Traumatic brain injury (TBI) is one of the leading causes of death and disability among children and adolescents worldwide. In the United States, it is estimated that pediatric TBI results in more than 630,000 emergency room visits, 60,000 hospital admissions, and 7,500 deaths annually.Reference Thurman 1 Sports and recreational activities account for a significant proportion of pediatric concussions and mild TBIsReference Cusimano, Cho and Amin 2 - Reference Zemek, Barrowman and Freedman 4 ; however, moderate and severe TBI in children and adolescents often result from other mechanisms such as motor vehicle collisions, falls, and nonaccidental trauma.Reference Thurman 1 , Reference Dewan, Mummareddy, Wellons and Bonfield 5 Although the modern management of acute pediatric moderate and severe TBI is aimed at addressing the primary and secondary pathological aspects of this injury,Reference Kochanek, Carney and Adelson 6 , Reference Scaife and Statler 7 the medical rehabilitation of surviving TBI patients requires a multidisciplinary, patient-centered approach to optimize functional recovery and patient outcomes.
In addition to motor, sensory, language, and cognitive impairments,Reference Anderson, Godfrey, Rosenfeld and Catroppa 8 - Reference Walker and Pickett 12 patients with TBI can also develop significant aerobic deconditioning that is a product of numerous factors including functional deficits, associated injuries, and prolonged immobility.Reference Bateman, Culpan, Pickering, Powell, Scott and Greenwood 13 , Reference Mossberg, Amonette and Masel 14 Previous studies have used a variety of exercise protocols to examine exercise tolerance in TBI patients, but these studies have been largely limited to adults who have undergone testing in the chronic stage of injury. These studies suggest that the aerobic capacity of adult TBI patients is reducedReference Bhambhani, Rowland and Farag 15 - Reference Jankowski and Sullivan 17 and that these patients demonstrate an impaired cardiorespiratory response to aerobic exercise.Reference Mossberg, Amonette and Masel 14 - Reference Mossberg, Orlander and Norcross 20 Despite these findings, there is no consensus on which exercise test or protocol is optimal for evaluating exercise tolerance in recovering adult or pediatric patients with moderate and severe TBI in the clinical setting.Reference Mossberg, Amonette and Masel 14
In contrast, graded aerobic treadmill testing has a well-established role in the evaluation and management of patients with concussion and persistent postconcussion syndrome. Evidence suggests that graded aerobic treadmill testing is a safe, well-tolerated, and clinically useful tool that can be used to evaluate exercise tolerance and guide the development of individually tailored submaximal exercise programs in adult and pediatric concussion patients.Reference Cordingley, Girardin and Reimer 21 - Reference Leddy and Willer 29 To date, however, there have been no studies that have examined the safety and tolerability of this clinical tool in recovering adolescent moderate and severe TBI patients.
Here, we present a retrospective case series of adolescent patients recovering from moderate and severe TBI who underwent graded aerobic treadmill testing at a multidisciplinary pediatric concussion program.
Methods
Ethics
This retrospective study was approved by the institutional research ethics board at the University of Manitoba.
Participants and Clinical Assessment
A retrospective chart review was performed for all consecutive pediatric moderate and severe TBI patients who underwent graded aerobic treadmill testing at the Pan Am Concussion Program at Pan Am Clinic, Winnipeg, Manitoba, Canada. The Pan Am Concussion Program is a multidisciplinary pediatric concussion program that primarily provides outpatient consultation and rehabilitation services to pediatric patients (age 19 years and younger) with sports- and nonsports-related concussion and mild TBI throughout the province of Manitoba and neighboring regions. The clinical team at the Pan Am Concussion Program comprises health care professionals with clinical training in TBI and neurorehabilitation such as experts in neurosurgery, neuropsychology, neurology, vestibular physiotherapy, exercise science, neuro-ophthalmology, and adolescent psychiatry. Therefore, the program occasionally receives referrals for pediatric patients recovering from moderate and severe TBI to aid in the optimization of out- and inpatient rehabilitation and guide return-to-learn and return-to-sports decision-making.
