Almost 2400 years ago, Hippocrates, the father of modern medicine, led the way by his famous aphorism ‘Let food be thy medicine and medicine by thy food’. Epidemiological evidence has shown that healthy dietary habits aid in maintaining good health; however, unhealthy habits have been related to several multifactorial non-communicable diseases(Reference Dominguez, Di Bella and Veronese1–Reference Elizabeth, Machado and Zinöcker4). The metabolic syndrome (MetS) corresponds to an early pathological condition that increases the risk of developing a plethora of chronic non-communicable diseases such as type 2 diabetes, CVD, including stroke(Reference Bohn, Samouda, Alkerwi, Hébert and Hofseth5–Reference Saklayen7), Alzheimer’s disease(Reference Zuin, Roncon and Passaro8) and cancer(Reference Lifshitz, Ber and Margel9). It affects approximately a quarter of the adult population worldwide(Reference Teklu, Zhou and Kapoor10) and also in developed countries. In Luxembourg, the prevalence is about 25 %(Reference Alkerwi, Donneau and Sauvageot11).
MetS is a constellation of several cardiometabolic risk factors and is diagnosed when three out of the five following conditions are met: (i) abdominal obesity, (ii) elevated blood pressure, (iii) elevated fasting blood glucose (FBG), (iv) reduced level of ‘good’ cholesterol (HDL-cholesterol) and (v) high levels of triglycerides (TAG)(Reference Balkau, Valensi and Eschwège6,Reference Saklayen7,Reference Lifshitz, Ber and Margel9,Reference Eckel, Grundy and Zimmet12) . It is estimated that the coexistence of all these factors increases the risk of type 2 diabetes by five times(Reference Beydoun, Chen and Jha13), a disease that affects 500 million people worldwide, including 60 million in Europe(Reference Gesteiro, Megía and Guadalupe-Grau14). In addition, it is estimated that the clustering of the set of MetS components increases by 1·7 the risk of CVD(Reference Beydoun, Chen and Jha13), leading to an annual death toll worldwide of about 18 million individuals(Reference Gesteiro, Megía and Guadalupe-Grau14). It is important to emphasise that other metabolic abnormalities characterise MetS, such as oxidative stress and chronic low-grade inflammation, even if they are not necessarily part of the traditional diagnostic criteria of this syndrome(Reference Zuin, Roncon and Passaro8,Reference Gesteiro, Megía and Guadalupe-Grau14) . Many researchers have though recommend the introduction of C-reactive protein, a marker of inflammation, into the diagnostic criteria for MetS, and oxidative stress markers, such as measured by F2-isoprostanes, are also frequently recommended(Reference Gesteiro, Megía and Guadalupe-Grau14,Reference van’t Erve, Kadiiska and London15) .
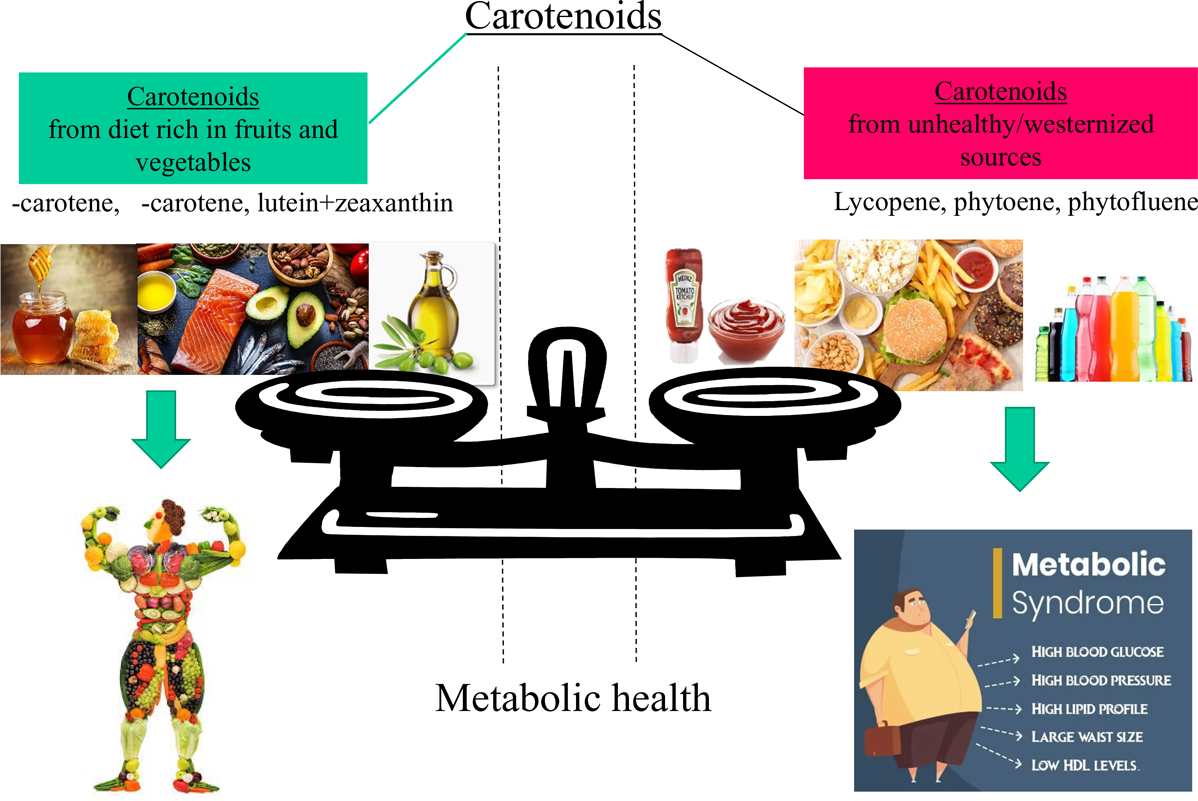
The prevalence of MetS and its cardiometabolic risk factors can be reduced by fostering a healthy lifestyle with increased physical activity and healthy dietary practices(Reference Dominguez, Di Bella and Veronese1,Reference Dreher2,Reference Saklayen7) . For instance, two meta-analyses found an inverse association between fruit and/or vegetable consumption and MetS risk(Reference Zhang and Zhang16,Reference Tian, Su and Wang17) . Health beneficial effects of diets rich in fruits and vegetables on MetS are attributed due to their low energy content, low total carbohydrate to fibre ratios, and also their bioactive constituents, including dietary fibre, vitamins, minerals, polyphenols and also carotenoids(Reference Dominguez, Di Bella and Veronese1,Reference Dreher2,Reference Beydoun, Chen and Jha13) .
