INTRODUCTION
The number of visits by seniors to emergency departments (ED) has increased by 30% in recent years, 1 a population that needs more attention from ED health professionals, especially those who are frail. 2 Frailty is characterized by multi-systemic dysfunction associated with abnormal aging. It is linked to an increased risk of adverse outcomes and can be related to cognitive impairment,Reference Rockwood, Song, MacKnight, Bergman, Hogan and McDowell 3 which may affect patients’ ability to perform daily activities.Reference Lin, O’Connor and Rossom 4 Moreover, seniors with undetected cognitive impairment are at higher risk of an unplanned return to the ED within 6 months of their discharge.Reference Lee, Sirois and Moore 5 Cognitive and functional impairment, frailty, and delirium are all elements of “the geriatric syndrome” that also includes falls, incontinence, or immobility.Reference Inouye, Studenski, Tinetti and Kuchel 6
The numerous benefits of adapting a medical approach to the specific needs of seniors were recently underlined by the Acute Care for Elders (ACE) strategy. 7 In 2014, the American College of Emergency Physicians (ACEP) published new practice guidelines, also endorsed by the Canadian Association of Emergency Physicians (CAEP), suggesting that ED professionals should be trained to screen seniors for cognitive and functional impairment and delirium. 8 Those guidelines suggest that a visit to the ED is an opportunity to detect these often undiagnosed elements of the geriatric syndrome and to refer patients to the appropriate resources during their hospitalization or after their discharge.Reference Bernstein 9
ED-friendly tools must be developed to better fit the fast-paced ED environment.Reference Lin, O’Connor and Rossom 4 The Bergman-Paris Question (BPQ) is a one-question screening test that was developed by Dr. Howard Bergman and involves asking a patient’s close relative if they would feel comfortable leaving the patient home alone for three months if other members of the family were also away. Caporuscio et al. have reported that this single question could detect the presence of dementia in patients followed in a memory clinic.Reference Caporuscio, Monette, Gold, Monette and O’Rourke 10 To our knowledge, no other study has evaluated the BPQ.
The objective of this study was to assess the BPQ as a screening tool for three geriatric syndromes in independent or semi-independent seniors in the ED. Specifically, we sought to explore the predictive capacities of the BPQ for cognitive and functional impairments, as well as frailty.
METHODS
Study design and setting
The prospective assessment of the BPQ was a planned substudy of the incidence and impact measurement of delirium induced by ED stay (INDEED) multicentre study. This prospective observational cohort study enrolled patients in four Canadian EDs (Hôpital de l’Enfant-Jésus [Québec City], Hôpital du Sacré-Cœur [Montréal], Centre Hospitalier Affilié Universitaire Régional [Trois-Rivières], and Centre Hospitalier Régional de Lanaudière [Joliette]) between March and July 2015.
Selection of participants
Patients were included if they: 1) were aged ≥65 years; 2) were independent or semi-independent (can perform five of the seven activities of daily living without any help); 3) spent ≥8 hours in the ED; and 4) were admitted to any hospital ward. Patients were excluded if they: 1) were living in a long-term care facility; 2) were unable to consent; 3) were unable to communicate in French or English; 4) were experiencing an unstable medical condition leading to their admission to the intensive care unit (ICU); 5) had a previous diagnosis of severe dementia or any other psychiatric condition; or 6) had delirium during their eight-hour ED stay.
Procedure
Potentially eligible patients were identified using the hospital or ED information system patient tracking software. After an ED stay of more than or equal to eight hours, trained research assistants (RA) obtained consent and assessed patients for eligibility directly in the ED when admission was confirmed. Sociodemographic data, medications, and information regarding the patient’s comorbidities were also collected. The Charlson index was used to assess comorbidities,Reference Rozzini, Sabatini, Barbisoni and Trabucchi 11 and the Acute Physiology and Chronic Health Evaluation II (APACHE II) was used to evaluate the physiological status upon ED admission.Reference Knaus, Draper, Wagner and Zimmerman 12 An available relative was asked the BPQ after the assessment. Close relatives who were not seen in person were contacted by phone with the patient’s authorization soon after enrolment. RAs assessed participants’ cognitive, functional, and frailty status and also screened for delirium during the initial interview. The latter was also assessed twice a day during the patients’ whole ED stay and up to 24 hours after their ward admission, with a minimum of six hours between each evaluation. The study was approved by the Research Ethics Board of the CHU de Québec.
