The colonic microbiota ferment endogenous and undigested dietary sources of carbohydrates and proteins. In the proximal regions (caecum and ascending colon) carbohydrates are readily digested, more distally carbohydrates are in short supply and protein fermentation predominates(Reference Macfarlane, Macfarlane and Gibson1, Reference Macfarlane, Gibson and Cummings2). Protein fermentation is associated with negative end products such as ammonia, amines, phenols and indoles(Reference Macfarlane, Gibson and Cummings2). Such compounds are undesirable due to their potentially toxic nature(Reference Bajka, Clarke and Cobiac3). Ammonia, for example, has cytopathic cellular effects(Reference Lin and Visek4), through increasing cellular turnover and increasing vulnerability to DNA damage(Reference Macfarlane, Macfarlane, Gibson and Macfarlane5).
Studies of the microbiota of populations over 60 years of age have frequently indicated an altered bacterial composition towards that of a more proteolytic one(Reference Woodmansey6, Reference Mäkivuokko, Tiihonen and Tynkkynen7). The faecal flora changes have been observed to differ between populations within different countries(Reference Mueller, Saunier and Hanisch8). Lower levels of Clostridium cluster XIVa and Faecalibacterium prausnitzii, which are known butyrate producers, have been observed in older volunteers(Reference Mueller, Saunier and Hanisch8, Reference Rajilic-Stojanovic, Heilig and Molenaar9). The use of traditional culturing has indicated reduced bifidobacteria numbers and diversity in ageing groups(Reference Hopkins, Sharp and Macfarlane10, Reference Hopkins and Macfarlane11). In more recent studies, with the use of molecular characterisation techniques, Zwielehner et al. (Reference Zwielehner, Liszt and Handschur12) showed reduced bifidobacterial diversity in a cohort of people aged 78–94 years. A study on an Italian population of adults, elderly and centenarians found only the centenarians to have significantly lower bifidobacteria(Reference Biagi, Nylund and Candela13); however, Italian populations have previously been observed to have higher levels of bifidobacteria than other populations(Reference Mueller, Saunier and Hanisch8). A study by Rajilić-Stajonović did not observe differences in bifidobacteria numbers in the microbiota of young adults and the elderly(Reference Rajilic-Stojanovic, Heilig and Molenaar9); however, the younger group was from five different European countries(Reference Lay, Rigottier-Gois and Holmstrøm14), which is likely to make an impact on the microbiota(Reference Mueller, Saunier and Hanisch8). Furthermore, different DNA extraction techniques were used for the two groups. Mäkivuokko observed low levels of bifidobacteria in all volunteers when characterising the microbiota of young adults and the elderly. The authors attributed these low levels to a mismatch in the bifidobacteria primers used(Reference Mäkivuokko, Tiihonen and Tynkkynen7). The elderly microbiota does appear to show temporal stability(Reference Claesson, Cusack and O'Sullivan15); however, the Actinobacteria group, which encompasses Bifidobacterium spp., has been observed to be temporally unstable(Reference Rajilic-Stojanovic, Heilig and Molenaar9). Therefore, microbiota in the elderly and young adults have been observed to show alterations that coincide with dietary changes including increased protein intake(Reference Cai, Clark and Croft16) and activity changes such as reduced exercise levels(Reference Woodmansey, McMurdo and Macfarlane17, Reference Bartosch, Fite and Macfarlane18). These microbial and fermentation changes and instability may have a role to play in some of the health changes that occur while ageing, as they reportedly often coincide with increased susceptibility to gastroenterological infection(Reference Saunier and Doré19), immune function and chronic diseases such as colon cancer(Reference Cai, Clark and Croft16).
