The capture and storage of CO2 produced by the combustion of fossil fuels is a technical challenge with great impact to the environment. CO2 uptake in metal-organic frameworks (MOFs) has been investigated, although under conditions—high pressure and low water vapor content—more relevant to pre-combustion CO2 capture from syngas (a mixture of H2, CO2, and CO) or natural gas reserves. In a recent investigation of nanoporous organic polymers for CO2 capture at ambient pressure, A.I. Cooper and co-researchers at the University of Liverpool postulated that rather than materials with very high surface areas, the introduction of tailored binding functionalities, and thereby increasing a material’s heat of adsorption, would increase the amount of CO2 adsorbed.
Cooper and co-researchers synthesized two conjugated microporous polymer (CMP) networks that incorporate primary amine and carboxylic groups, respectively. They found that the CO2 uptakes and isosteric heats of adsorption of these CMPs are consistent with previously published computations showing that incorporation of carboxylic acid groups in MOFs, rather than amine groups, leads to the highest isosteric heat of adsorption, suggesting to the researchers a common design principle that spans the two materials classes.
As reported in the May 6th online edition of Chemical Science (DOI: 10.1039/c1sc00100k), Cooper and co-researchers synthesized, isolated, and analyzed by Fourier transform infrared spectroscopy carboxylic acid and amine-functionalized CMP networks designated CMP-1-COOH and CMP-1-NH2, respectively. Insensitive to water, the CMP networks were also shown with thermal gravimetric analysis to be stable up to about 300°C. Scanning electron microscopy showed that the CMP network morphologies are similar to previous CMP networks. N2 adsorption/desorption isotherms, measured at 77 K for CMP-1-COOH and CMP-1-NH2 as well as for previously synthesized CMPs (see figure) are mainly Type I, that is, they show high gas uptake at low pressures (CMP-1-CH3 and CMP-1-OH2 are Type IV). From these isotherms the researchers calculated surface areas and micropore volumes.
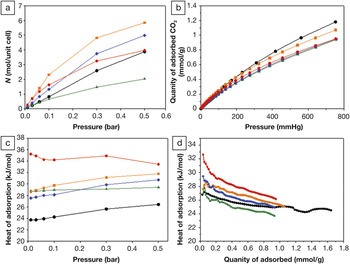
(a) Calculated CO2 isotherms at 298 K for substituted MIL-53 frameworks and (b) measured CO2 isotherms for conjugated microporous polymer (CMP) networks. (c) Calculated isosteric heats of adsorption for CO2 in substituted MIL-53 frameworks and (d) measured for CMP networks. Color-coding is as follows: unsubstituted networks (black); –(CH3)2 (green); –(OH)2 (orange); –NH2 (blue); and –COOH (red). Reproduced with permission from Chemical Science (DOI: 10.1039/c1sc00100k). © 2011 Royal Society of Chemistry
The researchers noted that CO2 uptake, measured at low pressure for the entire series of CMP networks, does not correlate solely with surface area or pore volume. However, the researchers found that the isosteric heats of adsorption depended on the functional group, increasing as: COOH > (OH)2 > NH2 > H > (CH3)2, with the heat of adsorption for CMP-1-COOH being substantially higher than all other CMP networks in this series, higher than activated carbon, but lower than for some MOF networks.
The researchers said that their data corroborates the fundamental computational conclusion that carboxylic acids are good targets for CO2 capture. Although the CO2 binding might be too strong for CO2 capture applications because of the energy penalty due to recycling the material, the researchers said that “the ability to fine tune CO2 affinity in this way [with the selection of functional groups] is of potential value for both CO2 capture and storage as well as gas separation.”


