Introduction
Alzheimer's disease (AD) has become the model disease for the whole dementia syndrome with predominant memory deficits (World Health Organization, 1994). Other forms of neurodegenerative dementias – dementia with Lewy bodies (DLB) and frontotemporal degeneration (FTD) – are characterized by different patterns of neuropsychological performance and behavioral disturbances (Neary et al., Reference Neary1998; McKeith et al., Reference McKeith2004). AD, DLB and FTD affect specific neuronal systems underlying different memory functions (Gabrieli et al., Reference Gabrieli1994; Moscovitch et al., Reference Moscovitch2005). Such differential effects on memory are associated with distinct effects on the perception of time. Concepts of time and their pathological change have not been considered as central issues in dementia research and care. This paper suggests that the altered perception of time in patients with different forms of dementia is largely neglected, and that clinical observations in dementia may improve our understanding of how the past, the present and the future are psychologically connected, a problem which philosophers have addressed repeatedly.
Hypotheses
I suggest the following two hypotheses:
(a) The pathology of different neurodegenerative diseases and their types of memory impairment determine the patients' concept of time and this is reflected in typical behavioral changes.
(b) A better understanding of these pathological conditions may contribute to the psychological and philosophical discussions on the nature of time.
Observations
Alzheimer's disease
Forgetfulness is the typical symptom early in the course of AD. It first affects declarative information, primarily “episodic” events, but also “semantic” content which has been experienced and learned, and which can – if remembered – easily be expressed in words (Tulving, Reference Tulving2002). A necessary prerequisite for explicit recall is the capability to learn, i.e. to obtain, understand and memorize information, and therefore an intact apparatus for the processing of declarative information (Takashima et al., Reference Takashima2006). Bottleneck structures of this apparatus in the medio-temporal lobe, entorhinal region and hippocampus are affected by Alzheimer-type plaque and neurofibrillary changes early in the course of this degenerative process (van Hoesen and Hyman, Reference van Hoesen and Hyman1990; Scahill et al., Reference Scahill2002). Therefore the learning of new information is impaired and these difficulties increase with ongoing neurodegeneration. During this process the intraneuronal neurofibrillary changes are far more focused on the structures of the temporal lobe responsible for declarative memory formation and retrieval than the widespread plaque pathology (Braak and Braak, Reference Braak and Braak1991). Activities of daily living are first impaired by an anterograde amnesia, which can also be verified by neuropsychological tests: from the onset of the first clinical symptoms most patients find it increasingly hard to keep track of what is going on, which leads to problems remembering events which have happened in the recent past, while remote history is usually well retained and accessible. After a longer course of illness with more intense and distributed brain changes, learning difficulties become more severe and include salient new events; remembering past events proves harder and other skills are also affected.
Several secondary deficits can be understood as logical consequences of amnesia (Harwood et al., Reference Harwood, Barker, Ownby and Duara2000). These include problems with temporal and spatial orientation, a process which relies on constant updates of what was intended and what has happened. This unsettling uncertainty of who (why, what, where, when) has done (become, happened, etc.) may cause anxiety, agitation and aggression (Förstl et al., Reference Förstl1993a; Reference Förstl1994). The patients' attempts at the best possible interpretation of this subjectively inexplicable experience can lead to delusional reasoning and to even greater cognitive and emotional turmoil. In advanced stages of AD, the access to large parts of the autobiographical memory are lost that normally provide personal continuity and the identity of the self (Levine, Reference Levine2004; Moscovitch et al., Reference Moscovitch2005). In emotional moments with focused attention, patients may become briefly aware of their lost past and personhood.
Familiar caregivers are not always recognized and must find a non-confronting style of comforting communication, which is preferably not based on spoken words, as they – if understood at all – would immediately be forgotten. Caregivers may find it difficult to cope with such declarative deficits during the early stage of dementia, but usually develop an appropriate understanding of the patients' main memory deficits in due course.
Dementia with Lewy bodies
A combination of Parkinson's and Alzheimer-type pathology may lead to a “dementia with Lewy bodies” (DLB) (McKeith et al., Reference McKeith2004; Perneczky et al., Reference Perneczky2007). Lewy bodies are the pathological hallmarks of Parkinson's disease just as plaques and tangles are the characteristics of AD. Clinically these patients suffer from the combination of a dementia syndrome together with recurring confusional states (Förstl et al., Reference Förstl1993b).