Accordingly, this study included patients who (1) were age 19 or younger, (2) were diagnosed with a moderate or severe TBI, and (3) underwent graded aerobic treadmill testing. Patients were not considered for graded aerobic treadmill testing if they were found to demonstrate motor, gait, or balance deficits that resulted in an inability to ambulate independently. Patients were also not considered for graded aerobic treadmill testing if they demonstrated language or cognitive deficits that resulted in an inability to provide informed consent or allow accurate rating of symptoms or perceived maximal exertion (RPE) during testing. In general, TBI patients were classified based on earliest reported Glasgow Coma Scale (GCS) score as moderate TBI patients (those with a GCS score of 9-12) and severe TBI patients (those with a GCS score of 8 or less). In cases were earliest reported GCS score was not documented in the medical records, TBI severity was based on the Mayo Clinic Classification for TBI severity, which includes other factors such as the presence of a loss of consciousness for 30 minutes or greater, posttraumatic amnesia for 24 hours or more, and the presence of traumatic abnormalities on neuroimaging.Reference Malec, Brown and Leibson 30
All patients underwent initial clinical consultation at the pediatric concussion program carried out by a single neurosurgeon. Emergency medical service, initial emergency department, and inpatient hospital records as well as neuroimaging studies were reviewed. All patients underwent a clinical history and physical examination, including evaluation of cranial nerve, motor, sensory, cerebellar, gait, balance, vestibulo-ocular, and cervical spine functioning. Clinical data points that were extracted included patient age at injury, sex, mechanism of injury, earliest reported GCS score, TBI severity, neuroimaging findings, associated cranial and extracranial injuries, functional motor and balance deficits on physical examination, and treadmill testing results.
Graded Aerobic Treadmill Testing
The objectives of graded aerobic exercise testing in this cohort were to (1) evaluate patient exercise tolerance, (2) establish safe intensity parameters for future exercise, and (3) develop an individually tailored submaximal aerobic exercise program for those who exhibited a symptom-limited threshold or evidence of aerobic deconditioning during treadmill testing.
Before graded aerobic treadmill testing, all patients underwent medical clearance by the neurosurgeon using the Physical Activity and Readiness Medical Examination.Reference Jamnik, Warburton and Makarski 31 Informed patient and parental consent was obtained before each test. Although previous research has shown that graded aerobic treadmill testing is safe and well tolerated in adolescent and adult concussion patientsReference Cordingley, Girardin and Reimer 21 , Reference Leddy, Baker, Kozlowski, Bisson and Willer 26 , Reference Leddy and Willer 29 as well as adult TBI patients,Reference Amonette and Mossberg 18 , Reference Mossberg, Ayala, Baker, Heard and Masel 19 , Reference Mossberg and Greene 32 there are limited studies of graded aerobic treadmill testing in pediatric moderate and severe TBI patients. All patients and their parents were informed of the potential risks of testing, which included temporary worsening or exacerbation of neurological symptoms, falling off the treadmill and sustaining further injury, seizure, and shortness of breath, and muscle soreness related to exercise.