Carotenoids are the most abundant secondary plant metabolites in the human body(Reference Bohn18,Reference Biehler, Alkerwi and Hoffmann19) . Though the consumption of carotenoids is relatively low, for example, it is 100 times lower than that of polyphenols, the total concentration of native carotenoids in plasma is about 0·9 to 2·5 µM, much higher than that of native polyphenols, which is typically in the nanomolar range(Reference Biehler, Alkerwi and Hoffmann19). Total blood carotenoids have been proposed as a cardiometabolic health index, with concentrations < 1 µM highly increasing the risk for cardiometabolic diseases(Reference Böhm, Lietz and Olmedilla-Alonso20). The optimum recommended concentrations of β-carotene to reduce the risk of ischaemic heart disease were set at > 0·4 µM(Reference McKay, Lyner and Linden21). In a recent cross-sectional study, it has been shown that plasma β-carotene levels decreased with increasing MetS severity in adult Polish with MetS(Reference Białkowska, Górnicka and Zielinska-Pukos22). Total carotenoid concentrations were also found significantly lower in adults with five MetS components(Reference Białkowska, Górnicka and Zielinska-Pukos22). In this regard, a recent meta-analysis related low circulating carotenoid levels to MetS with lipid disturbances(Reference Iqbal, Mendes and Finney23). In another recent meta-analysis, it was demonstrated that people with high levels of circulating carotenoids were significantly more protected from MetS compared with those with low levels(Reference Beydoun, Chen and Jha13). In addition to the inverse association between serum total carotenoids and MetS, the meta-analysis also revealed an inverse association between several individual carotenoids and MetS. For instance, this association was stronger for β-carotene than for α-carotene, followed by β-cryptoxanthin(Reference Beydoun, Chen and Jha13).
Evidence suggests that regarding MetS risk factors, carotenoids may play critical but differential roles in adipose tissue biology, including the control of adipogenesis, oxidative stress, and the production of adipokines and inflammatory mediators that affect central adiposity distribution and the occurrence of insulin resistance(Reference Beydoun, Chen and Jha13). Indeed, this is exactly what has been proposed in previous reviews(Reference Bonet, Canas and Ribot24,Reference Bonet, Canas and Ribot25) due to the involvement of especially PPAR receptors and adipogenesis, with PPAR being targeted by carotenoids and their apocarotenoid metabolites(Reference Bohn, Bonet and Borel26). Other mechanistic pathways via which carotenoids may be implicated in the aetiology of MetS and its components include direct antioxidant effects(Reference Bohn18,Reference Bouayed, Bohn, Bouayed and Bohn27) , their possible positive effects on the gut microbiota(Reference Böhm, Lietz and Olmedilla-Alonso20,Reference Eroglu, Al’Abri and Kopec28) , and their interactions with transcription factors such as NF-kB and Nrf2 as well as additional nuclear receptors, namely RAR/RXR(Reference Bohn, de Lera and Landrier29).
Carotenoids have been, in several epidemiological studies, considered as valuable indicators of a diet rich in fruits and vegetables(Reference Zheng, Sharp and Imamura30–Reference Ziegler33), being a characteristic of, for example, traditional Mediterranean diets, supplying also secondary plant metabolites at physiological ranges(Reference Bouayed, Bohn, Bouayed and Bohn27,Reference Bouayed and Bohn34) . For example, elevated levels of serum lycopene or intake including lycopene-rich tomatoes have been regarded as protective against MetS risk(Reference Iqbal, Mendes and Finney23,Reference Engelmann, Clinton and Erdman35–Reference Sluijs, Beulens and Grobbee37) . On the contrary, a high intake of lycopene from processed tomato-based products has also been regarded as an indicator of a westernised diet(Reference Böhm, Lietz and Olmedilla-Alonso20), which has been positively associated with MetS(Reference Fabiani, Naldini and Chiavarini38,Reference Rodríguez-Monforte, Sánchez and Barrio39) . Therefore, the role of especially lycopene in the diet and its association with MetS remains somewhat unclear.
In this study, we aimed to assess the possible associations of carotenoid dietary intake as well as their profiles with the MetS, its scores, its cardiometabolic components and anthropometric characteristics by using data from Luxembourg adults participating in the Observation des Risques et de la Santé Cardio-Vasculaire au Luxembourg (ORISCAV-LUX-2) study. More specifically, we hypothesised that the intake of lycopene and possibly other tomato-based carotenoids, due to their further association with westernised dietary patterns, may be differentially related to MetS compared with the intake of other carotenoids that rather characterise a healthy diet rich in fruits and vegetables.
Materials and methods
ORISCAV-LUX-2 study
In 2016–2017, an observational study, termed ‘Observation des Risques et de la Santé Cardio-Vasculaire au Luxembourg’ (ORISCAV-LUX-2), was carried out in Luxembourg(Reference Alkerwi, Pastore and Sauvageot40) as a second wave to the previously conducted ORISCAV-LUX study in 2006/2007(Reference Alkerwi, Sauvageot and Donneau41). A detailed description of the ORISCAV-LUX-2 study has been reported previously(Reference Alkerwi, Pastore and Sauvageot40,Reference Vahid, Brito and Le Coroller42,Reference Vahid, Hoge and Hébert43) . The flow chart of the participants’ sample progression is shown in Fig. 1. From all participants (n 1558) selected following sociodemographic criteria, including the district of residence, age and sex, 212 individuals with incomplete data, including FFQ and metabolic data, were removed from the present study (Fig. 1). Thus, 1346 participants (25–79 years) were retained in this cross-sectional study. Volunteers self-reported their lifestyle habits, including diet, drinking, physical activity and smoking, as well as sociodemographic aspects and health conditions such as job, income, marital status and medical history. Anthropometric measurements, including BMI and waist and hip circumference, were collected by trained nurses. BMI was estimated as weight in kg divided by the square of height in metres (kg/m2). Waist:hip ratio (WHR) was calculated by dividing the circumference of the waist by the circumference of the hip. Biological samples, including blood, were stored under appropriate conditions in the integrated BioBank of Luxembourg. Fasting blood tests were carried out to analyse the following markers: TAG, HDL-cholesterol and FBG. The commercial accredited company Ketterthill (Esch-Sur-Alzette) conducted medical analyses. The National Research Ethics Committee (CNER, No 201-505/12) and the National Commission for Private Data Protection (CNPD) approved the study’s design and the information collected.

Fig. 1. Flow chart of participants’ sample progression. ORISCAV-LUX, Observation des Risques et de la Santé Cardio-Vasculaire au Luxembourg; MetS, metabolic syndrome.
FFQ
The long-term habitual dietary intake of adult residents in Luxembourg was assessed employing a validated semi-quantitative 174-item FFQ, capturing food intake over the preceding 3 months(Reference Vahid, Brito and Le Coroller42). The FFQ was translated into the four frequently used languages in Luxembourg, namely French, English, German and Portuguese; self-reported food intake by adult residents was then back-translated into French to ensure linguistic validity. The FFQ consisted of nine food categories, namely vegetables (cooked and raw), fruits (including canned fruits, compotes, salted and unsalted dried fruits, fresh fruit juices, and fruit juices), meat-poultry-fish-egg, prepared dishes, dairy products, fats, starchy foods, miscellaneous and drinks, including total 174 food and beverage items. Trained nurses guided the respondents to fill out the auto-administered questionnaires and reviewed the correctness of their responses. Weekly and monthly frequency intakes were transformed into daily equivalent frequency intakes. The daily intake of each food (g/d) and drink (ml/d) item for each participant was obtained by multiplying the intake frequency (daily equivalents) by the portion size.