A confusional state can be considered as turbulence of the “stream of consciousness” (James, Reference James1890). One aspect of this stream of consciousness, the perception of the present, of the “moment”, is short-term-memory. When testing verbal or visual material, human short-term-memory covers around seven to eights seconds and seven to eight items. Outside critical situations and psychological laboratories, moments pulsating through our consciousness may be slightly shorter and hold less information (Pöppel, Reference Pöppel1997). When alert and attentive, humans can understand a complicated sentence with several clauses or reflect perceptions and intentions of several other individuals within a larger group. Language is one prominent attempt at making moments last. It can be compared with a “replay function,” which allows performers and listeners to repeat, learn and share experience. But all of these reflections take their own time, and the mirrors are never so well aligned that they can reflect one moment without distortion. Seemingly authentic recurrences of past events are infrequent and no sign of mental health (Aziz and Warner, Reference Aziz and Warner2005).
This stream of consciousness, with its big, “conscious” pictures usually accompanied by a running commentary and its minute minimal sensations (Leibniz, Reference Leibniz and Latta1714), corresponds to the momentary electrochemical oscillation of the whole brain. It includes the feeling, filtering, amplifying, comparing and eventually selecting of information most relevant to be attended to, and eventually even to be learned.
The decisive pharmacological deficit in DLB is a lack of the neurotransmitter acetylcholine, which is synthesized in the basal nucleus of Meynert (Perry et al., Reference Perry, Walker, Grace and Perry1999). This strategically important brainstem nucleus is affected not only by Alzheimer pathology but also by Lewy body pathology. Wakefulness, attention, filtering and learning are all critically dependent on acetylcholine, which in large parts of the brain can only be received from the basal nucleus. Acetylcholine improves attention and working memory performance (Furey et al., Reference Furey2000). Without acetylcholine, there is no “laminar” stream of consciousness, but disturbed vigilance, nightmares, and certainly no organized storage or retrieval of relevant information. Confusional states may initially manifest as disturbances of sleeping and dreaming, but do not usually cease after opening the eyes (Galanikis et al., Reference Galanikis2001). Patients will not usually remember explicitly what has happened during a confusional state, whereas caregivers will not easily forget such dramatic behavioral disturbances.
Frontotemporal degeneration
This neurodegenerative process, which is rarer than AD, affects the prefrontal cortex and frontal poles of the temporal lobes (Neary et al., Reference Neary1998; Johnson et al., Reference Johnson2005). They represent the phylogenetically youngest regions of the human neocortex dedicated to resolving interpersonal exchanges and taking care of one's future (Okuda et al., Reference Okuda2003). A large part of the brain's computational capacity is continuously devoted to controlling and improving social skills (Sodian et al., Reference Sodian2005). Sociobiologists discredit such perfectionist intentions of refining cooperation as attempts to improve the balance between our personal investment and benefit quite egotistically (Trivers, Reference Trivers1971). A vast fronto-dorsal cortico-subcortical circuit contributes to efforts usually summarized as intelligence (Alexander et al., Reference Alexander, Crutcher and Delong1990), its fronto-orbital counterpart deals with questions addressing reason, and the medial circuit produces behaviors generally associated with (free) will. Intelligence, or reason, or will are lost when these circuits are damaged selectively. If they collaborate in an intact brain, they achieve insight into complex social situations called “theory of mind” (Stone et al., Reference Stone, Baron-Cohen and Knight1998). The anterior paracingulate, superior temporal sulcus and bilateral anterior temporal poles are prominently involved in this complex task (Gallagher and Frith, Reference Gallagher and Frith2003), and they are predominantly affected in FTD. This “theory of mind” or “mentalizing” is the ability to see things from a different perspective, to understand how others view my actions, how they feel about it and will probably react to my potential actions (Buckner and Carroll, Reference Buckner and Carroll2006). Careful planning, taking others' actual views and collective experience accumulated over time into account means that an individual need not undergo every painful experience himself/herself. Imagining the potential consequences of one's intentions leads, more frequently than not, to inhibition, to refraining from certain actions.
Patients with FTD lose interest in many areas that require them to make a bit of an effort, but they do not shy away from faux pas (Gregory et al., Reference Gregory2002; Sturm et al., Reference Sturm, Rosen, Allison, Miller and Levenson2006). This loss of interest, of respect, and apathy toward the effect that one's actions may have, can occasionally lead to petty crime and rarely to more severe and fatal violations of social conventions (Diehl et al., Reference Diehl2006). These social deficits are associated with a lack of self-awareness (Eslinger et al., Reference Eslinger2005). On account of their “inability to will” and their lack of insight, these patients cannot be held responsible for what they have or have not done. This complete disintegration of future perspective and motivation in the course of the illness is usually accompanied by a loss of interest in the individual's personal past and basically in everything else (Diehl-Schmid et al., Reference Diehl-Schmid2006; Matuszewski et al., Reference Matuszewski2006). The patients' apathy or disinhibition, agitation and awkward behavior causes more caregiver distress than is observed with AD (de Vugt et al., Reference de Vugt2006), particularly because it appears almost incomprehensible that patients retain their knowledge and skills without hardly ever employing them.