Outcome and measurement
A trained RA asked patients’ relatives the BPQ: “Would you be comfortable leaving your family member home alone for three months if you had to go on a trip to Paris and no other family member or close friend was available?”Reference Caporuscio, Monette, Gold, Monette and O’Rourke 10 An answer such as “No, I would not be comfortable” represents a “positive BPQ,” suggesting that the patient may have an underlying geriatric syndrome. In contrast, a “Yes” from the patient’s relative indicated a “negative BPQ” and may lead to the conclusion that this patient would not benefit from further geriatric assessment.
Cognitive status was assessed using the Telephone Interview for Cognitive Status–modified (TICS-m).Reference Knopman, Roberts, Geda, Pankratz, Christianson and Petersen 13 This test can be administrated in person or by telephone and evaluates orientation, attention, language, and memory. Scores range between 0 (worst) and 50/50 (best).Reference Duff, Tometich and Dennett 14 In this study, we determined that a score of ≤27/50 with adjustment for education suggested cognitive impairment.Reference Knopman, Roberts, Geda, Pankratz, Christianson and Petersen 13
Functional status was assessed using the validated Older Americans Resources and Services scale (OARS).Reference Fillenbaum and Smyer 15 , Reference Haywood, Garratt and Fitzpatrick 16 Patients answered based on how they usually performed their daily activities before their ED visit. Scores range from 0 (dependent) to 28/28 (completely independent). A two-point decrease represents complete loss of independence for one activity of daily living or partial dependence for two activities of daily living.Reference Sirois, Émond and Ouellet 17 This test is usually used to detect a potential functional decline. In this study, we used this test as an image of our participants’ functional status. With no specific guideline available in the literature, our steering committee decided that a score of <26 would suggest a clinically significant functional impairment.
Frailty status was evaluated using the Clinical Frailty Scale (CFS).Reference Rockwood, Song, MacKnight, Bergman, Hogan and McDowell 3 This scale is based on category descriptors ranging from 1 to 7 (1, very fit; 2, well; 3, managing well with controlled medical problems; 4, vulnerable; 5, mildly frail; 6, moderately frail; and 7, severely frail).Reference Rockwood, Song, MacKnight, Bergman, Hogan and McDowell 3 In our study, patients with a score of 1, 2, 3, or 4 were considered robust. In contrast, patients with scores of 5, 6, or 7 were classified as frail.Reference Rockwood, Song, MacKnight, Bergman, Hogan and McDowell 3 , Reference Kahlon, Pederson, Majumdar, Belga, Lau and Fradette 18 A revised CFS scale adds scores 8 and 9 representing very severely frail and terminally ill patients. As our participants were all independent or semi-independent, these two categories were deemed unnecessary for this study.
Delirium was assessed using the Confusion Assessment Method (CAM), a validated measure with excellent sensitivity (94% to 100%) and specificity (90% to 100%).Reference Inouye, van Dyck, Alessi, Balkin, Siegal and Horwitz 19 , Reference Monette and Galbaud du Fort 20 The CAM assesses the following: acute onset and fluctuation, inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbance, abnormal psychomotor activity, and altered sleep-wake cycle.
Analyses
Descriptive analyses were conducted for sociodemographic, clinical, and outcome variables, which were compared according to the BPQ response (Fisher test and t-tests for categorical and continuous variables, respectively). The Kolmogorov-Smirnov test was used to compare the distribution of outcomes variables with the BPQ response. Raw sensitivity and specificity of the BPQ for the four outcomes and its positive and negative predictive values were estimated with their exact binomial 95% confidence interval (95% CI).Reference Bishop, Fienberg and Holland 21 Crude and adjusted (age and sex) areas under the receiver operating curve (AUC) were also computed with 95% CIs and obtained through a logistic regression.Reference Hosmer, Lemeshow and Sturdivant 22 The risk of presenting outcomes if the BPQ was negative was estimated using an odds ratio (OR) with a logistic regression.Reference Hosmer, Lemeshow and Sturdivant 22 All analyses were completed with Statistical Analysis System software (SAS Institute, Cary, NC, version 9.4).