Carotenoid intake assessment
The carotenoids most frequently consumed within the Luxembourgish diet, including β-carotene, α-carotene and β-cryptoxanthin (provitamin A species), and lycopene, lutein + zeaxanthin, astaxanthin, phytoene, phtofluene, neoxanthin and violaxanthin (non-provitamin A species), were assessed, by linking the FFQ data to several carotenoid databases. The concentrations (µg/100 g food item) of provitamin A species, lycopene, and lutein + zeaxanthin in food and drink items contained in the FFQ were extracted using the US Department of Agriculture (USDA) database (website: https://fdc.nal.usda.gov/, accessed in 2023) and the USDA-NCC Carotenoid Database(Reference Holden, Eldridge and Beecher44). As the other carotenoids were not included in the USDA database, additional sources were employed to estimate their intake: Concentrations of phytoene, phytofluene, neoxanthin and violaxanthin were estimated using a local database(Reference Biehler, Alkerwi and Hoffmann19). Concentration of astaxanthin in fish and seafood items was estimated using the EFSA database(45). Carotenoid intake (µg/d) was then computed by multiplying food intake (g/d) by carotenoid concentration (µg/100 g). Afterwards, the participant’s total daily intake for each individual carotenoid, provitamin A and non-provitamin A carotenoids, colourless carotenoids (phytoene + phtofluene), epoxycarotenoids (neoxanthin + violaxanthin), and the sum of all carotenoids consumed by each participant was obtained.
Metabolic syndrome criteria and calculation of its continuous scores
The dichotomous diagnosis of MetS in participants was established following the definition of the National Cholesterol Education Program – Adult Treatment Panel III (NCEP-ATP III)(Reference Saklayen7,Reference Alkerwi, Donneau and Sauvageot11) . Thus, the occurrence of three or more of the following cardiometabolic components were required for MetS diagnosis: (i) waist circumference: ≥ 102 cm for men and ≥ 89 cm for women; (ii) high TAG level: ≥ 150 mg/dl (1·7 mmol/l); (iii) reduced HDL-cholesterol: < 40 mg/dl (1·04 mmol/l) in men and < 50 mg/dl (1·3 mmol/l) in women; (iv) increased blood pressure (systolic blood pressure (SBP) or diastolic blood pressure (DBP)): ≥ 130 or 85 mmHg, respectively; and (v) impaired FBG: ≥ 100 mg/dl (5·6 mmol/l), or components were considered positive if any of them were treated by drugs (except for waist circumference).
A validated continuous score of MetS (siMetS), reflecting the metabolic status of all participants score, was computed employing the following formula(Reference Sebekova and Sebek46,Reference Soldatovic, Vukovic and Culafic47) that includes the cut-off values from the diagnosis of MetS:
In addition, a validated continuous score of MetS, derived from the siMetS score, was used to quantify metabolic risk (siMetS risk score), that is, the risk of cardio/cerebrovascular events, in all ORISCAV-LUX-2 participants(Reference Al Kudsee, Vahid and Bohn48). The calculation of the siMetS risk score relies on the age of participants, with an age of 45 years (for men) or 50 years (the average age of menopause) as a threshold for increased incidence of cardio/cerebrovascular events(Reference Soldatovic, Vukovic and Culafic47). In addition, the presence of family antecedents of cardiovascular events heightens the risk score of MetS by 20 %(Reference Soldatovic, Vukovic and Culafic47). siMetS risk score was computed employing the following formula(Reference Soldatovic, Vukovic and Culafic47):
siMetS risk score = (siMetS score × (Age /45 (men) or 50 (women)) × 1·2 or 1 (if the presence or not of the family antecedent of a cardiovascular event, respectively)).
The units of continuous variables used to calculate siMetS score were mmHg for SBP and DBP, centimetre (cm) for WC (waist circumference) and height, and mmol/l for FBG, TAG, and HDL-cholesterol. The siMetS and siMetS risk scores were calculated separately for women and men using different coefficients in the formulas.
Statistical analysis
The normality of the data distribution and homogeneity of variance was estimated by using Q-Q normality plots and box plots. All variables (unless otherwise noted) were transformed to a logarithmic scale to obtain a normal distribution. Bivariate correlations between intakes of carotenoids were analysed with Spearman’s rho.
For the descriptive analyses, two independent group comparisons were made to study log-transformed continuous data using the independent-samples t tests. The χ 2 test was employed for comparing categorical variables.
Four regression models were set up in our study to investigate the association of carotenoids, fruits, and vegetables with MetS and its related variables. Linearity between independent and dependent continuous variables was verified by plotting dependent variables v. standardised residuals using regression scatter plots and correlation coefficients. The first model was an unadjusted one (crude model). The second model was adjusted for age and sex (model 2, except for the risk score of siMetS). In addition, a set of the identified confounders was retained based on the literature. In model 3, fruits and vegetables were considered as independent variables in addition to carotenoids, and thus the retained confounders included age, sex (men/women), marital status (four categories), current smoking status (four categories), job status (four categories), income (eight categories), birth country (four categories) and total energy intake. Participants with missing income and job status values were included in the category ‘did not answer’. The fourth model was the fully adjusted one used to investigate the role of carotenoids independently from fruit and vegetable intake on MetS (model 4 = model 3 plus adjusted for fruit and vegetable intake).
Multivariable logistic regression was carried out to investigate the association between MetS (as a categorical dependent variable) and the consumption of carotenoids, fruits, and vegetables (log-transformed explanatory variables) using the four models. Multivariable linear regression was further carried out to seek the associations between dietary intake of carotenoids, fruits, and vegetables (log-transformed predictor variables) and continuous dependent variables, including the scores of MetS as well as its components and the different measured anthropometric characteristics, using the four models. Finally, sensitivity analyses were carried out for lycopene, phytoene and phytofluene, in which tomato-based convenience food items rich in these carotenoids (ketchup, lasagna with meat and tomato sauce, pizzas, and burgers) were included as confounding factors.
For all statistical analyses, a P-value below 0·05 (two-sided) was considered significant, and a non-significant P-value above this value but below 0·1 was considered tendency. The raw data were reported as median and interquartile ranges, and the categorical variables were reported as numbers and percentages. All statistical analyses were performed using SPSS 25.0 (SPSS Inc. IBM).
Results
Descriptive analysis (non-adjusted findings)
In the ORISCAV-LUX-2 survey, the overall percentage of MetS within the included individuals was 27·1 %. However, a significantly higher percentage was found for men (32·8 %) in comparison with women (22·1 %) (P < 0·001) (Table 1). Moreover, both continuous scores related to MetS (siMetS and siMetS risk scores) were significantly higher in men than women (P < 0·001). While the level of HDL-cholesterol was significantly higher in women compared with men (P < 0·001), all other MetS components, which were rather positively associated with MetS, were significantly elevated in men v. women (all P < 0·001). The same pattern was found when comparing MetS components between both men and women having MetS (all P < 0·001), except for FBG concentration (P = 0·109). Similarly, all MetS components were further found to be significantly different between both men and women without MetS (all P < 0·001). In this study, the number of women participants who had relatives (father, mother, brother or sister) having at least one cardiovascular event (myocardial infarction or stroke) was higher than men (Table 1).