Following medical clearance and informed consent, all patients underwent Buffalo Concussion Treadmill Testing (BCTT)Reference Kozlowski, Graham, Leddy, Devinney-Boymel and Willer 24 , Reference Leddy, Baker, Kozlowski, Bisson and Willer 26 performed by an experienced exercise physiologist and athletic therapist under the supervision of the neurosurgeon. All tests were performed by the same exercise physiologist and neurosurgeon. During testing, all subjects wore a Zephyr BioHarness (Zephyr Technology, Annapolis, MD) affixed to their chest that allowed continuous monitoring of heart rate (HR) throughout the test. Using a modified Balke protocol, patients performed an incremental exercise protocol until a symptom-limited threshold or maximal exhaustion was achieved (defined as an RPE of 18-20 on the Borg scale).Reference Borg 33 Previous studies using the BCTT have defined a symptom-limited threshold as a ≥3-point change in symptom report on a standardized Likert scale at any point during the test when compared with the patient’s pretest symptom level.Reference Leddy, Baker, Kozlowski, Bisson and Willer 26 , Reference Leddy and Willer 29 However, for use in this unique patient population, we chose to define a symptom-limited threshold as a ≥2-point change in symptom report compared with the patient’s pretest symptom level. Suggested starting speeds for the BCTT in concussion patients vary in the literature from 3.0 to 3.6 miles per hour (mph) and can be modified according to the patient’s size and fitness level.Reference Leddy, Baker, Kozlowski, Bisson and Willer 26 , Reference Leddy and Willer 29 , Reference Ellis, Leddy and Willer 34 For pediatric moderate and severe TBI patients, a starting speed of 3.2 mph was used but was modified on an individual basis depending on patient comfort. For each study, the test began with the patient walking at 0% grade for the first minute, after which the grade was increased by 1% per minute for the first 15 minutes. After 15 minutes, the speed was increased 0.2 mph/minute. Patients were asked to rate their symptoms and RPE every minute. Clinical reasons for test termination and complications of treadmill testing were also recorded. Total testing duration as well as maximal HR and blood pressure were collected following termination of treadmill testing. Age-predicted maximal HR was calculated using the equation (220 – patient’s age) and the HR measured at the time of test termination was used to calculate the percentage of age-predicted maximal HR achieved. Predicted VO2 peak was calculated using the American College of Sport Medicine walking equation. 35
Clinical Management and Follow-Up
Based on clinical presentation and testing results, selected patients were prescribed a tailored submaximal exercise program starting at 3 days and increasing to 5 days a week, which consisted of a 5-minute warm-up, 20 minutes of aerobic exercise at 80% of the maximum HR achieved during treadmill testing (or equivalent RPE), and a 5-minute cool down period. For patients who were receiving inpatient rehabilitation, patients were advised to perform the tailored submaximal aerobic exercise program under the supervision of a licensed heath care professional (i.e. physiotherapist). For those not receiving inpatient rehabilitation, patients were advised to perform the tailored submaximal aerobic exercise program under the supervision of a parent. Patient compliance with the submaximal exercise prescription was monitored through clinical follow-up and not formally with logbooks or digital watches. Follow-up appointments and repeat treadmill testing were scheduled as indicated by the treatment team and not according to a predesigned research protocol. Other targeted rehabilitative interventions were initiated as clinically indicated by the multidisciplinary concussion program team.
Statistical Analysis
The distributions of baseline characteristics for TBI patients who underwent graded aerobic treadmill testing were summarized using proportions for dichotomous and polytomous characteristics and means with standard deviations or ranges for continuous characteristics. Treadmill testing results and patient outcomes were tabulated.
Results
Study Participants
From October 22, 2014, to March 21, 2017, eight adolescent moderate or severe TBI patients were referred to the multidisciplinary pediatric concussion program. Seven patients were offered and agreed to undergo graded aerobic treadmill testing as part of their clinical management. The remaining patient was considered for graded aerobic treadmill testing, but was lost to follow-up before testing could be completed. Overall, the cohort included five females and two males with a mean age of 17.3 years (range, 16-19 years). Mechanisms of injury sustained by these patients included passenger-restrained motor vehicle collision (n=5), passenger-nonrestrained motor vehicle collision (n=1), and a fall from the third story of a building (n=1). Based on earliest reported GCS, one patient was classified as a moderate TBI patient and five were classified as severe TBI patients. Earliest reported GCS was not documented for one patient who was classified as a moderate TBI patient based on clinical history (40-minute loss of consciousness) and neuroimaging findings. Neuroimaging studies demonstrated evidence of structural brain injury in all patients (Figure 1). The most frequent neuroimaging findings were diffuse axonal injury (n=5), traumatic subarachnoid hemorrhage (n=3), intracerebral contusion (n=3), and acute subdural hematoma (n=3). Associated cranial injuries included petrous temporal bone fracture with conductive hearing impairment (n=1), occipital calvarial skull fracture and petrous temporal bone fracture with sensorineural hearing impairment and posttraumatic benign paroxysmal positional vertigo (n=1), stable occipital condyle fracture (n=1), complex maxillofacial and mandibular fractures requiring surgical reconstruction and tracheostomy (n=1), and frontal calvarial skull fracture and indirect traumatic optic neuropathy (n=1). Extracranial injuries among these patients included stable C1 vertebral fracture (n=1), subaxial cervical spine ligamentous injury, T4 vertebral transverse process fracture, spleen laceration (n=1), and nonoperative mesenteric root injury and pulmonary contusion (n=1). Based on physical examination findings, two patients were found to demonstrate subtle right-sided hemiparesis and incoordination, whereas five demonstrated mild impairments on balance testing. For additional features of the study cohort, see Table 1. The one patient diagnosed with posttraumatic benign paroxysmal positional vertigo underwent comprehensive evaluation by a vestibular physiotherapist and successful treatment with canalith repositioning before initial graded aerobic treadmill testing.