Consequences
Neurodegenerative dementias are experiments of nature that affect extended brain areas and their functions in a systematic manner. Such lesion models illustrate the essential importance of different areas of the brain for certain neuropsychological subfunctions and behaviors. However, it would be incorrect to assume that other parts of a healthy brain are completely uninvolved in any of these tasks.
This evidence suggests that different parts of the brain are particularly relevant for certain aspects of time-related experience and behavior:
Hypothesis (a) – the pathology of different neurodegenerative diseases and their types of memory impairment determine the patients' concept of time and this is reflected in typical behavioral changes.
Past
Areas critical for the storage of long-term memory predominantly affected in AD are the medio-temporal lobe and the temporal and parietal neocortex; further parts of the brain degenerate in more advanced stages of dementia. In the earlier stages, patients are not confused and they are interested in their environment and their future.
Present
Confusional states as observed in DLB lead to a temporary inability to store and retrieve information in an organized manner, and are usually associated with other disconcerting symptoms. Most patients are anxious and absorbed with understanding their immediate present. Brainstem and the limbic system suffer from a double pathology – they cannot provide sufficient acetyl-choline and conduct the outer layers of the central nervous system directing them toward relevant signals.
Future
A degeneration of the large and evolutionarily recent fronto-subcortical loops first reduces subtle social skills and can eventually lead to acts of gross misconduct. Perception and short-term memory are intact but underused in often stoic bouts of consumption of television or junk food. The patient shows little interest in his own past, even though long-term memory is readily available – but now it has no purpose.
Hypothesis (b) – a better understanding of these pathological conditions may contribute to the psychological of philosophical discussions on the nature of time.
Augustine, writing in AD 400, mentioned that time was divided into the past, the present and the future. On reconsideration he felt that neither the past nor the future existed, but only the present together with the present of the past and the present of the future. Dummett (Reference Dummett2004) has summarized several theoretical positions regarding the reality and relevance of the past, present and future – including their complete irrelevance and non-existence:
• Only the present is real – but what are the limits, what is the frame of a moment?
• Present and future are real, but not the past – this position appears biologically interesting, as our survival and success depends on a clever planning and coping with future tasks, which is basically only backed up by past experience.
• The past – but not the future – is part of our present and both are real and relevant. Past and present offer the basis of different options for future developments as we experience them. But if this were true, would it imply that our experience and reality must expand constantly with time?
• And finally, the past and the future are realms of our reality just like the present, “determined at any moment by our temporal perspective” (Dummett, Reference Dummett2004).
In order to re-examine these positions, we can attempt a neurobiological arrangement of cognitive disturbances due to AD, DLB and FTD (Table 1). These examples demonstrate that confusional states – as observed in DLB – intermittently inhibit the formation of long-term memory and preclude organized retrieval from long-term memory. In spite of a clear stream of consciousness, patients with FTD have no interest in their future and make little use of their long-term memory. Patients in the early stage of AD are primarily handicapped by their problems with declarative long-term memory, but pay attention to the present and relate to future perspectives. Dummett (Reference Dummett2004), like Augustine, favors the key role of the present, with the future as the runner-up, well ahead of the past, a position which is illustrated in various forms of neurodegenerative dementias. In even simpler terms, one may conclude that the present is essential and characterizes a wakeful individual, whereas the past appears primarily interesting with respect to the future, while pure knowledge without purpose is irrelevant.
Table 1 Different forms of neurodegenerative dementia illustrate the interdependence of memory functions.
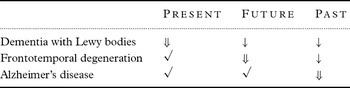
Note: ⇓ key deficit; ↓ secondary symptom; √ intact.
In summary, the frontal lobes are dealing with future aspects of behavior, posterior parts of the brain subserve the past, and the brainstem (including the limbic system) are essential for a laminar flow of consciousness. This simplistic interpretation is suggested by characteristic features of several prototypical forms of dementia. DLB is defined by recurrent confusional states, AD by difficulties of learning and recall, and FTD by a loss of imagination and responsibility. All parts of an intact brain are involved in perception, memory and behavior; observations on demented patients may, however, illustrate the distribution of complex tasks and the contribution of large brain areas to various aspects of behavior and underlying subjective experience. This may not only support Augustine's classical reasoning on the nature of time, but also improve the understanding of patients' “time frames” underlying their perception and behavior.