RESULTS
Participant’s characteristics
Of the 367 patients recruited in the INDEED study, 171 (47%) had a relative who answered the BPQ. There were no significant differences in sociodemographic data between patients for whom a BPQ answer was available and those without an answer. Patients’ characteristics were similar among the four centres. Table 1 details the participants’ characteristics. The BPQ was positive for 75% of the patients, indicating that most relatives were not comfortable leaving the participants home alone for three months. Patients with a positive BPQ were older (p=0.048). No significant differences in sex (p=0.21), living environment (p=0.21), or marital status (p=0.59) were found between patients with a “Yes” or “No” answer to the BPQ.
Table 1 Description of the sample
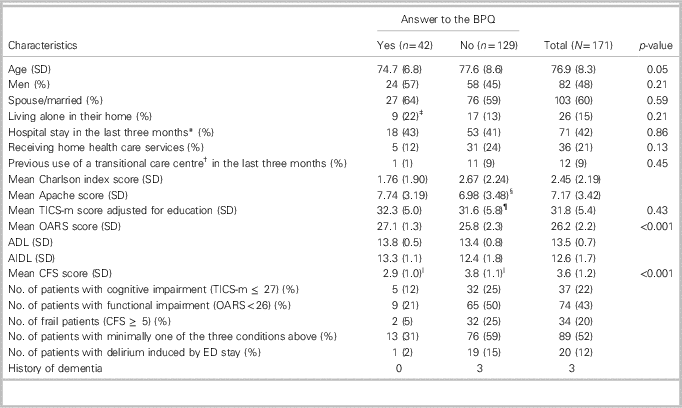
ADL=activities of daily living; CFS=Clinical Frailty Scale; IADL=instrumental activities of daily living; OARS=Older Americans Resources and Services; TICS-m=The modified Telephone Interview for Cognitive Status.
* Visit ED, day surgery, or hospitalization.
† Either rehabilitation centre, day hospital, or convalescence centre.
‡ One living environment missing.
§ Two data missing.
¶ One education missing.
ǁ One score missing.
BPQ screening capacity
To detect one of the three outcomes minimally, the BPQ had a sensitivity of 85.4% (95% CI 76.3-92.0), specificity of 35.4% (95% CI 25.1-46.7), positive predictive value (PPV) of 58.9% (95% CI 49.9-67.5), and negative predictive value (NPV) of 69.0% (95% CI 52.9-82.4). Incident delirium has not been included because this condition is a further consequence, and the BPQ was asked at the initial interview. Specifically, the predictive capacities of the BPQ for each outcome variables are reported in Table 2. The OR for a patient with a positive BPQ presenting with one of the three geriatric syndromes was 3.2 (95% CI 1.5-6.7), as compared with those with a negative BPQ.
Table 2 Predictive capacities of the BPQ for the four outcomes
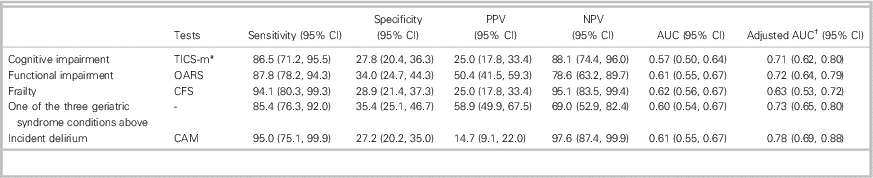
AUC=area under the curve; CFS=Clinical Frailty Scale; CAM=Confusion Assessment Method; NPV=negative predictive value; OARS=Older Americans Resources and Services; PPV=positive predictive value; TICS-m=Telephone Interview for Cognitive Status–modified.
* Adjusted for education.
† Adjusted for age and sex.