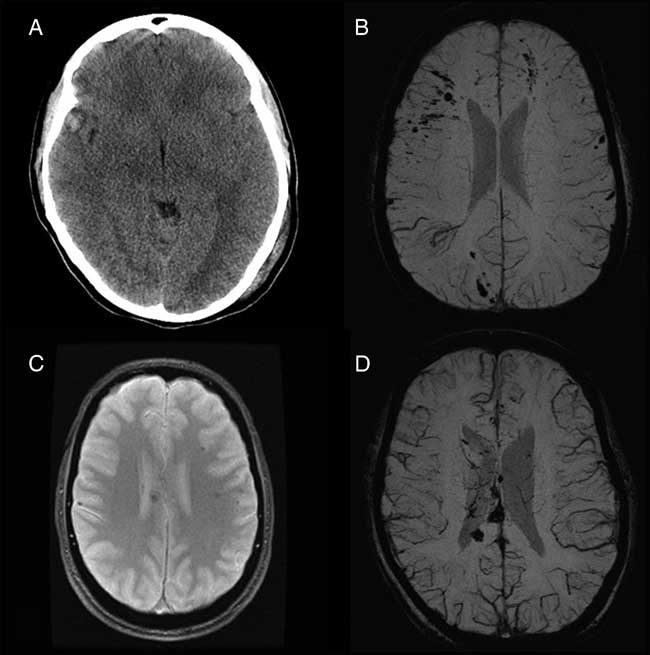
Figure 1 Representative neuroimaging findings among pediatric moderate and severe TBI patients who underwent graded aerobic treadmill testing. (A) Axial computed tomography scan in a 17-year-old female moderate TBI patient demonstrates a right temporal contusion (patient 3). (B) Axial susceptibility-weighted magnetic resonance imaging (MRI) scan in a 17-year-old female severe TBI patient demonstrates multifocal cortical and subcortical microhemorrhages consistent with diffuse axonal injury (patient 2). (C) Axial gradient recalled echo MRI in a 19-year-old male severe TBI patient demonstrates multiple hemorrhages within the corpus callosum and white matter consistent with diffuse axonal injury (patient 5). (D) Axial susceptibility-weighted MRI in an 18-year-old female severe TBI patient demonstrates multifocal microhemorrhages within the corpus callosum and white matter consistent with diffuse axonal injury (patient 6).
Table 1 Summary of demographics, clinical features, neuroimaging findings, and results from graded aerobic treadmill testing in pediatric moderate and severe TBI patients.