Table 1. Distribution of the metabolic syndrome (MetS), metabolic outcomes and anthropometric characteristics in Luxembourgish participants (n 1346) of the ORISCAV-2 study. Scores of the MetS and MetS components are reported as median and interquartile ranges

ORISCAV, Observation des Risques et de la Santé Cardio-Vasculaire au Luxembourg; NCEP-ATP III, National Cholesterol Education Program – Adult Treatment Panel III.
The MetS was diagnosed with the NCEP-ATP III criteria.
MetS [+] and MetS [−] account for the presence and absence of the MetS, respectively.
Distribution of MetS is reported as number and percentage (%).
siMetS score and siMetS risk score are continuous MetS scores used to quantify metabolic status and metabolic risk of cardio/cerebrovascular events, respectively.
χ 2 test was used to compare MetS numbers between groups.
Independent-samples t tests were used to compare between the metabolic outcomes of participants (log-transformed data).
All two group comparisons (in the same row) are significant (all P < 0·01), except for the two comparisons highlighted in the table with *P > 0·05).
Two group comparisons included all participants MetS [+] v. all participants MetS [−], men v. women, men MetS [+] v. women MetS [+] and men MetS [+] v. women MetS [+].
No statistical comparison was carried out for the number of participants with a history family of cardio/cerebrovascular events.
Family history of cardio/cerebrovascular events included father, mother, brother or sister having a cardiovascular event (myocardial infarction or stroke).
Daily intake of individual provitamin A species and the total sum of provitamin A species were significantly higher in women than men (all P < 0·05, Table 2). The same pattern was found for phytoene, phytofluene, phytoene + phytofluene, violaxanthin and epoxycartenoids (all P < 0·05). However, daily intake of lycopene (P < 0·001) and astaxanthin (P < 0·05) were significantly higher in men compared with women. Regarding the daily intake of neoxanthin, lutein + zeaxanthin, total non-provitamin A species and total carotenoids, no significant sex differences were found (all P > 0·05, Table 2).
Table 2. Daily intake of carotenoids (µg), fruits (g) and vegetables (g) reported as the median (interquartile range) of Luxembourgish participants (n 1346) of the ORISCAV-2 study

ORISCAV, Observation des Risques et de la Santé Cardio-Vasculaire au Luxembourg; MetS, metabolic syndrome.
The MetS was diagnosed with the NCEP-ATP III criteria.
MetS [+] and MetS [−] account for the presence and absence of the MetS, respectively.
Total provitamin A corresponds to the sum of α-carotene, β-carotene and β-cryptoxanthin.
Epoxycarotenoids is the sum of neoxanthin and violaxanthin.
Phytoene + phtofluene: the sum of these two carotenoids represents colourless carotenoids.
Total non-provitamin A species corresponds to the sum of lycopene, lutein + zeaxanthin, astaxanthin, phytoene, phytofluene, neoxanthin and violaxanthin.
Total carotenoids correspond to the sum of the total provitamin A carotenoids and total non-provitamin A carotenoids.
Carotenoid intakes were estimated by linking a validated 174-item FFQ to several databases, such as the USDA one.
Independent-samples t tests were performed on log-transformed data.
Significant (P < 0·05) and tendency (P < 0·1) P-values are given in bold. P-values in blue ink indicate a tendency, that is, P-value > 0·05 but < 0·1.
With respect to the consumption of fruits, data showed that participants with MetS ate significantly more fruit items than those without MetS (P < 0·01). Such a difference was also observed in men with MetS relative to those without MetS, though results were only marginally significant (P = 0·050). However, data showed that vegetables (P < 0·001) and fruits + vegetables (P < 0·01) were significantly more consumed by women than men (Table 2).
Regarding the intake of carotenoids in persons with v. without MetS, no markable differences were observed, even though intakes of neoxanthin and lycopene in persons with MetS were slightly but significantly higher (Table 2).
As for correlations between individual carotenoids (Fig. 2), the matrix showed that the intakes of all individual carotenoids were significantly correlated (all P < 0·001). However, the highest correlations were observed between phytoene and phytofluene (ρ = 0·997) and α- and β-carotene (ρ = 0·940), and the lowest correlation was between lycopene and α-carotene (ρ = 0·100) (Fig. 2).

Fig. 2. Correlation matrix showing Spearman’s correlation coefficients (ρ) between the intake of individual dietary carotenoids.
Multivariable linear regression analyses
Metabolic syndrome and metabolic syndrome scores (fully adjusted)
A marginal significant inverse association was found (P = 0·05) after full adjustment for the defined confounders (model 4), for total carotenoid intake and lower odds of MetS (dichotomous outcome, with participants without MetS as the reference group) (Fig. 3(a)). Besides, the fully adjusted binary logistic regression showed significant inverse associations between MetS and the dietary intake of α-carotene, β-carotene and total provitamin A carotenoids, that is, a protective effect (Fig. 3(a)).

Fig. 3. Forest plots showing the association of carotenoid intake on the OR of the metabolic syndrome (MetS) (a) and on the β coefficients and its CI of continuous MetS scores, reflecting metabolic status (siMetS score) (b) and metabolic risk (siMetS risk score) (c). The MetS was diagnosed with the NCEP-ATP III criteria. The fully adjusted model (model 4) was employed, adjusting for age, sex, marital status, current smoking status, job status, income, total energy intake, birth country and fruit + vegetable intake. This model was used to study the effect of carotenoids independently from fruit and vegetable intake. Regression analyses for the siMetS risk score did not include sex and age as confounders, as they were included in the formula of this score. Data for logistic regression are expressed as OR with its 95 % CI. Data for linear regression are expressed as β regression coefficient with its 95 % CI. NCEP-ATP III, National Cholesterol Education Program – Adult Treatment Panel III.
Similarly, the fully adjusted multiple linear regression model indicated significant inverse associations between continuous MetS status (siMetS score) and the intake of these carotenoids (α-carotene, β-carotene and total provitamin A species) and also that of lutein + zeaxanthin, that is, a protective effect (Fig. 3(b)). The robustness of the fully adjusted regression model was acceptable, with R2 ranging from 0·158 to 0·162.
In contrast, a higher lycopene intake was rather predictive of a higher siMetS score (P = 0·049, R2 = 0·156) (Fig. 3(b)). A tendency towards a positive association between metabolic status (siMetS score) and the intake of phytoene was also found, suggesting that a higher intake of this carotenoid may increase the risk of having severe MetS (Fig. 3(b)). Among all carotenoid species, only a high intake of α-carotene, β-carotene and total provitamin A carotenoid intakes was significantly associated with lower continuous MetS risk score (Fig. 3(c)). Nevertheless, a tendency towards protective effects against metabolic risk score was also found for violaxanthin, lutein + zeaxanthin and total carotenoid intakes (Fig. 3(c)).