A prebiotic is ‘a selectively fermented ingredient that results in specific changes, in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health’(Reference Gibson, Scott and Rastall20). Galacto-oligosaccharides (GOS) are prebiotics that have been shown in human feeding studies to selectively stimulate the growth of bifidobacteria(Reference Silk, Davis and Vulevic21–Reference Bouhnik, Raskine and Simoneau24) and lactobacilli(Reference Ito, Deguchi and Miyamori25, Reference Gopal, Prasad and Gill26). Indeed, human studies on infants have shown GOS to offer benefits in terms of stool consistency, while increasing bifidobacterial numbers(Reference Fanaro, Marten and Bagna27); in adults, GOS have also been seen to alleviate symptoms of irritable bowel syndrome(Reference Silk, Davis and Vulevic21).
The aim of the present study was to evaluate whether GOS lead to a beneficial shift in the faecal microbiota in a population of men and women over 50 years of age, while beneficially affecting fermentation characteristics. This was assessed in vitro and in vivo.
Experimental methods
A total of thirty-nine volunteers aged 50–81 years (58·9 (sd 5·9) years), with a BMI of 19·7–38·4 kg/m2 (26·05 (sd 3·63) kg/m2), were recruited for the study. None of their first-degree relatives had bowel cancer while under 50 years of age. Volunteers were excluded on the grounds of receiving antibiotic therapy or medication active on the gastrointestinal tract within 3 months of the start of the study. Volunteers were asked not to consume prebiotic supplemented foods and to refrain from consumption of probiotics, such as yoghurts, before and during the study and for the run-in period of 4 weeks before the start of the study. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Reading Research and Ethics Committee. Written informed consent was obtained from all subjects. Of the volunteers, twenty-one were female and the remaining eighteen were male, of which three were current smokers. Two of the volunteers (both male) consumed antibiotics during the second treatment of the study; therefore, their results have not been included in the mean data or statistical analyses.
The prebiotic used was GOS (Vivinal GOS; FrieslandCampina Domo, Zwolle, The Netherlands). Volunteers were given this prebiotic within an orange juice preparation at a concentration of 4 g GOS per serving (comparable to 9 g Vivinal GOS syrup: 59 % GOS, 21 % lactose, 19 % glucose, 1 % galactose on DM with degrees of polymerisation ranging between 2 and 8). The unsupplemented placebo orange juice product had an identical taste and mouthfeel; however, it contained approximately 4 g less simple sugars than the Vivinal GOS juice preparation. The juices were consumed twice daily by the volunteers, preferably in the morning and evening (2 × 250 ml/d).
The present study was a double-blind, placebo-controlled crossover feeding trial. Volunteers were randomly assigned into two groups; one starting with the placebo juice preparation, the other with the GOS-containing juice. Volunteers consumed the products for 3 weeks, followed by a 3-week washout period (no treatment products consumed); the remaining treatment was consumed over the next 3 weeks, preceded by a final 3-week washout period. Volunteers were required to visit the University of Reading (Reading, UK) on six occasions in total and to provide a faecal sample at each visit (1 week before the start; on day 0; and after 3, 6, 9 and 12 weeks).
Volunteer diaries
Volunteers were asked to maintain a normal diet throughout the study and to complete detailed food diaries during any 3 consecutive study days. These diaries were analysed using Foodbase 3.1 (Institute of Brain Chemistry and Human Nutrition, University of North London, London, UK). Daily diaries with general questions concerning bowel habit and mood were also given to volunteers to complete.
Collection of faecal samples
Volunteers were provided with plastic containers in which to provide a fresh faecal sample on site at the Department of Food and Nutritional Sciences. Samples were placed into an anaerobic cabinet (H2:CO2:N2, 10:10:80 by volume at 37°C) and processed within 30 min of voiding. In the event that a volunteer was unable to provide a sample, a further day's supplement was issued and the volunteer returned a day later.
Faecal water isolation
A 50 % (w/v) faecal slurry in ice-cold PBS (0·1 mol/l, pH 7) was homogenised for 3 min. The slurry was immediately frozen at − 70°C where it was stored for up to 2 weeks. The sample was ultra-centrifuged at 65 000 g for 2 h at 4°C. The remaining supernatant was filtered initially through a 0·2 μm PVDF filter (Whatman, Kent, UK). Samples were stored at − 70°C until commencement of the comet assay.