Cognitive impairment
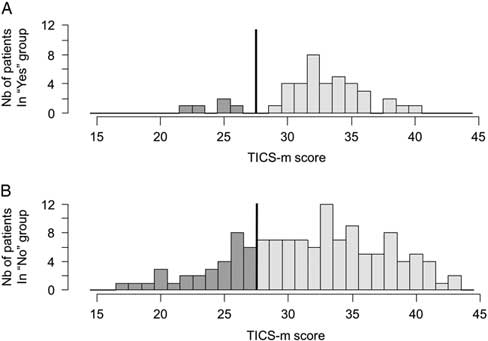
The BPQ had a sensitivity of 86.5% (95% CI 71.2-95.5) to detect cognitive impairment with a specificity of 27.8% (95% CI 20.4-36.3), a PPV of 25.0% (95% CI 17.8-33.4), and an NPV of 88.1% (95% CI 74.4-96.0). The distribution of TICS-m scores according to the relative’s response to the BPQ is shown in Figure 1. Because of the small sample size, they were not significantly different (p=0.10). However, in the negative BPQ group, the majority of patients were above the cut-off of 27/50 (Figure 1A), and the positive BPQ group had a heterogeneous distribution (Figure 1B). The TICS-m mean scores were neither statistically nor clinically significant between the yes and no groups (p=0.43).

Figure 1 Distributions of TICS-m values according to a negative (A) or positive (B) BPQ. The line illustrates the cut-off score of ≤27/50; the darker tone represents patients with possible cognitive impairments.
The OR for patients with a positive BPQ presenting with cognitive impairment was 2.5 (95% CI 0.9-6.8), as compared with those with a negative BPQ. However, approximately one-eighth of the patients with cognitive impairment remain unrecognized. Of note, three participants in our cohort had a past history of mild dementia. All three had a negative BPQ.
Functional impairment
Patients with functional impairment were efficiently detected using the BPQ with a sensitivity of 87.8% (95% CI 78.2-94.3), a specificity of 34% (95% CI 24.7-44.3), an NPV of 78.6% (95% CI 63.2-89.7), and a PPV of 50.4% (95% CI 41.5-59.3). Likewise, there were significant differences in mean OARS scores between seniors with a negative BPQ (mean [M]=27.1, standard deviation [SD]=1.3) and those with a positive BPQ (M=25.8, SD=2.3) (p<0.001). As shown in Figure 2A and 2B, 78% of patients with a negative BPQ were independent in their daily life (OARS score from 26 to 28/28), v. 48% if the BPQ was positive (p=0.009). The OR for patients with a positive BPQ presenting with functional impairment was 3.7 (95% CI 1.7-8.4), as compared with a negative BPQ.
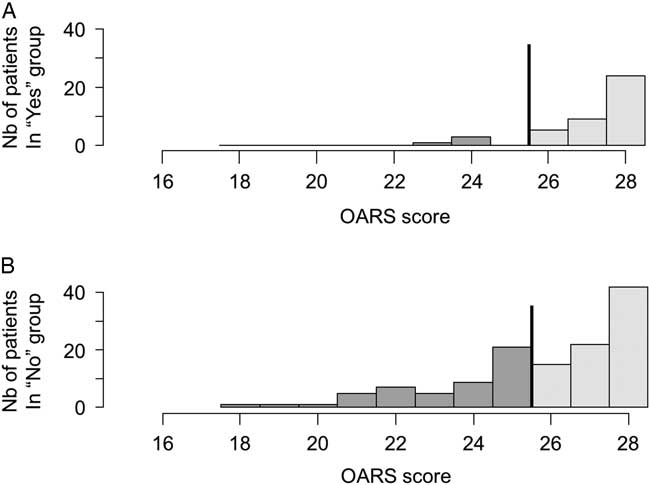
Figure 2 Distributions of OARS values according to a negative (A) or positive (B) BPQ. The line illustrates the cut-off score of <26/28; the darker tone represents patients with possible functional impairments.
Frailty
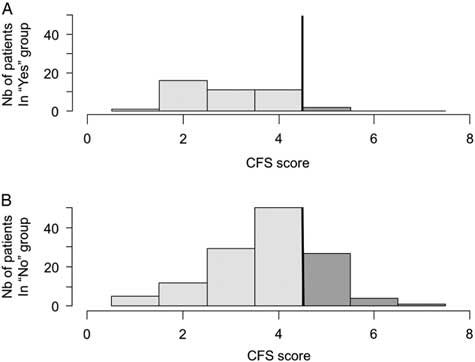
The BPQ detected almost all frail patients with a sensitivity of 94.1% (95% CI 80.3-99.3) but with a specificity of 28.9% (95% CI 21.4-37.3). People with a negative BPQ were almost all independent according to the NPV of 95.1% (95% CI -83.599.4). However, the BPQ had a PPV of 25.0% (95% CI 17.8-33.4) for frailty. Patients with a negative BPQ were more robust than those with a positive answer to the question (M=2.9, SD 1.0 v. M=3.8, SD 1.1, respectively, p<0.001). The distributions of frail patients across a negative and positive BPQ were significantly different (p=0.003) (Figure 3). The OR for being frail if the BPQ was positive was 6.5 (95% CI 1.5-28.6), as compared with a negative BPQ.