Components of metabolic syndrome and additional anthropometric characteristics (fully adjusted)
The fully adjusted multiple linear regression model indicated an inverse association between FBG levels and the intake of total carotenoids, phytoene + phytofluene and α-carotene (Table 3). Furthermore, a tendency towards a negative association between FBG levels and either the intake of β-carotene, total provitamin A species, phytoene or phytofluene was also found, that is, a protective effect (Table 3).
Table 3. Fully adjusted logistic and linear regression models associating carotenoid intake with components of the metabolic syndrome (MetS) and additional anthropometric measurements of Luxembourgish participants (n 1346) of the ORISCAV-2 study

ORISCAV, Observation des Risques et de la Santé Cardio-Vasculaire au Luxembourg; WHR, waist:hip ratio.
The fully adjusted model (model 4) was used to study the effect of carotenoids independently from fruit and vegetable intake while considering the effects of age, sex, marital status, current smoking status, job status, income, total energy intake, birth country and fruit+vegetable intake.
All predictor variables were log-transformed before multivariable regression models.
Among anthropometric measurements, including BMI and WHR, only waist circumference is considered to be a component of MetS.
Data for logistic regression are expressed as OR with its 95 % CI.
Data for linear regression are expressed as β regression coefficient with its 95 % CI.
Total pro-vitamin A species corresponds to the sum of α-carotene, β-carotene and β-cryptoxanthin.
Epoxycarotenoids is the sum of neoxanthin and violaxanthin.
Phytoene+phtofluene: the sum of phytoene and phtofluene represents colourless carotenoids.
Total non-provitamin A species corresponds to the sum of lycopene, lutein+zeaxanthin, astaxanthin, phytoene, phytofluene, neoxanthin and violaxanthin.
Total carotenoids correspond to the sum of the total provitamin A carotenoids and total non-provitamin A carotenoids.
Significant (P < 0·05) and tendency (P < 0·1) P-values are given in bold. P-values in blue ink indicate a tendency, that is, P-value > 0·05 but < 0·1.
A significant association was found between higher intake of phytofluene and lower DBP, though not for any other carotenoid intake and blood pressure. However, a marginally significant association was found for astaxanthin (inverse association with SBP), neoxanthin (positive association with SBP), phytoene and phytoene + phytofluene (negative associations with DBP), (Table 3).
As for HDL-cholesterol, the fully adjusted multivariable regression model indicated inverse associations between its blood levels and the intakes of lycopene, phytoene, phytofluene, phytoene + phytofluene and total non-provitamin A carotenoids, suggesting potential negative health effects regarding MetS. However, a positive association between HDL-cholesterol levels and carotenoid intake was found only for α-carotene, suggesting potential protective health effects regarding MetS.
Regarding TAG, high intakes of α-carotene, β-carotene, total provitamin A carotenoids and lutein + zeaxanthin were associated with decreased levels of TAG. In contrast, high lycopene intake was positively associated with increased levels of TAG, suggesting potential negative health effects regarding MetS (Table 3).
While high intake of α-carotene showed a tendency to protect from central obesity, intakes of phytoene, phytofluene and phytoene + phytofluene had, however, a tendency to be positively associated with waist circumference. As for BMI, not strictly a component of MetS, after full adjustment for confounders, multiple linear regression models indicated significant inverse associations between this anthropometric characteristic and the intake of the α-carotene, β-carotene, and total provitamin A carotenoids, that is, a protective effect. However, positive associations were found between BMI and intake of phytoene, phytofluene, and tendency for phytoene + phytofluene (Table 3).
A high intake of the same carotenoids, that is, phytoene, in tendency phytofluene and phytoene + phytofluene, was further associated with higher WHR (Table 3). However, high intake of α-carotene was associated with lower WHR.
α- and β-Carotene and the total sum of provitamin A species were the carotenoids most strongly and inversely associated with a high number of components of MetS, with α-carotene being ranked the first, as it was associated with four components in the four models of regression analyses used in this study (Fig. 4). Phytoene, phytofluene and phytoene + phytofluene were also among the carotenoids associated with a high number of MetS components, up to four components in certain regression models (Fig. 4); however, their higher intake was predictive for lower FBG and DBP but also lower HDL-cholesterol and higher waist circumference (Table 3). Lycopene was adversely associated with MetS, significantly associated with two components (Fig. 4), including lower HDL-cholesterol and higher TAG (Table 3).

Fig. 4. Number of significant associations (and those with a tendency, that is, P-value > 0·05 but < 0·1), both inversely related to MetS (bottom part) and those with a positive health impact on MetS (upper part) with the five components of MetS and carotenoid intake, with unadjusted and adjusted linear multivariable regression models. Model 1 was unadjusted; model 2 was adjusted for age and sex; model 3 was further adjusted for marital status, current smoking status, job status, income, total energy intake and birth country; model 4 was further adjusted for fruit + vegetable intake. The five MetS components were central obesity (waist circumference), hypertension (SBP and/or DBP), hyperglycaemia (FBG), TAG and HDL-cholesterol. The number of both associations, either favouring or not MetS, were reported. MetS, metabolic syndrome; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose.
Additional sensitivity analyses – multivariable regression models for lycopene, phytoene and phytofluene containing as confounders tomato-based convenience foods
While analyses showed that higher intake of lycopene predicted severe MetS (siMetS scores), higher TAG and lower HDL-cholesterol (Table 3 for the final adjusted model, and online Supplementary Tables 1–3 for non-adjusted or partially adjusted models), regression analyses considering processed tomato-based food items (pizzas, burgers, ketchup, and lasagna with meat and tomato sauce) as additional confounders demonstrated the absence of any remaining association between lycopene intake and MetS components, in the four models (Table 4 for final adjusted model, and online Supplementary Table 4 for non-adjusted or partially adjusted models). In addition, this adjustment for processed tomato-based items revealed a stronger inverse association of the intake of the colourless carotenoids with FBG and hypertension, particularly in model 3 (Table 4), that is, a protective effect. However, even when adjusting for processed tomato-based items, adverse health associations remained with waist circumference (positive association) and HDL-cholesterol (inverse association), and additional positive associations appeared for BMI and WHR (Table 4).
Table 4. Additional sensitivity analysis for fully adjusted models of logistic and linear regression for tomato-based carotenoids (lycopene, phytoene and phytofluene), taking into account potential confounding factors, that is, processed tomato-based food items, associating them with metabolic syndrome (MetS), its scores, components and additional anthropometric measurements of Luxembourgish participants (n 1346) of the ORISCAV-2 study

ORISCAV, Observation des Risques et de la Santé Cardio-Vasculaire au Luxembourg; WHR, waist:hip ratio.
* Regression analyses for the siMetS risk score did not include sex and age as confounders, as they were included in the formula of this score.
The MetS was diagnosed with the NCEP-ATP III criteria.
siMetS score and siMetS risk score are continuous MetS scores used to quantify metabolic status and metabolic risk of cardio/cerebrovascular events, respectively.
Model 4: adjusted for age, sex, marital status, current smoking status, job status, income, total energy intake, birth country and fruit + vegetable intake. This model was used to study the effect of carotenoids independently from fruit and vegetable intake.
