systems would be cost-effective for individual facilities or healthcare systems, such as the VHA. A large gap also needs to be bridged between accuracy of the data and intelligence of the system, with issues identified regarding an automated system’s lack of intelligence during clinical emergencies when high hand hygiene compliance may not be achievable. Reference Boyce, Laughman, Ader, Wagner, Parker and Arbogast52
Hand hygiene bundles remain effective tools for increasing hand hygiene compliance in the hospital setting. These bundles are multifaceted interventions that include increased access to hand hygiene products, education, audit and feedback, and administrative support. Reference Glowicz, Landon and Sickbert-Bennett49 Although these bundled interventions have been proven to be very effective in the short term, there is mixed evidence on what is needed to make them more sustainable. Some studies have found that champions and consistent re-enforcement have proven highly effective in sustaining hand hygiene compliance for several years. However, other studies have found that auditing and consistent meetings with healthcare workers did not sustain high hand hygiene compliance numbers. More work is needed to determine what factors influence long term sustainment of hand hygiene compliance.
One of the drawbacks to current hand hygiene solutions is that they are short-acting. There have been some recent trials on a new longer-acting hand prep solution which has been shown in clinical trials to inactivate COVID-19 and bacteria up to 4 hours after application with no reports of skin irritation. Reference Shevachman, Mandal, Gelston, Mitragotri and Joshi53 This product and others should be evaluated using mixed-method hybrid study designs in various clinical settings to establish effectiveness and optimal implementation strategies.
Environmental cleaning/disinfection and management
The healthcare environment plays a key role in the transmission and persistence of healthcare-associated pathogens. Reference Peters, Schmid and Parneix65 For instance, several recent publications have highlighted that patients are at higher risk of C. difficile infection (CDI) if the prior occupant of the room they are in in had CDI. Reference Cohen, Cohen, Løyland and Larson66 Environmental management is a critical aspect of effective infection prevention, and it is essential for infection prevention and control teams to collaborate and partner closely with environmental management services (EMS) staff. The recent SHEA compendiums on MRSA and CDI prevention and the 2022 update on CDI prevention Reference Popovich, Aureden and Ham12,Reference Kociolek, Gerding and Carrico67 provide a set of recommendations for cleaning of patient rooms and acknowledge that the quality of evidence for many of the recommendations is low. Cleaning and disinfection of patient rooms is a complex activity that is an interplay of several possible discrete tasks (daily vs at discharge, high-touch surfaces vs all surfaces), tools (such as microfiber cloths and a variety of available products), technologies (such as ultraviolet light), healthcare personnel (nursing vs EMS), and physical layout (single vs multiple-occupancy rooms, isolation vs non-isolation patients). Moreover, environmental cleaning and disinfection of a patient’s room must often be completed under intense time pressure to have the room ready for the next patient. EMS staff, the personnel at the center of this complex set of behaviors, are often underappreciated and undertrained.
In the prior research agenda, several research gaps were identified, some of which have been addressed, but new, important questions have arisen (Table 4). For example, although it is increasingly evident that cleaning and disinfection are important for reducing the risk of infection to hospitalized patients, the intensity, frequency, technique, choice of product, and the role of novel existing and emerging technologies are all unanswered questions. Reference Rutala, Donskey and Weber68,Reference Donskey69 For example, Ultraviolet technology has been studied with positive results for reducing some, but not all, pathogens. In the VHA System, use of Ultraviolet C (UV-C) was associated with a 19% lower incidence of hospital-onset gram-negative bloodstream infection, Reference Goto, Hasegawa, Balkenende, Clore, Safdar and Perencevich70 but no decrease in hospital-onset CDI. Similarly, daily and at post-discharge UV-C added to standard cleaning and disinfection did not reduce VRE or CDI rates in non-VA cancer and solid organ transplant units. Reference Rock, Hsu and Curless71
Table 4. Veterans Healthcare Administration (VHA) research agenda for transmission prevention research: environmental cleaning/disinfection and management
Future studies should systematically examine the set of complex interventions that constitute environmental management with input by stakeholders to address barriers to effective cleaning/disinfection and incorporate innovations in this area. These questions may be well suited to mixed-methods approaches. Moreover, a fundamental gap exists in our understanding of what constitutes effective environmental cleaning and disinfection as it relates to the risk of pathogen transmission and what are the optimal monitoring methods to adopt. It is also important to identify whether or not sporicidal agents are needed for routine daily cleaning and disinfection or whether they should be targeted for high risk areas or for certain pathogens such as C. difficile. Given the permutations possible in the various environmental cleaning bundles, simulation modeling could be very useful to identify and narrow down promising approaches for further testing in trials (ideally cluster-randomized trials).
Special populations and settings
Transmission prevention strategies vary by healthcare settings. Most research has focused on acute care settings, but patients receive care across multiple settings such as in nursing homes, Reference Sturm, Flood, Montoya, Mody and Cassone83,Reference Wong, Huang, Wei, Wong and Kwok84 ambulatory care, Reference Harris, Chandramohan, Awali, Grewal, Tillotson and Chopra85,Reference Reynolds, Sexton, Pivo, Humphrey, Leslie and Gerba86 home care, Reference Keller, Hannum and Weems87–92 and specialty units such as dialysis Reference D’Agata, Lindberg and Lindberg93–Reference Johansen, Gilbertson, Wetmore, Peng, Liu and Weinhandl98 and rehabilitation or spinal cord injury. Reference Hughes, Evans and Ray99–Reference Ramanathan, Fitzpatrick and Suda101 Policies and general acute care guidelines focusing on prevention of healthcare-associated infections (HAIs) and MDROs may not be appropriate for these patient populations and settings. For example, patients with limited mobility such as those with spinal cord injury may depend on healthcare workers to enter rooms more frequently and have many more opportunities for patient contact than patients with more mobility. As a result, standard protocols may need to be modified for these types of interactions when MDROs are involved. Reference Lones, Ramanathan and Fitzpatrick102 The evidence base on infection prevention in special populations or alternative care settings has evolved over the past five years, but significant research gaps remain.