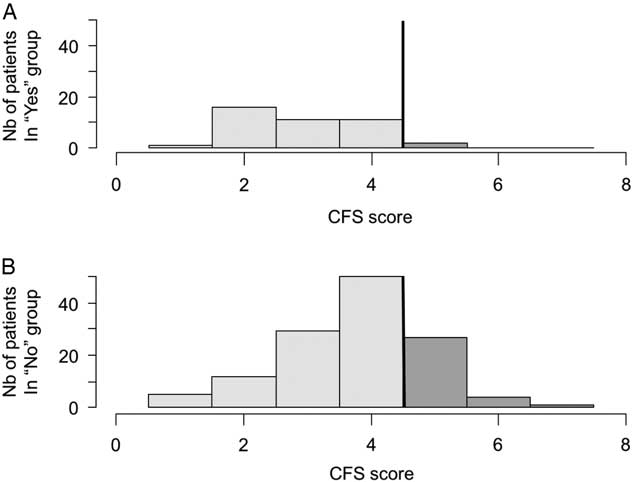
Figure 3 Distribution of CFS values according to a negative (A) or positive (B) BPQ. The line illustrates the cut-off score of <4/7; the darker tone represents possible frail seniors.
Incident delirium
A total of 19 of the 20 patients with an episode of incident delirium were detected by the BPQ leading to a sensitivity of 95.0% (95% CI 75.1-99.9]) and specificity of 27.2% (95% CI 20.2-35.0) (Table 2). From the 20 patients with delirium induced by an extended ED stay, only four were cognitively normal at the initial interview.
DISCUSSION
Key results
The BPQ had good sensitivity but a low specificity for detecting the three geriatric syndromes in our prospective cohort. Regarding cognitive impairment, our sensitivity result (86.5%) was similar to that of Caporuscio et al.,Reference Caporuscio, Monette, Gold, Monette and O’Rourke 10 but our specificity dropped significantly (27.8%) in our general ED population. They had a sensitivity of 95% (95% CI 82.9-99.2) and specificity of 63% (95% CI 40.8-80.4) for the diagnosis of dementia in a memory clinic, with a PPV of 0.820 (95% CI 0.681-0.910) and an NPV of 0.882 (95% CI 0.623-0.979). This could be explained by differences in our methodologies and populations. Their study was conducted in an outpatient clinic comprising patients with a higher prevalence of cognitive impairment than those in our study. Their study focused on dementia, but we broadened the aim of the study to expose four major geriatric syndromes. Furthermore, the diagnosis of cognitive impairment was completed by a geriatrician or neurologist comparatively to a screening tool (the TICS-m) in our study.
People with a negative BPQ were mostly independent and robust. These results suggest that these people are less likely to need further investigation for any geriatric syndrome. Logically, dependent patients with a lot of comorbidities will not be left alone by their relatives. Cognitive impairment, functional status, frailty, and delirium are all concepts linked together and regrouped as a geriatric syndrome. Unsurprisingly, our results demonstrate a link between these notions.
Clinical usefulness of the BPQ
In the ED, cognitive impairment detection is still not systematically done in day-to-day practiceReference Caporuscio, Monette, Gold, Monette and O’Rourke 10 , Reference Parke, Beaith, Slater and Clarke 23 possibly because of the important time and resource constraints in the ED. To address this gap, many validated tests are available, but they are lengthy. For example, the Mini Mental State Examination (MMSE) has a duration of approximately 10 minutes; even if it seems to be short, it can be excessive in the time-pressured ED environment.Reference Cullen, O’Neill, Evans, Coen and Lawlor 24 Some shorter tests such as the Brief Alzheimer’s Screen, Short Blessed Test, or Ottawa 3DY could be interesting alternatives, but all of these have a low specificity despite their high sensitivity.Reference Carpenter, Bassett and Fischer 25
The BPQ could be seen as a “red flag” to help emergency physicians focus on seniors more at risk. If a patient seems to be functional but has a positive screen per the BPQ, some explanation and further evaluation are probably required depending on the reason given as to the underlying problem.