Sensitivity of the model 4 was increased, considering the following processed tomato-based food items as confounders: pizza, burger, ketchup, and lasagna with meat and tomato sauce.
All predictor variables were log-transformed before multivariable regression models.
Among anthropometric measurements, including BMI and WHR, only waist circumference is considered to be a component of MetS.
Data for logistic regression are expressed as OR with its 95 % CI.
Data for linear regression are expressed as β regression coefficient with its 95 % CI.
Total provitamin A species corresponds to the sum of α-carotene, β-carotene and β-cryptoxanthin.
Epoxycarotenoids is the sum of neoxanthin and violaxanthin.
Phytoene + phtofluene: the sum of phytoene and phtofluene represents colourless carotenoids.
Total non-provitamin A species corresponds to the sum of lycopene, lutein + zeaxanthin, astaxanthin, phytoene, phytofluene, neoxanthin and violaxanthin.
Total carotenoids correspond to the sum of the total provitamin A carotenoids and total non-provitamin A carotenoids.
Significant (P < 0·05) and tendency (P < 0·1) P-values are given in bold. P-values in blue ink indicate a tendency, that is, P-value > 0·05 but < 0·1.
Multivariable regression models with fruits and vegetables as independent variables and not confounders
When fruits and vegetables were considered as independent variables (models 1–3, online Supplementary Tables 1–3), associations between the intake of individual carotenoids and total carotenoids, including total provitamin A carotenoids and total non-provitamin A carotenoids, and MetS, its scores, as well as its components, were significantly found. These associations remained significantly associated when fruits and vegetables were considered as confounders (model 4, Table 3).
Adjusted multiple linear regression model without fruits and vegetables as confounders (model 3, fruits and vegetables were considered as independent variables) indicated that fruit consumption was not significantly associated with MetS, its scores, its components and different anthropometric measurements (Table 5). However, high vegetable consumption was inversely associated with lower MetS scores, DBP and BMI, that is, a protective effect. In addition, high vegetable or fruit + vegetable consumption displayed an inverse tendency with FBG, SBP, TAG and waist circumference. Consumption of fruits + vegetables was also negatively associated with metabolic status and metabolic risk (both P < 0·05), and in tendency with BMI (Table 5), that is a protective effect.
Table 5. Adjusted models of logistic and linear regression associating fruit and vegetable intake with metabolic syndrome (MetS), its scores, components and additional anthropometric measurements of Luxembourgish participants (n 1346) of the ORISCAV-2 study

ORISCAV, Observation des Risques et de la Santé Cardio-Vasculaire au Luxembourg; WHR: waist:hip ratio.
* Regression analyses for the siMetS risk score did not include sex and age as confounders, as they were included in the formula of this score.
The MetS was diagnosed with the NCEP-ATP III criteria.
siMetS score and siMetS risk score are continuous MetS scores used to quantify metabolic status and metabolic risk of cardio/cerebrovascular events, respectively.
Regression model (model 3) was adjusted for age, sex, marital status, current smoking status, job status, income, total energy intake and birth country.
All predictor variables were log-transformed before multivariable regression models.
Fruits + vegetables correspond to the combined intake of fruits and vegetables.
Among anthropometric measurements, including BMI and WHR, only waist circumference is considered to be a component of MetS.
Data for logistic regression (only for MetS ATP III) are expressed as OR with its 95 % CI.
Data for linear regression are expressed as β regression coefficient with its 95 % CI.
Significant (P < 0·05) and tendency (P < 0·1) P-values are given in bold. P-values in blue ink indicate a tendency, that is, P-value > 0·05 but < 0·1.
Discussion
The results of our study highlight that carotenoid intake was bidirectionally associated with MetS and its components. A high intake of total carotenoids, in particular of provitamin A species α- and β-carotene, as well as the non-provitamin A carotenoids lutein + zeaxanthin predicted reduced odds of MetS and its cardiometabolic components, decreased MetS scores (siMetS status score) and metabolic risk (siMetS risk score), as well as anthropometric indicators such as waist circumference, BMI and WHR. By contrast, higher consumptions of the tomato-based carotenoids lycopene, phytoene and phytofluene were rather predictive of lower HDL-cholesterol as well as higher MetS status (siMetS), TAG levels and anthropometric characteristics, possibly due to their relation to a more westernised diet. Moreover, linear regression models showed that these effects persisted, even when considering fruit and vegetable consumption as confounders. To our knowledge, this bidirectional relation has thus far not yet been clearly demonstrated.
Carotenoids are lipophilic pigments occurring in the human diet(Reference Bouayed, Bohn, Bouayed and Bohn27) and may exert several beneficial effects, including improving cardiovascular health(Reference Voutilainen, Nurmi and Mursu31) and thus reducing the risk of non-communicable diseases(Reference Beydoun, Beydoun and Fanelli-Kuczmarski49–Reference Cheng, Koutsidis and Lodge51), including MetS(Reference Beydoun, Chen and Jha13,Reference Iqbal, Mendes and Finney23,Reference Leermakers, Darweesh and Baena52) . The majority of diet-derived carotenoids originate from vegetables and fruits, even though other sources such as fish, eggs and milk may contribute to a lesser extent to their dietary intakes(Reference Voutilainen, Nurmi and Mursu31,Reference Holden, Eldridge and Beecher44,45) . Researchers have reported significant positive associations between their intake and blood concentrations, and β-carotene and lycopene are among the most frequently consumed dietary carotenoids and display the highest circulating concentrations of carotenoids(Reference Böhm, Lietz and Olmedilla-Alonso20,Reference Bohn, Desmarchelier and El53) .
The present findings showed that β-carotene was the most frequently consumed carotenoid (6·5 mg/d), followed by lycopene (3·6 mg/d) and α-carotene (2·5 mg/d) (Table 2). A high correlation was found between α-and β-carotene (ρ = 0·94), indicating that β-carotene-rich foods are also a good source of α-carotene (Fig. 2). However, the correlation between lycopene and α-and β-carotene was rather weak (ρ = 0·10 and ρ = 0·14, respectively), indicating different food sources of these carotenoids. Compared with men, women’s median daily intakes of β-carotene (6·9 v. 6·1 mg) and α-carotene (2·6 v. 2·4 mg) were significantly higher, while lycopene consumption was significantly lower (3·2 v. 4·2 mg) (Table 2). Consistent with the literature, it has been reported that women consume higher amounts of provitamin A species than men, resulting in higher blood concentrations(Reference Böhm, Lietz and Olmedilla-Alonso20), and this correlation could likely be stronger when considering the higher daily energy intake in men than in women(Reference Bennett, Peters and Woodward54). Regarding lycopene, both higher and lower concentrations in blood have been reported in men v. women(Reference Böhm, Lietz and Olmedilla-Alonso20).