Bacteriology
A 10 % (w/v) faecal slurry in anaerobic PBS (0·1 mol/l, pH 7) was made for assessment of bacteriology. The slurry was homogenised for 2 min. Faecal slurry (1 ml) was centrifuged for 5 min at 13 400 g. The pellet was frozen and used for DNA extraction, for quantitative PCR analysis. For fluorescence in situ hybridisation, the samples were processed and the analysis was conducted according to the method used by Probert et al. (Reference Probert, Apajalahti and Rautonen28), using the probes (Erec482, target group Eubacterium rectale–Clostridium coccoides 52°C: 5′-GCTTCTTAGTCAGGTACCG-3′(Reference Franks, Harmsen and Raangs29)) and CHIS150, target group Clostridium perfringens–histolyticum subgroup 50°C: 5′ TTATGCGGTATTAATCT(T/C)CCTTT 3′(Reference Franks, Harmsen and Raangs29)).
Bacterial DNA extraction
Bacterial DNA was isolated from the frozen pellet using a Qiagen (West Sussex, UK) stool kit according to the manufacturer's instructions. Initially, the pellet was incubated at 37°C for 40 min in enzyme solution (3 mg lysozyme, 100 units mutanolysin in 100 μl Tris–EDTA buffer (10 mm-Tris, 1 mm-EDTA)) to aid breakdown of the cell walls. Then, the solution was mixed with 1·4 ml stool lysis buffer and the Qiagen protocol followed.
Quantitative PCR
The quantitative PCR was conducted from an adapted method of Ritalahti et al. (Reference Ritalahti, Amos and Sung30). Briefly, 5 μl of DNA samples/standards were applied to each well. To this, 20 μl mastermix solution (Applied Biosystems, Foster City, CA, USA) including relevant primer sets and probes with 6-carboxyfluorescein (6-FAM) as a reporter fluorophore on the 5′ end, with dihydrocyclopyrroloindole tripeptide minor groove binder quencher on the 3′ end (Table 1). For total bacteria, SYBR Green mix was used(Reference Ritalahti, Amos and Sung30). The plate was covered with an optical adhesive cover (Applied Biosystems) and placed into the AB 7700 sequence detector (Applied Biosystems), which was used in conjunction with Sequence Detector System software (Applied Biosystems). Temperatures used for starting, denaturing, annealing and final temperatures were 50°C, 95°C, 60°C and 50°C, respectively. However, total bacteria had an annealing temperature of 58°C and lactobacilli an annealing temperature of 64°C. The denaturing and annealing sequence was repeated forty times. Primers used in the present study were designed by Nauta et al. (Reference Nauta, van den Heuvel and Veurink31, Reference Nauta, van den Heuvel and Veurink32), based on the 16 s ribosomal RNA sequences derived from the ribosomal project database.
Table 1 Quantitative PCR (qPCR) primer probes and reaction mixtures used for microbial enumeration*

* Reaction mixtures, 20 μl, were mixed with 5 μl of the DNA sample in a ninety-six-well plate, before commencement of qPCR. The probe and primer concentrations stated are final concentrations.
Faecal water genotoxicity
The single-cell gel electrophoresis assay was conducted on the baseline faecal water sample for all volunteers. The five volunteers whose baseline samples caused the most DNA damage to the HT29 cells were investigated for the remaining time points. Briefly, HT29 cells were harvested at a concentration of 2·5 × 107 cells/l. From this, 350 μl of cell suspension were mixed with 150 μl faecal water (i.e. 30 % as determined by calibration) or with controls. PBS solution was used as a negative control and H2O2 (75 μmol/l) as the positive damaging control. The comet assay was conducted according to the in vitro method of Gill et al. (Reference Gill, Haldar and Porter33). Within 48 h of completion of the assay, the cells were viewed using an epi-fluorescence microscope (Nikon epifluorescence; Nikon, Surrey, UK) at 400 × magnification and 100 cells scored for DNA intensity in head and tail area using Komet 5 software (Andor Technologies, Nottingham, UK). Results are expressed in terms of tail DNA intensity, which is the percentage of DNA that is able to migrate outside the general cell mass. The more the migration that occurs, the greater the DNA tail intensity and the greater the DNA damage.