Special populations have cross-cutting themes across healthcare settings and populations in relation to MDROs. For example, there is a need for additional surveillance activities and definitions of infections in home care. The growing burden and morbidity due to multidrug-resistant gram-negative infections are of particular significance in patients with spinal cord injury and those in long-term care where there is a need to assess prevalence and risk factors and identifying interventions to reduce or interrupt transmission for infections caused by these organisms. Other areas of continued research needs include understanding outbreaks and using lessons learned from the COVID-19 pandemic (long-term care/rehabilitation, dialysis), developing evidence-based practice for maintenance, stopping use of long-term devices (home care) including dialysis catheters, and designing interventions that incorporate patient engagement (dialysis). Additionally, emerging evidence suggests inequities exist in who is affected by HAIs and MDROs (eg, by race, ethnicity, and rurality), but further study is needed to understand the drivers of these inequities. Reference Chen, Khazanchi, Bearman and Marcelin103 Table 5 highlights the aforementioned research themes needed for special population settings.
Table 5. Veterans healthcare administration research agenda for transmission prevention research: special populations and settings
Biosurveillance
Biosurveillance, a systematic framework for the comprehensive monitoring, collection, analysis, interpretation, and dissemination of health-related data, plays a foundational role in MDRO epidemiology research. Reference Wagner, Moore and Aryel104 Integrating large healthcare systems with comprehensive electronic health records (EHRs) opened the door for near-real-time data collection from diverse care settings, with VHA leading the nation by establishing the Corporate Data Warehouse (CDW), which includes health data from across the country. 105,Reference Kolodner106
CDW also includes microbiology data and has become a fundamental resource in MDRO epidemiology research and operations, including early detection of resistant strains, identifying hotspots and risk factors, tracking antimicrobial use, and evaluating the effectiveness of programs and policies. Reference Goto, O’Shea and Livorsi5,Reference Khader, Thomas and Huskins107–Reference Nelson, Evans and Simbartl109 VHA’s informatics infrastructure can serve as a model for many large, multi-facility healthcare systems.
There are also several areas where VHA may be able to improve upon its groundbreaking efforts. First, most current biosurveillance activities within the VHA continue to rely on structured data elements from the EHR. However, there is a vast quantity of unstructured information also present in the EHR. Expanding utilization of unstructured data elements, such as free-text documentation by healthcare providers, is a potential avenue to conducting more comprehensive surveillance. Second, the expansion of the VHA community care program in recent years allowed Veterans to seek care outside of VHA and improved access to care, Reference Schlosser, Kollisch, Johnson, Perkins and Olson110 but the lack of integration across information systems potentially creates fragmentation of care and gaps in health information, making longitudinal surveillance more challenging. Reference Tsai, Orav and Jha111 Lastly, the data sets currently accessible to researchers are largely limited to patient-level health data and structural information (eg, facility characteristics). Although VHA collects operational data for healthcare environments (eg, facility water quality monitoring) or daily operations activities (eg, availability and consumption of PPE), Reference Gamage, Jinadatha and Coppin112,113 research access to those data sources and integration with patient care data are relatively limited at this point.
To support advancement in healthcare epidemiology, there are several biosurveillance areas where improvements may be beneficial. First, the aforementioned unstructured data can be harnessed using natural language processing and large language models. VHA established the National Artificial Intelligence Institute (NAII) to facilitate the adaptation of advanced analytic technologies, which should be expanded to include healthcare epidemiology research. Reference Atkins, Makridis, Alterovitz, Ramoni and Clancy114 Second, the timely integration of healthcare data from VHA and non-VHA community partners through improved interoperability and information exchange can help researchers understand the global picture of the VHA patient population. Third, expanded research access to existing environmental and operational data, as well as new modalities of disease surveillance if adopted by VA (eg, facility wastewater monitoring), Reference Gamage, Jinadatha and Rizzo115 may help facilitate a better understanding of transmission dynamics. Fourth, expanded access to computational resources within the VHA firewall to support the efforts mentioned above is needed to advance science while protecting privacy and data security. 116 VHA recently launched the VA Enterprise Cloud, partnering with Amazon Web Services and Microsoft Azure platforms, 117 and expanding access to these cloud-based elastic computational resources can accommodate the needs of cutting-edge research. Lastly, VHA is in the midst of a monumental and unprecedented transition of its EHR system with a sequenced roll-out nationwide. These processes could potentially cause disruptions in data access and raise the need for new models of data collection, especially during the roll-out period, projected to last several years. This may hinder the ability of VHA researchers to conduct comprehensive and longitudinal analyses for some time into the future.
Conclusion
This document represents collaboration between national research and operations experts to identify key research goals in MDRO transmission prevention for 2024–2028. Subtopics include AS, contact precautions, hand hygiene, environmental cleaning/disinfection, special populations, and biosurveillance. There is great need for additional research in these areas, and the VHA is well suited to be a national leader in these MDRO transmission prevention domains.
Acknowledgments
The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of the Veterans Health Administration, the US Government, and the listed academic affiliates.
Financial support
This work was supported in part by funds and facilities provided by the Center for Access and Delivery Research and Evaluation (CADRE) at the Iowa City Health Care System and by a Department of Veterans Affairs Quality Enhancement Research Initiative grant (QUE 20-016 to MR, CE, ENP). C.J.D reports research funding to his institution from Clorox and Ecolab.
Competing interests
All authors report no conflicts of interest relevant to the content of this manuscript.