Carotenoids may reflect a diet rich in fruits and vegetables(Reference Voutilainen, Nurmi and Mursu31–Reference Ziegler33), which would at least contribute to the observed health benefits, following additive or synergist actions of complex mixtures of non-nutrients and nutrients(Reference Bouayed, Bohn, Bouayed and Bohn27,Reference Bouayed and Bohn34) . However, independently from fruit and vegetable intake, the fully adjusted logistic regression model indicated that total carotenoids and total and individual provitamin A species decreased the odds of MetS. A higher dietary intake of these carotenoids, including α-and β-carotene, reduced 31 % to 48 % of the odds of the MetS, respectively (Fig. 3(a)). This is in agreement with the results of Beydoun et al.(Reference Beydoun, Chen and Jha13), indicating an inverse correlation between MetS and serum total carotenoids, with the strongest one observed for β-carotene, followed by α-carotene. In another recent meta-analysis of observational studies, MetS with disturbances in lipid metabolism was linked to reduced serum levels of carotenoids, with the strongest reduction for β-carotene(Reference Iqbal, Mendes and Finney23). However, the lowest relation was found for α-carotene and β-cryptoxanthin(Reference Iqbal, Mendes and Finney23). In a cross-sectional study, it has been reported that low levels of serum carotenoids were associated with higher odds of MetS as well as with an increasing number of any of the five MetS components being present(Reference Kanagasabai, Alkhalaqi and Churilla55). In this cross-sectional study, α- and β-carotene and the total sum of provitamin A species were the carotenoids most strongly and inversely associated with the number of components of MetS (Fig. 4). In light of the above, it can be suggested that carotenoids individually (e.g. β-carotene) and collectively (e.g. total provitamin A carotenoids) appeared to be protective against the incidence of MetS.
However, most often, the diagnosis of MetS is based on a dichotomous decision (presence or not), which may result in the loss of valuable information on the metabolic status of participants, such as those with borderline values or with < 3 positive cardiometabolic risk factors, conditions that impede the clinical diagnosis of MetS. Thus, a validated continuous score of MetS (siMetS score) was calculated to quantify metabolic status, as well as the degree and severity of cardiometabolic affliction in all participants, including those not diagnosed with MetS(Reference Sebekova and Sebek46,Reference Soldatovic, Vukovic and Culafic47) . Findings indicated that the intake of total and individual provitamin A carotenoids (α- and β-carotene) were inversely related to metabolic status (siMetS score) (Fig. 3(b)). In accordance with these findings, daily high consumption of these carotenoids were related to a higher protection of participants from developing high BMI and higher levels of cardiometabolic components of MetS, including FBG and TAG (Table 3). Furthermore, the metabolic risk (siMetS risk score) was quantified for participants, considering both age and heredity (family background of cardiovascular events). The findings indicated that daily intake of total and individual provitamin A carotenoids (α- and β-carotene) significantly reduced the severity of MetS (Fig. 3(b)) and also its risk (Fig. 3(c)), while a low intake enhanced severity and risk.
MetS (dichotomous outcome) was not significantly related to β-cryptoxanthin or any non-provitamin A carotenoids, as well as total carotenoids, despite an existing tendency for the latter. However, as for continuous dependent variables, marginal, even significant beneficial effects of the combined intakes of lutein and zeaxanthin on MetS status score (siMetS), siMetS risk score and TAG were found. Lutein and zeaxanthin can be considered as indicators of a healthier diet, as the major sources of these carotenoids are leafy green vegetables such as broccoli, peas and green beans(Reference Holden, Eldridge and Beecher44). Several health benefits have been attributed to lutein and zeaxanthin, including effects on eye health and cardiometabolic health(Reference Iqbal, Mendes and Finney23,Reference Leermakers, Darweesh and Baena52,Reference Mares56) . For example, a meta-analysis of observational studies(Reference Iqbal, Mendes and Finney23) showed that low blood concentrations of lutein + zeaxanthin were associated with developing MetS, concomitantly with disturbances in lipid metabolism. Another meta-analysis of trials, cohort, cased control and cross-sectional studies showed that higher lutein intake or blood concentrations were related to a reduced likelihood of developing stroke, CHD and MetS(Reference Leermakers, Darweesh and Baena52). Interestingly, there is some evidence that the combined dietary intake of lutein/zeaxanthin, circulating concentrations of lutein/zeaxanthin or lutein alone were related to high levels of physical activity and inversely with sedentary behaviour(Reference Crichton, Elias and Alkerwi57–Reference Thomson, Coates and Howe60). The relationship with physical activity was thought to be causative, which would warrant further investigation of the action of lutein-rich vegetables, possibly related to positive changes in lifestyle factors, following its direct action on the brain, though this remains speculative(Reference Crichton, Elias and Alkerwi57–Reference Gruber, Chappell and Millen59).
Astaxanthin is a red pigment mostly consumed via aquatic species such as salmon, trout, shrimp and crustaceans(45,Reference Leung, Chan and Tam61) . In the ORISCAV-LUX-2 study, astaxanthin was the lowest-consumed carotenoid by participants (median of 47 µg/d). Several health benefits have been attributed to this carotenoid, including its protective role on the cardiovascular system(Reference Leung, Chan and Tam61,Reference Donoso, González-Durán and Muñoz62) . Leung et al.(Reference Leung, Chan and Tam61) pointed out in a meta-analysis of randomised controlled trials its protective effect on individuals at risk of MetS, reducing in tendency total cholesterol, SBP, and at a significant level, LDL-cholesterol. The fully adjusted linear regression model also indicated a marginal significant and, thus, protective effect of astaxanthin on SBP (P = 0·06) (Table 3). However, when fruits and vegetables were not considered as confounders, a significant inverse association between astaxanthin intake and SBP, as well as marginal on DBP, was found (online Supplementary Table 3). Due to the very low intake of astaxanthin, likely, this component acted primarily as an indicator for the intake of certain types of fish and seafood, such as trout and shrimp, which have been inversely associated with MetS(Reference Tørris, Småstuen and Molin63–Reference Karimi, Heidari and Firouzi65).
In this study, women consumed significantly more fruits, vegetables and fruits + vegetables than men. Linear regression models showed that either vegetable or fruit + vegetable consumption were inversely associated with MetS scores and its components, that is, suggesting a protective effect (Table 5). However, fruits alone failed to demonstrate an inverse relation with MetS and its components in the adjusted models. The unadjusted model even indicated a positive relation to MetS in the participants (online Supplementary Table 1), which could be related to the high sugar content in all fruit items or be due to inverse causality, that is, that persons with MetS made sure to consume sufficient fruits per d.