Three-stage continuous culture fermentation
A second baseline sample of freshly voided faeces from three randomly selected volunteers was taken and used in three parallel three-stage continuous culture systems (adapted from Macfarlane & Gibson(Reference Macfarlane, Gibson, Mackie and White34)). The samples were diluted to a concentration of 20 % (w/v) in pre-reduced 0·1 mmol/l, pH 7·4, PBS solution, homogenised for 3 min, and then 100 ml of this faecal slurry added to each of three vessels of the three in vitro systems. The vessels were held at operating volumes of 280, 300 and 300 ml and pH values of 5·5, 6·2 and 6·8, respectively, representing the proximal, transverse and distal regions of the colon in terms of pH, transit time and nutrient availability. Anaerobic conditions were established by the pumping of oxygen-free nitrogen gas (15 ml/min) through each vessel of the continuous culture system. The bacteria were allowed to establish in each of the vessels for 24 h before the medium feed pump was started.
The medium used was as described by Macfarlane & Gibson(Reference Macfarlane, Gibson, Mackie and White34). Continuous culture systems were conducted at a retention time of 36 h. Samples were taken from the models after 13 d, and SCFA profiles monitored; steady state was determined through stabilising of the SCFA and branched chain fatty acid concentrations over 3 consecutive days. Steady state was reached following a minimum of ten turnovers (15 d) and the model was dosed with 4 g GOS, twice daily.
SCFA analysis
SCFA profiles were determined by GC as done previously by Pereira & Gibson(Reference Pereira and Gibson35).
Statistical analysis
By the use of a statistical power calculation, it was determined that at a significance level of 5 % (two-sided), a log change of 0·33 bacterial numbers could be detected, at a power of 90 %, with thirty-nine volunteers. This calculation was based on the assumption that baseline standard deviation is 0·42, which was as observed by Tuohy et al. (Reference Tuohy, Kolida and Lustenberger36) in younger adults.
It was calculated that for the comet assay, using samples from five volunteers would enable detection of a 15 % difference in tail DNA following treatment, at a probability of 93 %. This was based on the assumption that the within-subject standard deviation of genotoxicity was 5 %(Reference Nauta, van den Heuvel and Veurink32).
Before analysis, bacteriology data were log transformed. Bacteriology, genotoxicity and SCFA data were analysed using paired-sample t tests to compare the outcomes following consumption of the treatment and placebo preparations. Additionally, a general linear model was performed on the human subjects trial bacteriology data, using a post hoc Tukey test to determine changes following consumption of the placebo and treatment preparations.
For assessing the influence of different factors, such as carbohydrate intake or BMI, volunteers were grouped accordingly, and the groups were split based on their medians. On these smaller groups, a paired t test was conducted to assess the significance of changes post-placebo to post-treatment. When using these smaller groups (n 19), it was determined that at a significance level of 5 % (two-sided), a log change of 0·44 bacterial numbers could be detected, at a power of 90 %.
Results are reported as means with standard deviations. When the P-value was < 0·05, results were considered to be statistically significant. Minitab 14 (Lead Technologies Inc., Charlotte, NC, USA) was used for the statistical analysis.
Results
A dosage of 4 g GOS twice daily was very well tolerated, as there were no significant differences in stool consistency, intestinal bloating, abdominal discomfort, flatulence severity and frequency during GOS and control treatments (data not shown). Markers of mood remained the same throughout the two treatment periods. It was confirmed that there was no carry-over from the first leg of the crossover study by statistical evaluation using an ANOVA with a post hoc Tukey test, as there were no significant differences regarding the sequence of the placebo or GOS treatments for all of the factors analysed within the study.
Prebiotic treatment led to significantly more faecal bifidobacteria (9·16 (sd 1·09) log10 colony-forming units (CFU)/g) as compared to the placebo: (8·64 (sd 0·95) log10 CFU/g) (P =0·024 – Tukey test) (Table 2).