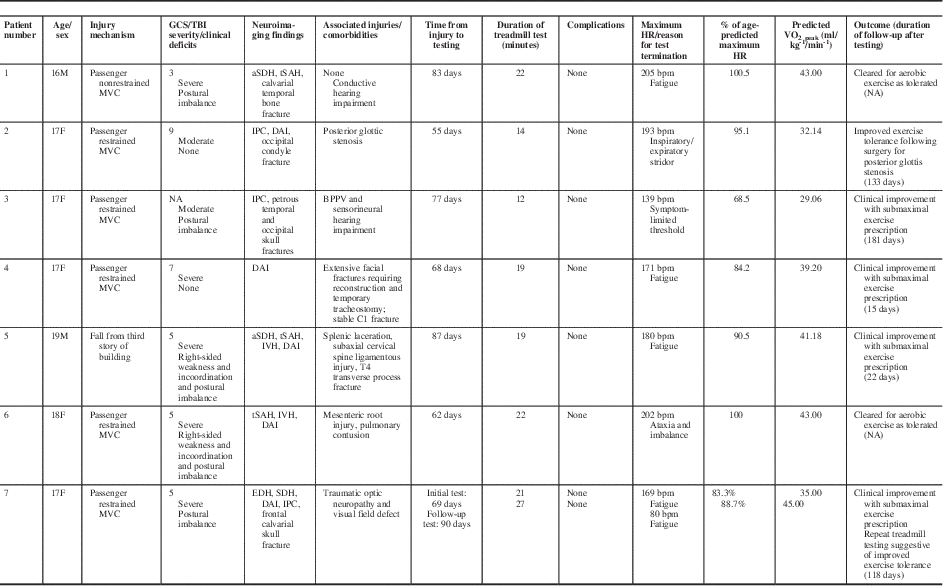
aSDH=acute subdural hematoma; BPPV=benign paroxysmal positional vertigo; DAI=diffuse axonal injury; EDH=epidural hematoma; F=female; IPC=intraparenchymal contusion; IVH=intraventricular hemorrhage; M=male; MVC=motor vehicle collision; NA=not available; tSAH=traumatic subarachnoid hemorrhage.
Safety and Tolerability of Graded Aerobic Treadmill Testing
In this study, six adolescent TBI patients completed a single graded aerobic treadmill test and one patient underwent two tests. The mean time from injury to initial treadmill testing for the entire cohort was 71.6 days (range, 55-87 days).
There were no complications related to treadmill testing among this cohort. The mean duration of all treadmill tests was 19.5 minutes (range, 12-27 minutes). Overall, 6/8 (75%) tests were well tolerated and allowed an accurate assessment of exercise tolerance. During initial treadmill testing, four patients terminated the test because of volitional exhaustion and one patient achieved a symptom-limited threshold during testing. In two patients, testing was terminated by the treatment team. One test was terminated for precautionary reasons because of patient ataxia and imbalance during light jogging after 22 minutes of exercise at an RPE of 17 (i.e. near maximal exhaustion). Another test was terminated early because of the development of mild inspiratory and expiratory stridor after 14 minutes of exercise. The mean maximum HR achieved during initial treadmill testing was 180 bpm (range, 139-205) resulting in a mean percentage-predicted maximum HR of 88.9% (range, 68.5%-100.5%) achieved during testing for the entire cohort. The percentage-predicted maximal HR achieved among patients who terminated the test because of fatigue ranged from 83.3% to 100.0%. The one patient who experienced a symptom-limiting threshold during testing achieved 68.5% of their age-predicted maximal HR. Although one test was terminated because of ataxia and imbalance, the patient achieved 100% of her age-predicted maximal HR. The patient whose test was terminated for stridor achieved 95% of her age-predicted maximal HR; however, it was unclear to what extent an increased work of breathing may have contributed to an increase in HR. The mean predicted VO2peak for this cohort during initial treadmill testing was 37.5 (range, 29.1-43 ml/kg-1/min-1).