Most importantly, findings from the present study suggested that lycopene, phytoene and phytofluene were positively associated with MetS severity (continuous scale) (Fig. 3(b)). These carotenoids have been especially associated with convenient, processed and fast food containing processed tomato products such as pizza, ketchup, sauces and burgers. Thus, unlike α- and β-carotene, lutein and zeaxanthin, lycopene, and to a lesser degree, due to lack of data, phytoene and phytofluene, have been associated with rather westernised dietary patterns(Reference Böhm, Lietz and Olmedilla-Alonso20). For example, 5 g of ketchup may contain 439 µg of lycopene, which is at least three times higher than that of raw tomato(Reference Biehler, Alkerwi and Hoffmann19), not yet considering also its much higher bioavailability(Reference Unlu, Bohn and Francis66). The fully adjusted linear regression model showed that higher intake of lycopene was predictive of higher metabolic severity (siMetS) score and TAG, as well as lower levels of HDL-cholesterol. It is worth noting that the consumption of lycopene-rich tomatoes in some studies was related to reduced likelihood of several chronic diseases, including CVD, for which the risk may diminish due to, for example, cholesterol-lowering effects(Reference Engelmann, Clinton and Erdman35,Reference Løchen67) . Likewise, elevated serum lycopene levels or intake were associated with a reduced risk of MetS(Reference Iqbal, Mendes and Finney23,Reference Sluijs, Beulens and Grobbee37) . In addition, a meta-analysis of human interventional trials revealed that lycopene intake significantly ameliorated HDL-cholesterol levels(Reference Inoue, Yoshida and Sasaki36). Therefore, adverse effects of lycopene on the metabolic health of participants of the ORISCAV-LUX-2 study may be attributed to its indicator properties for high consumption of processed tomato-based foods and thus as a marker of a rather westernised diet. However, when pizzas, burgers, ketchup, and lasagna with meat and tomato sauce were included as confounders in the model, no physiologically adverse associations remained between lycopene consumption and the severity of MetS and its components (Table 4). This suggests that lycopene by itself is not a ‘bad’ carotenoid, but it is rather a valuable indicator for unhealthy/westernised diets. In general, it has been pointed out that dietary patterns in Luxembourg, as in other westernised countries, have been developing towards more westernised patterns, marked by lower fruit and vegetable intake and increased intake of meat, sauces, drinks, and fast, processed, and convenient foods(Reference Vahid, Brito and Le Coroller42) rich in salt, sugar, fat, and additives, and characterised by low quantities of micronutrients(Reference Bouayed and Bohn3,Reference Ifland, Preuss and Marcus68) . These aspects may have suppressed any beneficial direct effects that lycopene may have had on the subjects in this study.
Phytoene and phytofluene are typically not included in food carotenoid databases despite their large presence in plant-based foods such as oranges, prunes, apricots, peaches, watermelon, and especially tomatoes and tomato products(Reference Engelmann, Clinton and Erdman35,Reference Meléndez-Martínez, Mapelli-Brahm and Benítez-González69) . These precursors of other carotenoids are found in human samples, including plasma, milk and tissues(Reference Meléndez-Martínez, Mapelli-Brahm and Benítez-González69). However, epidemiological evidence is missing to confirm their putative health advantages observed in in vitro and animal studies(Reference Meléndez-Martínez, Mapelli-Brahm and Benítez-González69). In the present study, the median daily intake of the colourless phytoene and phytofluene in participants (3·2 mg) was only slightly lower than that of lycopene (3·6 mg) (Table 2). Even though the fully adjusted logistic regression models did not confirm any significant association with MetS, higher intake of phytoene and/or phytofluene was positively associated with metabolic severity score and anthropometric measures, including waist circumference, BMI and WHR. In addition, significant inverse associations were found between phytoene and/or phytofluene consumption and HDL-cholesterol. These results suggest that phytoene and phytofluene may be, similarly as to lycopene, associated with westernised dietary patterns, as they also occur in lycopene-rich foods such as ketchup/pizza. However, consumption of these colourless carotenoids was found to be inversely associated with FBG and DBP, that is, a protective effect. The fact that these colourless carotenoids still presented adverse associations with MetS components even after adjusting for processed tomato products containing foods (Table 4) may suggest a differential action of phytoene/phytofluene or merely reflect a broader dietary origin compared with lycopene. This is in line with the modest correlation found between lycopene and both phytoene (ρ = 0·38) and phytofluene (ρ = 0·36) (Fig. 2), suggesting that lycopene-rich foods are only a part of food items that supplied these colourless carotenoids for participants of ORISCAV-LUX-2 survey.
The main strength of this study is the combination of various databases for completing carotenoid containing food items, with the most frequently consumed carotenoids being included. Several potential confounders were considered, including fruit and vegetable intake. The number of participants in this study is fairly high, especially given that Luxembourg is a small country. In addition, the FFQ was applied by trained nurses. However, the main limitation of this investigation is capturing food intakes by FFQ, as this technique – as other dietary recalls, is prone to recall bias(70). In addition, the cross-sectional nature of this study does not allow for drawing causative conclusions. Another limitation of our study is the lack of data regarding the study participants’ use of carotenoid supplements, as well as the lack of serum measurements related to the intake of carotenoids. It is essential to consider these factors in future studies to investigate the effects of carotenoids comprehensively.
In conclusion, this study showed divergent effects of carotenoid intake on metabolic status, risk and syndrome, and its cardiometabolic components in ORISCAV-LUX-2 participants, depending on the carotenoid type. While α- and β-carotene, total provitamin A carotenoids, and lutein + zeaxanthin were associated with improved MetS-related health outcomes, high intake of lycopene, phytoene and phytofluene were associated with worse outcomes. As lycopene and uncoloured carotenoids dietary sources in a westernised diet (same as our study) are mainly from processed tomato-containing foods, including pizza, burgers, puree, sauces and ketchup, they may be considered as new indicators of an unhealthy and westernised dietary pattern. Future investigations are warranted to explore the role of lycopene and the uncoloured carotenoids phytoene and phytofluene as markers of dietary patterns or their direct impact on components of the MetS.
Acknowledgements
The authors appreciate the dedication of all participants who took part in the ORISCAV-LUX 2 study. The authors thank the research nurses involved in the ORISCAV-LUX 2 study. A special thanks to Torsten Bohn for his help encompassing the idea generation, writing of this MS and his advice for the interpretation. The authors are finally very much indebted to all members of the ORISCAV-Working Group who contributed to the planning and conduction of the ORISCAV-LUX studies: Ala’a Alkerwi, Stephanie Noppe, Charles Delagardelle, Jean Beissel, Anna Chioti, Saverio Stranges, Jean-Claude Schmit, Marie-Lise Lair, Marylène D’Incau, Jessica Pastore, Gloria Aguayo, Gwenaëlle LeCoroller, Michel Vaillant, Hanen Samouda, Brice Appenzeller, Laurent Malisoux, Sophie Couffignal, Manon Gantenbein, Yvan Devaux, Laetitia Huiart, Dritan Bejko, Guy Fagherazzi, Magali Perquin, Maria Ruiz-Castell and Isabelle Ernens.
The ORISCAV-LUX 2 data collection was funded by the LIH (Ministry of Higher Education and internal research funding). The outgoing international mobility of Jaouad Bouayed to LIH was supported by Lorraine Université d’Excellence (LUE) – Widen Horizons.
J. B. conducted statistical analyses and wrote the manuscript. F. V. supported the statistical analyses and reviewed the paper. All authors have read and agreed to the published version of the manuscript.
The authors declare no conflict of interest.
All participants were informed and consented to take part in the study.
Data will be available on request from the corresponding authors. Due to our institute’s rules and laws, the data are not publicly available.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114524000758