Table 2 Changes in faecal bacteriology in volunteers over 50 years undergoing 3-week placebo and 3-week galacto-oligosaccharides (GOS) intervention (4 g twice daily)*
(Mean values and standard deviations, n 37)

CFU, colony-forming units.
a,b Mean values with unlike superscript letters within a row were significantly different for each treatment (P < 0·05).
* Analysed by one-way ANOVA with Tukey's post hoc test.
Four Clostridium species – C. perfringens, C. paraputrificum, C. tertium and C. butyricum – were enumerated by quantitative PCR; however, they were found to be below the limit of detection (log 4·2 CFU/g); therefore, these are not included in the results.
The bifidobacterial increase following GOS consumption differed between men and women. With men, 0·32 (sd 1·23) log10 CFU/g more bifidobacteria were observed following intervention (n 16); with women, 0·67 (sd 1·16) log10 CFU/g more bifidobacteria were observed (n 21). The bifidogenic response was not significant in the men (P =0·31), but it was significant in the women (P =0·015) (data not shown).
The impact of BMI on the bifidogenic effects was assessed. Individuals under BMI 25 kg/m2 had significantly more bifidobacteria after GOS consumption (9·20 (sd 0·75) log10 CFU/g), relative to placebo (8·74 (sd 1·00) log10 CFU/g; P =0·05; n 24, fourteen females and ten males), while those over BMI 25 kg/m2 did not (9·08 (sd 0·59) log10 CFU/g compared to placebo 8·45 (sd 1·26) log10 CFU/g; P =0·12; n 13, seven females and six males) (data not shown).
Compared to the guideline daily amounts, the average nutritional intake of the volunteers was much lower for total energy and carbohydrate consumption, but was greater for protein (Table 3). The responses of volunteers ingesting more than the median of 200 g carbohydrate daily differed from those who ate less. In those eating more than 200 g carbohydrate daily, there were 0·28 (sd 1·44) log10 CFU/g more bifidobacteria following GOS intervention (P =0·46; n 15, eight females and seven males), and in those who ate less, there were 0·68 (sd 0·99) log10 CFU/g more bifidobacteria (P =0·0039; n 22, thirteen females and nine males), relative to placebo. The opposite was observed with those eating more protein. Those eating more than the median of 70 g protein daily had 0·65 (sd 1·12) log10 CFU/g more bifidobacteria following GOS intervention (P =0·025; n 19, twelve females and seven males), and those ingesting less had 0·40 (sd 1·26) log10 CFU/g more relative to placebo, which was not statistically significant (P =0·18; n 18, nine females and nine males) (data not shown).
Table 3 Nutritional intake of volunteers over 24 h, from data of 3 consecutive days and guideline daily amounts (GDA)
(Mean values and standard deviations, n 21 for women and n 18 for men)
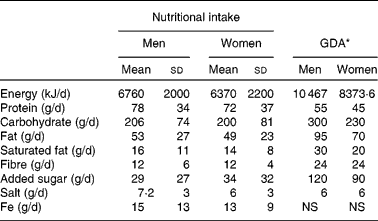
NS, not specified.
* Nutritional intake guide as published by the Institute of Grocery Distribution(65).
From the comet assay, tail DNA intensity ranged from 9·5 to 76·5 % (35·2 (19·1) %). Out of the thirty-seven volunteers, ten had a tail DNA intensity of < 50 %, five of which were < 30 %. The average tail DNA intensity of the three smokers was 23·9 % (range 19·3–27·2; sd 4·0 %). The comet assay was conducted on all samples from the five volunteers with the highest tail DNA intensity (all non-smokers).
There was variation in faecal water genotoxicity of all the volunteers (Table 4). Changes in genotoxicity did not always coincide with GOS treatment; however, with volunteers A, C and E, it did. A decline was seen before the treatment period for volunteer B and genotoxicity levels of volunteer D remained high throughout. The genotoxicity results showed no significant changes.