As a result of graded aerobic treadmill testing, two patients were cleared to participate in aerobic exercise as tolerated. Four patients were treated with an individually tailored submaximal exercise prescription resulting in subjective improvement in residual symptoms (i.e. headaches, fatigue) and/or exercise tolerance on follow-up interview (Table 1). One patient treated with an individually tailored submaximal aerobic exercise program underwent repeat treadmill testing using the same test parameters and demonstrated results suggestive of improved exercise tolerance including longer test duration and higher maximum HR and VO2 peak achieved during testing. The patient whose treadmill test was terminated because of mild stridor was referred to an ear, nose, and throat surgeon, leading to a diagnosis of posterior glottic stenosis that was likely secondary to intubation during the patient’s initial hospital admission. This patient had been discharged from an inpatient rehabilitation hospital and had returned to exercise before undergoing graded aerobic treadmill testing. This condition was treated surgically resulting in complete resolution of the exercise-induced stridor. In clinical follow-up, the patient was able to resume aerobic exercise without any limitation or postconcussion symptoms.
Discussion
The results of this study provide preliminary evidence that graded aerobic treadmill testing is a safe, well-tolerated, and clinically useful tool to evaluate exercise tolerance in carefully selected adolescent moderate and severe TBI patients.
Exercise tolerance and aerobic capacity can be estimated in the clinical and laboratory setting by measuring cardiorespiratory function while a patient or subject is exposed to an incremental increase in aerobic exercise. Standardized tests used in TBI patients have included timed walking tests and treadmill- or exercise bike–based protocolsReference Bhambhani, Rowland and Farag 15 , Reference Hunter, Tomberlin, Kirkikis and Kuna 16 , Reference Amonette and Mossberg 18 , Reference Mossberg, Ayala, Baker, Heard and Masel 19 , Reference Mossberg and Greene 32 , Reference Mossberg 36 - Reference Vitale, Jankowski and Sullivan 38 ; however there is no accepted clinical standard for evaluating exercise tolerance in this patient population.Reference Mossberg, Amonette and Masel 14 Over the past 5 to 10 years, standardized graded aerobic treadmill testing has emerged as a safe, well-tolerated, and clinically useful tool that can be used to evaluate exercise tolerance in adult and pediatric concussion patients.Reference Cordingley, Girardin and Reimer 21 , Reference Darling, Leddy and Baker 22 , Reference Kozlowski, Graham, Leddy, Devinney-Boymel and Willer 24 - Reference Leddy and Willer 29
Previous research has shown that the BCTT demonstrates good test-retest reliability for evaluation of symptom-limiting HR in concussion patients with poorer reliability observed among other variables such as maximal systolic and diastolic blood pressure measurements and maximum reported perceived exertion. Importantly, the test demonstrates a sensitivity of 99% for identifying subjects with a symptom-limited threshold and a specificity of 89% for ruling out concussion symptoms with high interrater reliability (95%) for test interpretation across a variety of professionals.Reference Leddy, Baker, Kozlowski, Bisson and Willer 26 To date, however, the authors are not aware of any studies that have examined graded aerobic exercise testing among an exclusive cohort of adolescent moderate and severe TBI patients. In this study, there were no serious complications or adverse events among the adolescent moderate and severe TBI patients who underwent graded aerobic treadmill testing. Six of the eight tests were completed and allowed an accurate assessment of exercise tolerance. Although it is important for all pediatric concussion and TBI patients to undergo medical screening by a physician before graded aerobic treadmill testing, this is especially important in pediatric moderate and severe TBI patients who may have motor, sensory, cognitive, or balance impairments that can affect the safety of exercise testing. Furthermore, TBI patients can also harbor coexisting orthopedic, vascular, pulmonary, or hematological conditions that may also limit exercise capacity.Reference Alali, Scales and Fowler 39 - Reference Ravindra, Riva-Cambrin, Sivakumar, Metzger and Bollo 43 In this study, two treadmill tests were terminated by the treatment team. One test was terminated late in the study because of patient ataxia and imbalance secondary to previously known right-sided weakness. In another case, treadmill testing was terminated early because of onset of mild inspiratory and expiratory stridor resulting in early detection and management of previously unknown posterior subglottic stenosis. Taken together, these findings suggest that graded aerobic treadmill testing is safe and well tolerated in selected adolescent patients with moderate and severe TBI, but requires rigorous medical screening performed by a physician with clinical training in TBI and trauma and careful patient monitoring during testing by an experienced exercise physiologist.