What is making the BPQ more interesting than these other tests is the evaluation by a family member. Most geriatric patients come to the ED with a relative. Those relatives are a precious resource to an emergency physician or another health professional to obtain a good portrait of the patient’s status and to add information if necessary.Reference Goldstein, Travers, Hubbard, Moorhouse, Andrew and Rockwood 26
Despite its low specificity, its good sensitivity for geriatric syndromes may make the BPQ a good rapid front-line screening tool for ED professionals. The good NPV (79% to 98%) suggests that patients with a negative BPQ may not need further geriatric syndrome evaluation.
LIMITATIONS
There are limitations in this study. The presence of the patients at the moment when the BPQ was asked may have biased the relatives’ answer because the relatives may have been uncomfortable answering “No.” Conversely, most patients visiting EDs have an acute issue, and it probably overestimated the “No” results because of the anxiety and emotional involvement of family members regarding the actual condition.
Our point of reference to detect cognitive impairment was the TICS-m. This test is not the best tool to diagnose mild cognitive impairment or dementia.Reference Herr and Ankri 27 The choice of this tool and, moreover, uncertainty regarding the optimal cut-off could have led to the over- or under-diagnosis of cognitive impairment. With the high proportion of patients with a positive TICS-m in our population, a larger number of “No” responses to the BPQ would be expected. The same issues were experienced with the OARS scale cut-off. No study has fixed a score for patients with functional impairment. Currently available literature suggests a loss of two points indicates a functional decline, but our study was cross-sectional for functional status. Because our patients were relatively independent, we used this cut-off even if it may have overestimated the number of impaired seniors.
The response rate for the BPQ was 47%. The high rate of missing data is mostly because of the ED environment in which visitors are not always allowed for extended periods of time. As our patients were independent or semi-independent, not all of them were accompanied by a family member. Some patients also did not allow us to contact their family members for various personal reasons that were not obtained. However, potential selection bias has been rejected because no differences were found in patient characteristics between those who did and those who did not answer the BPQ. Finally, the inclusion and exclusion criteria may have reduced the prevalence of cognitive impairment. Because of the framework of the larger INDEED study, our study participants were independent or semi-independent, and all of them were admitted that is not representative of the general geriatric ED population.
A receiver operating characteristic (ROC) model adjusted for age and sex is better than the BPQ alone for cognitive impairment, functional status, and delirium. For frailty, the AUC for the BPQ alone is the same as the fitted model. Age is a confounding factor that is significantly associated with the BPQ.
In the future
This ED-tailored screening tool could be helpful in the overcrowded ED to determine which admitted patient should not have any further evaluation because patients with a negative BPQ have been identified as unimpaired and robust. The results of this study coincide with our primary hypothesis that the BPQ may have the ability to screen patients for major geriatric syndromes. We must not forget that the BPQ is only a screening test that can help the emergency physician in obtaining a second opinion from a person who knows the patient better. The usefulness of the BPQ for discharged patients should be assessed, and the question should be validated in all ED patients in future studies. Such validation could meet the need of emergency physicians to ensure that elderly patients can safely be discharged home. More investigations will be needed to determine the placement of the BPQ in the toolbox of emergency health professionals.
Acknowledgements: We would like to thank Pierre-Hugues Carmichael for performing the statistical analyses and all the RAs who participated in the recruitment of patients for this study. MÉ had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. He was responsible for the design, funding, and conduct of the study. AL led the analyses and wrote the manuscript. MJS, MCO, and MG were involved in the statistical analysis and data interpretation. MP, RD, EG, and ME were responsible for recruitment at all four sites. SB, MM, TTMV, JL, MR, and LJ are all collaborators of the INDEED project. VB managed the study and reviewed the manuscript. AAB, SB, MP, TTMV, VB, MG, MCO, MJS, PV, RD, and ME reviewed and approve the manuscript.
Competing interest: This study was funded by the Fonds de Recherche en Santé du Québec (FQRS 29307). A. Laguë holds a student salary award from the Canadian Frailty Network. Sponsor’s role: none.