Table 4 Faecal water genotoxicity changes in volunteers over 50 years undergoing 3-week placebo and 3-week galacto-oligosaccharides (GOS) intervention (4 g twice daily)*
(Mean values and standard deviations, n 3 from screening with three technical replications, indicating assay variability)
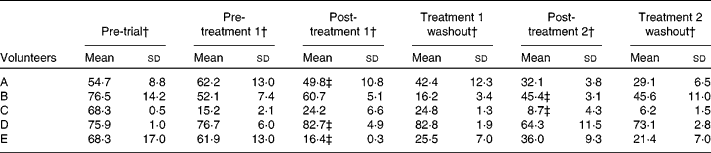
* Samples were analysed throughout the trial from five volunteers (A–E).
† Percentage of tail DNA.
‡ The highlighted values indicate the faecal water genotoxicity post-GOS treatment.
Multiple-stage continuous culture systems
Following GOS intervention within the continuous culture systems (Table 5), significant increases in bifidobacteria were identified in the three vessels (P =0·033; 0·033; 0·004, respectively, t test). In vessel 1, Lactobacillus significantly increased following the addition of GOS to the system (P =0·001). Within vessel 2, the number of Escherichia coli significantly declined (P =0·026).
Table 5 Bacterial populations as determined by quantitative PCR and fluorescence in situ hybridisation in in vitro continuous culture systems using galacto-oligosaccharides (GOS) as a substrate at 4 g twice daily
(Mean values and standard deviations, n 3 from three continuous culture systems; three different baseline volunteer faecal samples provided the bacterial inoculum)

CFU, colony-forming units.
* Mean values were significantly different from steady state 1 (pre-treatment; P ≤ 0·05).
† Steady state before treatment.
‡ Steady state following GOS treatment.
There was significantly more butyrate in all three vessels of the continuous culture system following dosing with GOS (Table 6). In vessel 3, significant reductions in propionate (P =0·008) and in the branched chain fatty acid iso-valerate (P =0·008) were observed following GOS dosing.
Table 6 SCFA profiles as determined by GC in in vitro continuous culture systems using galacto-oligosaccharides (GOS) as a substrate at 4 g twice daily
(Mean values and standard deviations, n 3 from three continuous culture systems; three different baseline volunteer faecal samples provided the bacterial inoculum

* Mean values were significantly different from steady state 1 (pre-treatment; P ≤ 0·05).
† Steady state before treatment.
‡ Steady state following GOS treatment.
Discussion
Intervention with prebiotics and synbiotics in a maturing population has previously been observed to offer potential benefits(Reference Vulevic, Drakoularakou and Yaqoob22, Reference Bouhnik, Achour and Paineau37–Reference Surakka, Kajander and Rajilić-Stojanović39). The present study used in vitro models alongside a prebiotic intervention human subjects trial. This study has utilised modern molecular techniques for microbial enumeration(Reference Wilson and Blitchington40), to provide a more quantifiably accurate approach to plating(Reference Apajalahti, Kettunen and Nurminen41).
A significant bifidogenic effect was observed following consumption of 4 g GOS twice daily, for this cohort of men and women over 50 years of age. Moreover, the increase was highest in subjects with the lowest basal levels of bifidobacteria, which corresponds well with previous studies(Reference Silk, Davis and Vulevic21–Reference Bouhnik, Raskine and Simoneau24). A difference of 0·5 log was observed between the placebo and the prebiotic; such a change is considered a major shift in the gut microbiota towards a potentially healthier composition(Reference Kolida and Gibson42). Unexpectedly, the bifidobacteria increase in the faecal microbiota of men was not significant (P =0·31); however, a 0·3 log10 CFU/g increase was observed.
The placebo product contained about 4 g less simple sugars, as compared to the GOS juice preparation/250 ml. This, however, is unlikely to make an impact on the results of the present study, as these sugars are likely to be digested in the upper gastrointestinal tract.