Using a variety of exercise testing protocols, previous studies have demonstrated impairments in exercise tolerance and aerobic capacity as well as alterations in the cardiorespiratory response to maximal and submaximal exercise among TBI patients. It is estimated that the peak aerobic capacity is reduced to 65% to 74% of normal among adult TBI patients.Reference Bhambhani, Rowland and Farag 15 - Reference Jankowski and Sullivan 17 These patients also exhibit lower peak responses in HR, oxygen pulse, oxygen consumption, minute ventilation during exercise, and lower ventilatory anaerobic thresholds.Reference Mossberg, Amonette and Masel 14 - Reference Mossberg, Ayala, Baker, Heard and Masel 19 One study performed graded treadmill testing in a sample of adult TBI patients a mean of 10.4 months postinjury and found that only 61.5% of patients were able to reach greater than 90% of their age-predicted maximal HR during treadmill testing.Reference Mossberg, Ayala, Baker, Heard and Masel 19 The level of aerobic deconditioning and exercise intolerance observed among TBI patients is highly variable and is likely dependent on a number of factors, including patient age, preinjury fitness levels, TBI severity, associated neurological and extracranial injuries that limit mobility, length of initial hospitalization and immobility, the degree to which aerobic exercise is incorporated into postinjury rehabilitation, and the time at which the patient undergoes exercise testing relative to the date of injury. To date, most of the studies examining exercise tolerance in TBI patients have been limited to adults with moderate and severe injuries who have undergone testing on average 10 months to years after injury.Reference Mossberg, Amonette and Masel 14 - Reference Mossberg, Ayala, Baker, Heard and Masel 19 In this study of exclusive adolescent patients with predominantly severe TBI assessed less than 3 months postinjury, we found surprisingly good aerobic exercise tolerance in most patients. Although we did not perform comprehensive assessment of cardiorespiratory physiology, all patients were capable of exercising for periods that were similar to those achieved by adult and pediatric concussion patients during treadmill testing,Reference Cordingley, Girardin and Reimer 21 , Reference Kozlowski, Graham, Leddy, Devinney-Boymel and Willer 24 , Reference Leddy, Kozlowski, Donnelly, Pendergast, Epstein and Willer 28 and four of the seven patients reached greater than 90% of their age-predicted maximal HR. However, predicted VO2 peak values were below those reported in healthy adolescents,Reference Grassi, Turci and Sforza 44 suggesting that there is a level of deconditioning in adolescent patients with moderate and severe TBI.
In concussion patients with clinical evidence of aerobic exercise intolerance (i.e. autonomic/physiological postconcussion disorder), emerging evidence suggests that tailored aerobic exercise may be an effective treatment option to reduce concussion symptoms and enhance clinical and physiological recovery. Laboratory studies suggest that exercise may promote TBI recovery through the anti-inflammatory, neuroprotective, and neuroplastic effects of proteins such as brain-derived neurotrophic factor as well as through restoration of autonomic nervous system and cerebrovascular function.Reference Leddy, Hinds, Sirica and Willer 25 , Reference Archer 45 - Reference Griesbach 47 In adult and pediatric concussion patients, the results of graded aerobic treadmill testing can be used to design individually tailored submaximal exercise programs. Studies suggest that submaximal exercise prescription leads to a high rate of symptomatic improvement and return to full functioning in adult and pediatric autonomic/physiological postconcussion disorder patients (70%-90%).Reference Cordingley, Girardin and Reimer 21 , Reference Leddy, Hinds, Sirica and Willer 25 , Reference Leddy, Kozlowski, Donnelly, Pendergast, Epstein and Willer 28 , Reference Baker, Freitas, Leddy, Kozlowski and Willer 48 Preliminary research also suggests that these clinical improvements correlate with improvements in objective measures of physiological recovery including exercise tolerance, cerebral blood flow, and cerebrovascular reactivity.Reference Clausen, Pendergast, Willer and Leddy 49 , Reference Mutch, Ellis and Ryner 50 Although some studies have demonstrated significant improvements in exercise capacity and peak cardiorespiratory responses among adult moderate and severe TBI patients treated with aerobic exercise programs,Reference Bateman, Culpan, Pickering, Powell, Scott and Greenwood 13 , Reference Jankowski and Sullivan 17 , Reference Chin, Chan, Woolstenhulme, Christensen, Shenouda and Keyser 51 , Reference Mossberg, Masel, Gilkison and Urban 52 there has been limited focus on this therapeutic intervention in adolescent patients with TBI. Based on the results of graded aerobic treadmill testing, four patients in this study were prescribed individually tailored submaximal aerobic exercise programs. Although repeat testing was not uniformly undertaken to confirm improvements in exercise tolerance, all patients reported an improvement in residual symptoms and/or exercise tolerance following submaximal aerobic exercise prescription. Repeat treadmill testing in one patient performed 1 month after treatment with an individually tailored submaximal aerobic exercise program was suggestive of improved exercise tolerance as reflected by a longer test duration and higher maximum HR and VO2 peak achieved during testing.