The lower-than-guideline daily amounts intake of energy and carbohydrate of the volunteers could be a reflection of inaccurate recording, or dietary modification over the days of diary entries. Volunteers were asked to maintain their normal diets throughout the study and to keep the diary recordings over 3 consecutive days, as this could help combat dietary alterations (as an individual is less likely to modify their diet for 3 consecutive days). There was a higher-than-guideline daily amounts intake of protein. A high protein intake is generally associated with a negative impact on the gut microbiota(Reference Bajka, Clarke and Cobiac3, Reference Toden, Bird and Topping43).
It has previously been observed that increased carbohydrate consumption leads to more carbohydrate reaching the colon(Reference Langkilde, Champ and Andersson44). In the present study, those consuming more carbohydrate were also consuming more fibre (P =0·013; n 19, twelve females and seven males). The bifidogenic effect in those eating < 200 g of carbohydrate daily, while not in those eating more, was therefore possibly a consequence of greater carbohydrate yields reaching the colon in the latter group. Previous studies on overweight and obese individuals have shown a reduced intake of carbohydrate to be accompanied by a decrease in bifidobacterial numbers(Reference Brinkworth, Noakes and Clifton45, Reference Duncan, Belenguer and Holtrop46). Lower bifidobacteria levels were not observed in the present study; however, it seems plausible that carbohydrate persisting to the large intestine non-selectively influences the fermentation characteristics within(Reference Muir and O'Dea47). As a consequence, additional fibre was not able to have the same magnitude of effect in >200 g/d carbohydrate consumers as in those consuming less.
It was observed that a significant bifidogenic effect occurred with greater protein consumption (>70 g/d; P =0·026), while the effect was not significant when less protein was consumed. Therefore, it is likely that more protein was reaching the distal colonic regions of these individuals(Reference Evenepoel, Claus and Geypens48), and was available for fermentation by proteolytic organisms. An increase in carbohydrate through prebiotic intake may therefore have had a greater impact on the nature of fermentation occurring within; and subsequently exerted a greater impact on the gut microbiota. As GOS acts in a selective way, these effects may be particularly on beneficial groups such as bifidobacteria. It therefore seems that GOS consumption led to an altered fermentation profile, particularly when higher protein levels were consumed, to a potentially more beneficial one. Duncan et al. (Reference Duncan, Belenguer and Holtrop46) have previously observed that in obese volunteers, diets rich in protein and low in carbohydrate lead to reduced numbers of bifidobacteria. Thus prebiotic intervention may have a greater impact in such individuals. The greater impact of prebiotics was confirmed in the present study.
When looking at the bifidogenic effect in terms of BMI, those under 25 kg/m2 saw a significantly bifidogenic effect, while those of the higher BMI group did not. An impact of the microbiota on metabolism has been previously observed (Cani & Delzenne(Reference Cani and Delzenne49)). Thus such differences may also influence the effect of a prebiotic. Future assessment of prebiotics as to whether a higher dose is required for those of a greater BMI may be of benefit.
Faecal water genotoxicity provides information on the carcinogenic potential within the distal colonic content(Reference Benassi, Leleu and Bird50, Reference Singh, McCoy and Tice51). This provides an indication of how diet may affect carcinogenesis risk(Reference Pearson, Gill and Rowland52). Cigarette smokers were not monitored throughout the study due to differences in the way they may respond to treatments(Reference Glei, Habermann and Osswald53). The faecal water data were inconclusive, as no significant changes were determined; this may be partially due to the variability of genotoxicity results obtained, and it seems likely that other lifestyle factors could be responsible for such changes(Reference Glei, Habermann and Osswald53). Indeed, the work of Pearson et al. (Reference Pearson, Gill and Rowland52) has shown that many studies indicate such variations. The present study allowed volunteers to continue their normal dietary and alcohol habits, although such levels of faecal water variation have also been observed in volunteers with controlled diets(Reference Oßwald, Becker and Grimm54). Further work on a greater number of individuals with higher baseline genotoxicity levels may better enable changes to be determined.
The use of an in vitro model provides a tool for looking at potential changes in other colonic areas, where bacterial growth at different pH, transit times and nutrient availability can be assessed. SCFA, which would normally be absorbed, can also be assessed, thus giving more of an indication of the effects of dietary intervention on SCFA levels. The in vitro data show significant bifidogenic effects in all three vessels of the continuous culture system, re-emphasising the ability of GOS to stimulate this potentially beneficial group of bacteria. The increase of lactobacilli observed in vessel 1 of the model is a potentially positive change, as this group is largely associated with positive health outcomes. Enhanced lactobacilli numbers following GOS consumption have previously been observed in adult volunteers(Reference Ito, Deguchi and Miyamori25, Reference Gopal, Prasad and Gill26). The group E. coli includes some opportunistic pathogens, and subsequently the decrease in this bacterial group in vessel 2 is also considered to be potentially positive. A decrease in E. coli in the presence of prebiotics has also been observed by Sharp et al. (Reference Sharp, Fishbain and Macfarlane55); such changes could be potentially caused by the inhibitory properties of bifidobacteria(Reference Gibson and Wang56).
A significant increase in the amount of butyrate in all three vessels of the continuous culture system provides further evidence of persistence. Bifidobacteria do not produce butyrate; however, Belenguer et al. (Reference Belenguer, Duncan and Calder57) observed that certain known butyrate producers (Eubacterium hallii and Anaerostipes caccae) utilise lactate from Bifidobacterium adolescentis during butyrate production, indicating the existence of a cross-feeding pathway. In addition, this observed butyrate stimulation could potentially result from an increase in acetate production, as acetate is utilised by some key butyrate-producing bacteria, such as F. prausnitzii and Roseburia spp.(Reference Duncan, Barcenilla and Stewart58). Butyrate is the preferred energy source for colonocytes(Reference Roediger59, Reference Hill60, Reference Singh, Halestrap and Paraskeva61) and has been linked to anti-cancer activities and inducing of apoptosis in damaged cells(Reference Knudsen, Serena and Canibe62). Similar increased concentrations of butyrate has been seen to lead to enhanced activities of butyrate as an inhibitor of histone deacetylation, hence assisting the regulation of the cell-cycle events(Reference Pool-Zobel, Selvaraju and Sauer63). Therefore, such an increase is potentially beneficial to the host.
Branched chain fatty acids are indicative of protein fermentation(Reference Macfarlane, Gibson and Cummings2), and thus their decline indicates that less proteolysis is occurring. Overall, it could be seen that the SCFA concentrations increased following GOS treatment, and that branched chain fatty acid concentrations decreased; thus a more saccharolytic environment was achieved. Therefore it seems, in vitro, GOS caused a distal shift in fermentation, to an environment considered more beneficial to the host.
Overall, the combined in vitro and in vivo results of the present study show bifidogenic and saccharolytic effects and the potential to act within more distal regions of the gastrointestinal tract. This indicates that GOS can be used as a selective ingredient to beneficially affect gut bacterial composition and to reduce the risk factors associated with shifts in the colonic microbiota and fermentation resultant of ageing. Consequently, GOS could promote well-being in a more mature population.
Acknowledgements
This project was made possible by the financial support of a Biotechnology and Biological Sciences Research Council, FrieslandCampina – research case studentship award. The product tested within this research was provided by the case funders for this project. E. G. H. M. v. d. H. and M. H. W. K. both worked as employees for the case funders. G. E. W., K. M. T., R. A. R. and G. R. G. have no conflicts of interest. G. E. W., K. M. T., G. R. G., R. A. R. and E. G. H. M. v. d. H. designed the research and were involved in the preparation of the manuscript; G. E. W. conducted the research; M. H. W. K. developed the quantitative PCR technique and associated probes used in this research. G. E. W. analysed the data; G. E. W., G. R. G. and E. G. H. M. v. d. H. wrote the paper. G. E. W. had primary responsibility for the final content. All the authors read and approved the final manuscript.