The present pilot study has several limitations. First, the sample size is small and was limited to older adolescents. Second, this study was carried out at a multidisciplinary pediatric concussion program that likely selected for moderate and severe TBI patients who had achieved a good functional outcome postinjury and may not be representative of a more generalized pediatric moderate and severe TBI population. Third, patients did not go undergo comprehensive physiological monitoring of cardiorespiratory function during treadmill testing. Fourth, although all study participants were active adolescents prior to injury, baseline exercise tolerance and fitness level data were not available for comparison to postinjury graded aerobic treadmill testing results. Fifth, compliance with submaximal aerobic exercise programs was not rigorously monitored with digital watches or logbooks and repeat graded aerobic treadmill testing was not performed in the majority of patients. Sixth, this study did not include a control group. Taken together, these factors limit conclusions regarding the independent effect of exercise on patient symptoms and exercise tolerance but highlight factors that should be considered in future prospective studies. Future work is also needed to examine the interrater and test-retest reliability of graded aerobic treadmill testing in this unique TBI population.
In conclusion, preliminary results from this pilot study suggest that graded aerobic treadmill testing is safe, well tolerated, and can be used to evaluate exercise tolerance in appropriately selected adolescent moderate and severe TBI patients. Optimizing the use of graded aerobic treadmill testing requires a multidisciplinary team with clinical training and experience in TBI, trauma, and exercise physiology who can facilitate comprehensive patient screening and monitoring for neurological and cardiorespiratory factors that may limit exercise tolerance. Although standardized BCTT is commonly used in the management of pediatric concussion patients, modifications to the starting speed and test termination symptom threshold should be considered when applying this technique to adolescent moderate and severe TBI patients. Despite the encouraging preliminary results presented here, future studies are needed to confirm the safety and tolerability of graded aerobic treadmill testing in a larger sample of TBI patients including younger adolescents and children. Longitudinal studies using continuous monitoring of cardiovascular, autonomic, and respiratory functioning as well as cerebral blood flow during exercise testing are also needed to understand the pathophysiological mechanisms that mediate aerobic exercise intolerance following pediatric TBI.
Acknowledgments
All phases of this study were supported by a grant from the Pan Am Clinic Foundation, Winnipeg Jets True North Foundation and a generous donation by Leonard and Susan Asper.
DMC, RG, JL, MPM, K. Reimer, K. Russell, and MJE report grants from Pan Am Clinic Foundation, grants from Winnipeg Jets True North Foundation, and grants from Donation to Pan Am Clinic Foundation by Leonard and Susan Asper, during the conduct of the study.
Statement of Authorship
DMC, JL, K. Russell, and MJE conceptualized and designed the study, carried out the data collection and analysis, drafted the initial manuscript, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. RG, MPM, and K. Reimer carried out data collection and analysis, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.




